Abstract
Lifestyle interventions may reduce inflammation and lower breast cancer (BrCa) risk. This randomized trial assessed the impact of the Sistas Inspiring Sistas Through Activity and Support (SISTAS) study on plasma C-reactive protein (CRP), interleukin-6 (IL-6) and Dietary Inflammatory Index (DII).
This unblinded, dietary and physical activity trial was implemented in 337 obese (body mass index [BMI] ≥30 kg/m2) African American (AA) women recruited between 2011 and 2015 in South Carolina through a community-based participatory approach with measurements at baseline, 3 months, and 12 months. Participants were randomized into either intervention (n=176) or wait-list control group (n=161). Linear mixed-effect models were used for analyses of CRP and IL-6.
Baseline CRP was significantly higher in those with greater obesity, body fat percentage, and waist circumference (all p-values <0.01). No difference was observed between groups for CRP or IL-6 at 3 or 12 months; however, improvements in diet were observed in the intervention group compared to the control group (p-value=0.02) at 3 months but were not sustained at 12 months.
Although the intervention was not successful at reducing levels of CRP or IL-6, a significant decrease was observed in DII score for the intervention group, indicating short-term positive dietary change.
Clinical Trial Number: NCT02144571.
Trial Status: Completed.
Keywords: African Americans, breast cancer, C-reactive protein, Dietary Inflammatory Index, inflammation
Introduction:
Breast cancer (BrCa) is the most frequently diagnosed cancer among women in the United States (US), as well as in South Carolina (SC), where it accounts for 32% of all cancer cases in women (Siegel, Miller, and Jemal 2016). In the US overall, European-American (EA) and African-American (AA) women have approximately equal incidence rates of BrCa, but AAs have both higher mortality rates and shorter 5-year-survival (Siegel, Miller, and Jemal 2016). In SC, EA women have higher BrCa incidence rates (129.8/100,000 compared to 111.6/100,000 for AAs), whereas BrCa mortality is 1.5-times higher in AA women (Adams et al. 2006).
Factors that increase BrCa risk include reproductive factors, such as age at first pregnancy, total number of pregnancies, and lactation history; older age; family history of BrCa (primarily for premenopausal BrCa); obesity; and lifestyle factors, including diets high in omega-6 polyunsaturated fatty acids and low levels of physical activity (PA) (Adams et al. 2006). It is probable that the role of diet in BrCa works at least partially through its effect on inflammation (Kuczmarski et al. 2013). It is well-established that inflammation has a role in the initiation of cancer and is a key factor in tumor progression (Coussens and Werb 2002). Chronic inflammatory conditions predispose to cancers (An and Kulkarni 2015). Obesity, which has higher prevalence rates among AA women, is acknowledged as a metabolically-induced chronic state of inflammation, which is linked with a general increase in cancer incidence (An and Kulkarni 2015). In the United States, about 60% of AA women are obese compared with about 30% of non-Hispanic European American (Dietze, Chavez, and Seewaldt 2018). AA women also have a high incidence of insulin resistance and premenopausal triple negative breast cancer (TNBC). Some studies have shown associations among obesity, insulin signalling, and aggressive subtypes of TNBC. (Dietze, Chavez, and Seewaldt 2018). Obesity promotes tissue inflammation, which in turn leads to high levels of inflammatory markers (e.g., IL-6, IL-8, TNF-α, and leptin). The process of signalling for some of these inflammatory markers (e.g., IL-6 and IL-8) in turn initiates STAT3, NF-κB, and EZH2 signalling and predicts poor prognosis in women with TNBC (Dietze, Chavez, and Seewaldt 2018).
Diet and PA can play a key role in reducing inflammation and lowering cancer risk; indeed, higher levels of PA and fruit and vegetable intake, both of which contribute to lower levels of systemic inflammation, have been shown to reduce the risk of BrCa, particularly among AA women (Shivappa et al. 2017), Recent work has shown that the Dietary Inflammatory Index (DII®) is a reliable measure of the inflammatory potential of an individual’s diet and is a good predictor of risk for breast and endometrial cancers (Shivappa et al. 2017, Shivappa et al. 2016).
A frequent problem among diet and PA interventions is sustainability, with behavioral changes during the study period often not translating into long-term successes (Greaves et al. 2011). Health interventions that use community involvement, as well as those that are implemented within a group setting, may help create lasting lifestyle changes (Cowart et al. 2010) through a variety of proposed mechanisms, including group and environmental support. The Sistas Inspiring Sistas Through Activity and Support (SISTAS) study was a community-developed and community-implemented dietary and PA intervention for AA women (Adams et al. 2015, Malcolm Bevel et al. 2018). The purpose of this investigation was to assess the impact of the SISTAS study on two biological markers of chronic inflammation: plasma C-reactive protein (CRP) and interleukin-6 (IL-6). CRP was selected as serological marker of inflammation in this study was because of its previous use as a marker of inflammation in cancer risk research (Gunter et al. 2006). It is an acute-phase protein produced primarily by the liver in response to stimulation by IL-6 and correlates with the magnitude and severity of inflammation (Gabay and Kushner 1999). Plasma cytokine IL-6 was examined because increase in IL-6 has been reported to be correlated with disease status among cancer patients and because of its role along the signalling pathway of CRP (Chung and Chang 2003).
Methods
The SISTAS study was a randomized, wait-list control, dietary and PA intervention implemented among obese (body mass index [BMI] ≥30 kg/m2) AA women. All protocols and consent forms were approved by the Institutional Review Board at the University of South Carolina, and all participants provided written informed consent. This Clinical trial was registered at ClinicalTrials.gov (NCT02144571), and the status is completed. The name of the trial registration is Sistas Inspiring Sistas Through Activity and Support. No adverse effects were reported by participants in this trial.
Recruitment
The SISTAS study was marketed to the general AA community in multiple ways, including flyers posted throughout the community (physician offices, hospitals, etc.), brochures at stores and other places of business that AA women visit frequently, church bulletins and announcements, Facebook™, employee listservs, and, for later waves, word of mouth of participants in previous waves. The study period was July 2011 to February 2015. To qualify for inclusion, participants had to: self-identify as AA; be ≥30 years of age; be obese (BMI >30 kg/m2, calculated from self-reported height and weight); be willing to be randomized to the intervention or control group; not have a previous cancer diagnosis, have no inflammatory-related conditions; and have stable hormone replacement therapy usage. BMI was later confirmed at baseline using digital scale and stadiometer measurements.
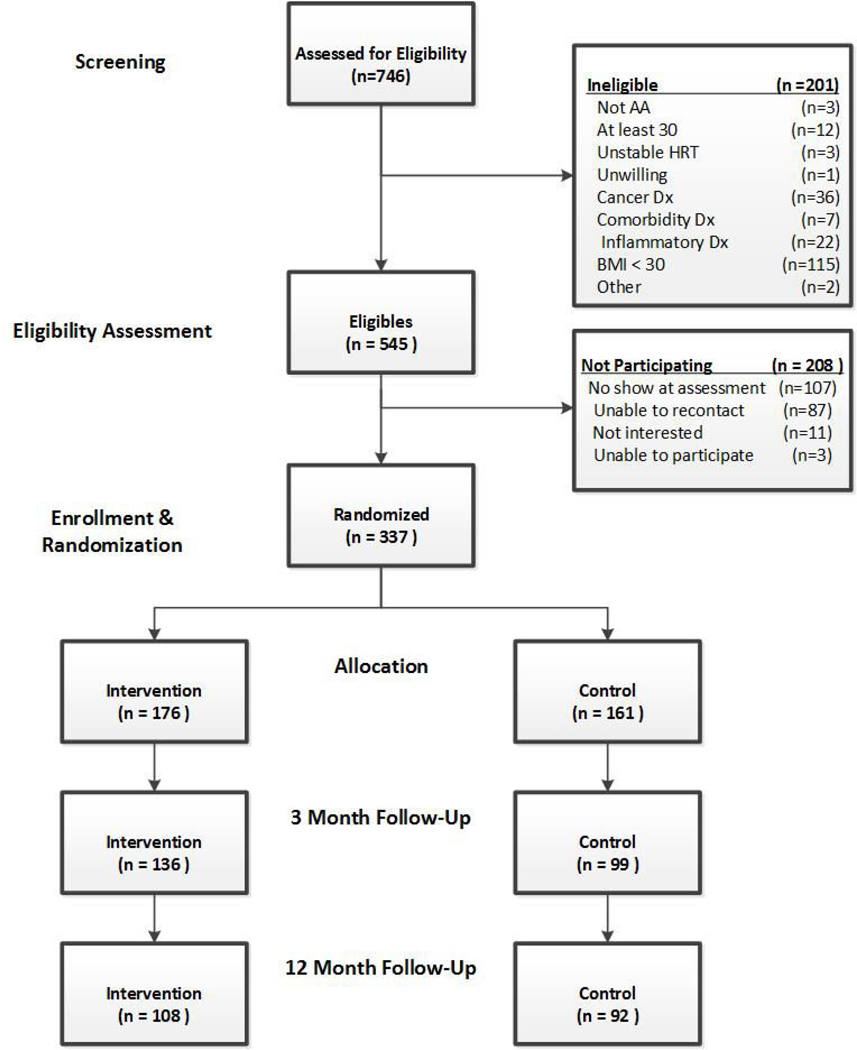
A total of 746 potential participants were screened for eligibility, 545 were eligible, and 337 were recruited [See Figure 1 for the CONSORT diagram]. The trial was not blinded but all assessments were conducted by staff without access to or knowledge of the treatment Access® 2010 database.
Figure 1:
SISTAS CONSORT Diagram.
Additional details about recruitment can be found elsewhere (Adams et al. 2015). The numbers of participants that were analyzed were 176 in the intervention group and 161 in the control group.
Intervention design
The SISTAS study was developed as a year-long dietary and PA intervention using community-based participatory approaches for engaging AA leaders and community members as partners in the intervention design (Babatunde et al. 2016, Hebert et al. 2013). The intervention consisted of three months of weekly classes (two hours per week), followed by nine months of monthly ‘booster’ sessions (two hours per month). The dietary targets for the intervention were increasing the consumption of whole foods (fruits and vegetables, legumes, and whole grains) and decreasing the consumption of calorically dense, high-fat (especially saturated and trans-fat) foods. More specifically, attention was focused on reduced consumption of high-fat pro-inflammatory foods (especially fast foods containing omega-6 polyunsaturated fatty acids and saturated fatty acids) and increasing consumption of anti-inflammatory foods, such as beans, brassica vegetables, and whole grains. The curriculum consisted of classes focusing on hands-on healthy cooking experiences, along with PA and stress reduction exercises.
The PA targets for this intervention were consistent with previously released PA recommendations for adults (Haskell et al. 2007). Participants were encouraged to obtain at least 30 minutes of moderate-intensity PA (equivalent to brisk walking) on at least five days of the week and engage in at least two sessions of strength training each week. To ensure safety, participants were instructed to progress gradually in increasing the intensity and duration of PA. The control arm did not attend any classes but received biweekly correspondence of small participation gifts for the first three months and monthly materials for the following nine months.
Measurements
All participants were assessed at baseline, three months, and 12 months. Assessments were conducted at the same locations where the intervention classes were held. Body habitus measurements (height, weight, body composition, waist and hip circumference), and blood samples were taken at all three time points by trained staff. Weight and fat mass were measured using a Tanita® TBF 300As electronic scale precise to 0.1 kg and 0.1% fat, respectively. Height was measured using a stadiometer, and waist and hip circumference were measured using a Gulick™ measuring tape. BMI was computed as weight (kg)/(height, m)2, and waist-to-hip ratio (WHR) was also computed. Additionally, participants completed detailed questionnaires ascertaining information on basic demographics, medical history, self-efficacy for diet and exercise, perceived discrimination, perceived stress, social support, and quality of life (Franklin et al. 2007). Within two weeks of this visit, participants also completed a telephone-administered dietary and PA 24-hour recall (24HR).
Estimates of energy, nutrient, and food group intakes were derived from the 24HR, considered an imperfect “gold standard” for estimating dietary intake (Kristal AR 2001) in a nutrition intervention to calculate group-level mean dietary intake (Willett) and centiles of the distribution using standard statistical techniques (Hebert et al. 2000). The Nutrient Data System for Research software (NDSR; current version), licensed by the Nutrition Coordinating Center (NCC) at the University of Minnesota (Minneapolis, Minnesota), was used to conduct the dietary interviews. In this study, the 24HRs were collected by a team of experienced (>6 years using NDSR) registered dietitians specifically trained in using the NCC protocol. This protocol employs the multi-pass approach that uses prompting to reduce omissions and standardizes the interview methodology across interviewers (Dwyer et al. 2001). Calculation of the DII has been described previously (Shivappa et al. 2014). More positive DII scores represent more pro-inflammatory diets; more negative values indicate more anti-inflammatory diets (Shivappa et al. 2014).
Physical activity data was obtained using a previous-day recall (PDR) A previous-day recall (PDR) was used to collect information on physical activity. This instrument has been validated as a measure of physical activity and sedentary behaviors. (Matthews et al. 2013) PDR interviews were conducted by the same dietitians who conducted the 24HR dietary recall and who were trained to complete the PDR using a standardized protocol. Interviewers led participants chronologically through the previous day (midnight to midnight) using a semi-structured interview. These data were summed across all individual sedentary and active behaviours that were reported based on estimates of the duration of the activity. Time spent in each type activity was also summarized into light intensity (1.5 to less than 3 METs), moderate intensity (3 to less than 6 METs), and vigorous intensity (6+ METs). (Bassuk and Manson)
Blood Processing and Assays
All blood samples collected were assayed for CRP and high-sensitivity IL-6. Peripheral whole blood samples were collected in EDTA and centrifuged for 15 minutes at 1,000g immediately following collection. Samples were then aliquoted, put on liquid nitrogen, and stored at −80ºC until analysis. Samples were analyzed using ELISA kits (R & D systems, Minneapolis, MN). The minimal detectable dose in the ELISA kits ranges from 0.005 to 0.022 ng/mL for CRP and 0.016 to 0.110 pg/mL for IL-.6.
Data Analysis
All questionnaire-derived data were collected via an optically scannable form developed in Teleform™. Questionnaires were visually verified at the assessment sessions and then scanned by study personnel using the Teleform™ software. All data files were output to an ASCII file format that was then imported into SAS® v9.4 for data cleaning and creation of the primary analytic dataset.
All analyses were run using SAS® v9.4 (Cary, NC). Summary descriptive statistics were created for all relevant variables, including means and frequency tables. Comparisons were made using t-tests or chi-square tests, as appropriate. A linear, mixed-effects model (Proc Mixed in SAS) was used to model the effect of the intervention assignment on biomarker levels after adjusting for the baseline level of the biomarker. Initial analyses used an intent-to-treat approach and included all participants enrolled and randomized into the study. Additional analyses examined intervention fidelity using dietary and anthropometric measures (weight, BMI, body fat percentage, waist-to-hip ratio, DII).
The main purpose of these intent-to-treat analyses was to determine the effect of the SISTAS intervention on CRP and IL-6 levels. In the analyses that we present in the tables, the main factor that we adjusted for in our analyses was age at baseline due to the high correlation with level of inflammatory markers. One participant refused to report her age and was excluded from all analyses; otherwise, we had complete follow-up data for all participants who came back for 3-month and 12-month assessments. We therefore used a listwise deletion method (complete case analyses). As this was an intent-to-treat analysis, no other confounding variables were included in our models.
Of those randomized to receive the intervention (n=176), 60% of participants attended 50% or more of the intervention classes (>5 classes). We ran sensitivity analyses to assess the effect that the attendance in classes had on the value of the two main outcomes, i.e., CRP and IL-6 among the intervention group and found no statistically significant relationship between either CRP or IL-6 level (at the 3-month assessment) and attendance in classes (p=−0.168 and 0.09, respectively).
Results
A total of 337 participants were randomized; intervention group (176) and control group (161). At three months follow-up, there were 136 (77%) and 99 (61%) participants retained in the intervention and control groups respectively. At 12 months follow-up, there were 108 (61%) and 92 (57%) participants retained in the intervention and control groups respectively.
Overall, no significant differences in demographic and lifestyle characteristics were observed between those randomized to the intervention versus the control arm with the exception of BMI (Table 1). As the overall mean level of inflammatory markers for our population was especially high in comparison to other reported findings (Ahonen et al. 2012, Bellia et al. 2013, Fonseca and Izar 2016, Gode et al. 2011, Imayama et al. 2012, Kubota et al. 2010, Sarac et al. 2007, Tsai et al. 2012), we further explored baseline differences in the inflammatory markers by anthropometric and dietary factors (Table 2). Mean CRP was significantly higher in those with greater obesity as measured by BMI, body fat percentage, and waist circumference (all p-values <0.01). No significant differences were noted in mean baseline IL-6 levels by body size or DII score (Table 2).
Table 1.
Population and Lifestyle Characteristics at Baseline by Intervention Status, SISTAS, 2010–2015.
| Characteristic | Intervention Mean±SDa or n (%) N=175 | Control Mean±SDa or n (%) N=161 | p-value |
|---|---|---|---|
| Age (years) | 50 ± 11 | 49 ± 11 | 0.70 |
| Marital Status | |||
| Married or living with partner | 69(51.11) | 66(48.89) | 0.91 |
| Widowed | 10(52.63) | 9(47.37) | |
| Divorced or separated | 51(55.43) | 41(44.57) | |
| Single, never married | 46(50.55) | 45(49.45) | |
| Educational Status | |||
| High school or less | 31(44.29) | 39(55.71) | 0.21 |
| Some college | 80(56.74) | 61(43.26) | |
| Complete college | 34(57.63) | 25(42.37) | |
| Postgraduate | 31(46.27) | 36(53.33) | |
| Employment Status | |||
| Full-time | 92(50.27) | 91(49.73) | 0.84 |
| Part-time | 22(57.89) | 16(42.11) | |
| Retired | 28(45.90) | ||
| Not employed | 28(51.85) | 26(48.15) | |
| Perceived Health | |||
| Excellent or very good | 55(56.12) | 43(34.21) | 0.33 |
| Good | 97(52.72) | 87(47.28) | |
| Fair or poor | 24(43.64) | 31(56.36) | |
| Smoking Status | |||
| Current or former | 55(50.46) | 54(49.54) | 0.62 |
| Never | 121(53.30) | 106(46.70) | |
| Alcohol Use | |||
| Yes | 121(54.02) | 103(51.79) | 0.31 |
| No | 54(48.21) | 58(51.79) | |
| Body Mass Index (kg/m2) | 38.31±7.03 | 39.89(7.77) | 0.05 |
| Waist-to-Hip Ratio | 0.88±0.07 | 0.88±0.09 | 0.89 |
| Body Fat Percentage | 45.67±5.81 | 46.70±5.39 | 0.09 |
| C-Reactive Protein (mg/l) | 9.84±23.34 | 8.68±8.13 | 0.55 |
| Interleukin-6 (pg/ml) | 7.10±33.04 | 3.94±5.30 | 0.22 |
SD-standard deviation
Table 2.
Descriptive Statistics of Baseline Inflammatory Markers Stratified by Anthropometric and Dietary measures, SISTAS, 2010–2015.a
| CRP (mg/L)a | IL-6 (pg/mL)a | |||
|---|---|---|---|---|
| Mean±SDa | p-value | Mean±SDa | p-value | |
| BMI (kg/m2) | ||||
| Low (25.0–37.5) | 5.91±5.91 | <0.01 | 9.97±48.41 | 0.54 |
| High (37.6–70.0) | 11.07±10.20 | 6.24±17.07 | ||
| Body Fat Percentage (%) | ||||
| Low (20.0–45.4) | 5.91±5.46 | <0.01 | 12.03±50.24 | 0.18 |
| High (45.4–70.0) | 11.07±10.20 | 3.96±3.50 | ||
| Waist-to-Hip Ratio | ||||
| Low (0.40–0.87) | 8.30±8.71 | 0.80 | 11.03±49.21 | 0.36 |
| High (0.88–1.10) | 8.67±8.46 | 5.27±15.35 | ||
| DII Score | ||||
| Low (−6.0 – 0.4) | 8.08±8.91 | 0.57 | 8.36±46.16 | 0.93 |
| High (0.4 – 6.0) | 8.90±8.23 | 7.85±22.61 | ||
| Waist Circumference (inches) | ||||
| Low (30.0–43.0) | 6.57±7.41 | <0.01 | 10.87±48.58 | 0.32 |
| High (43.1–70.0) | 10.82±9.39 | 4.74±5.23 |
WHR-waist-to-hip ratio; DII-Dietary Inflammatory Index; BMI-body mass index; CRP-C reactive protein; IL-6-Interleukin-6; SD-standard deviation.
No significant differences were noted for CRP or IL-6 by treatment arm at either 3 or 12 months; however, the intervention group did demonstrate significant changes in measures of intervention (Table 3). At three months, the intervention group had significantly lower (i.e., more anti-inflammatory) DII scores (−0.14 vs 0.85, p-value=0.02) compared to the control arm; however, this difference was not sustained at 12 months (Table 3). WHR was marginally significantly lower in the intervention group at three months (0.86 vs. 0.88, p-value=0.09), but this difference was not sustained at 12 months.
Table 3:
Inflammatory and Anthropometric Outcomes by Intervention Status at the 12-Week and 1-Year Follow-Up Timepoints, SISTAS, 2010–2015.a
| 3-month Follow-Up | 12-month Follow-Up | |||||
|---|---|---|---|---|---|---|
| LSM (95% CI)a | Difference between Intervention and Control | p-value | LSM (95% CI)a | Difference between Intervention and Control | p-value | |
| OUTCOME | ||||||
| CRP (mg/l) | ||||||
| Intervention | 7.93 (7.31, 8.53) | −1.54 | 0.12 | 8.22 (7.52, 8.92) | 0.82 | 0.46 |
| Control | 9.47 (8.70, 10.24) | 7.40 (6.55, 8.25) | ||||
| IL-6 (pg/ml) | ||||||
| Intervention | 1.00 (0.93, 1.07) | −0.06 | 0.59 | 0.92 (0.83, 1.01) | −0.22 | 0.12 |
| Control | 1.06 (0.97, 1.15) | 1.14 (1.03, 1.25) | ||||
| Weight | ||||||
| Intervention | 225.24 (224.64, 225.84) | −1.08 | 0.27 | 225.93 (224.43, 227.43) | −1.18 | 0.62 |
| Control | 226.32 (225.56, 227.08) | |||||
| BMI (Kg/m2) | ||||||
| Intervention | 39.19 (39.04, 39.34) | −0.15 | 0.55 | 39.71 (39.41, 40.01) | −0.2 | 0.67 |
| Control | 39.34 (39.15, 39.53) | 39.91 (39.54, 40.28) | ||||
| Fat Percentage | ||||||
| Intervention | 45.93 (45.48, 46.38) | 0.09 | 0.91 | 46.19 (45.67, 46.71) | 0.59 | 0.48 |
| Control | 45.84 (45.27, 46.41) | 45.60 (45.96, 46.24) | ||||
| Waist-to-Hip Ratio | ||||||
| Intervention | 0.86 (0.85, 0.87) | −0.2 | 0.09 | 0.87 (0.86, 0.88) | 0.0 | 0.61 |
| Control | 0.88 (0.87, 0.89) | 0.87 (0.86, 0.88) | ||||
| DII Score | ||||||
| Intervention | −0.14 (−0.40, 0.12) | −0.99 | 0.02 | −0.55 (−0.94, −0.16) | −0.56 | 0.35 |
| Control | 0.85 (0.52, 1.18) | 0.01 (−0.44, 0.45) |
WHR-waist-to-hip ratio; DII-Dietary Inflammatory Index; BMI-body mass index; CRP-C reactive protein; IL-6-Interleukin-6; LSM, least squares mean; CI, confidence interval.
Discussion
Although mean baseline inflammatory markers of our participants provide evidence for a population with some of the highest levels of chronic inflammation yet observed(Ahonen et al. 2012, Bellia et al. 2013, Fonseca and Izar 2016), our intervention was not successful at reducing levels of CRP or IL-6 through PA and dietary changes. Interestingly, DII scores were significantly decreased in the intervention group at three months, relative to the control group, an indicator of positive dietary behavior change, but this difference was not sustained at 12 months.
All results presented here describe the intent-to-treat analysis of all participants who were randomized and for whom we had complete data. Upon closer inspection, we found evidence for significant contamination among our control group; indeed, we observed a higher frequency of any weight loss during the study period in our control group compared to the intervention arm (59.5% vs 49.1%) (Hébert et al. 2016). Although participants specifically consented to being randomized, and those randomized to the comparison group were offered the intervention upon completion of the follow-up period, our data suggest that ultimately these women who volunteered for a dietary and PA behavioral trial were motivated to make immediate changes and were unwilling to wait, consistent with prior observations (Hébert et al. 2016). This is understandable, given that the psychological literature supports that readiness to change is high in volunteers for this type of study (Jordan et al. 2013). This observation also suggests that randomized controlled trial designs (stipulated by the funding agency) are not ideal for community-based participatory research-developed trials such as this in which community partners and potential participants are engaged about making positive lifestyle changes. Although randomized controlled trials are cited as the “gold standard” for rigorous scientific methodology, this certainly highlights the weaknesses of this design for this type of behavior change (Adams et al. 2015).
The participants’ neighborhood environments may have influenced the impact of our intervention. Although previous studies in a systematic review reported mixed results, physical environments that support healthy lifestyles, including access to healthy foods, neighborhood safety, and neighborhood walkability, are facilitators of or barriers to adopting healthy behaviors (Caspi et al. 2012). In a post-hoc analyses, the impact of the SISTAS intervention on dietary intake differed by the food access level of the participant’s residential area.(Choi et al. 2014) Participants living in areas with limited access to healthy foods and resources for engaging in PA may have found it difficult to make the behavior changes learned through the intervention. Similarly, for those participants making behavior changes, it could be more challenging to maintain these healthier behaviors without a supportive environment (Services 2001). Understanding the complex interaction between the intervention and the neighborhood environments of participants in both the intervention and control arms will be helpful in considering the design and tailoring of interventions in the future.
Our findings should be interpreted within the context of several study limitations. First, we were only able to assess two biomarkers of inflammation. Although the biomarkers chosen are those with the largest body of literature supporting their importance in carcinogenic pathways (Ahonen et al. 2012), they by no means represent a comprehensive evaluation of inflammation. Second, our study assessed plasma levels of inflammation, which may not always reflect underlying gene expression changes at the cellular level. In addition, given that CRP levels were already high in our population of AA women, our results may not be generalizable to women of other racial minorities. Another limitation was the convenience nature of our sampling, which may have reduced the representativeness of the sample and thus the generalizability of results.
Additionally, because we recruited 337 out of the 548 eligible participants and because only 60% of participants attended at least 50% of the classes, participation bias potentially affected the results from this study. We are unable to speculate about the eligible 211 participants that were not randomized. The predominant reason for not randomizing an eligible participant (who indicated verbal agreement to the study) was not that the participant did not attend the baseline assessment. We implemented numerous strategies to maximize attendance including increasing the frequency and timing of telephone reminders, mailing reminder postcards, emphasizing monetary incentives, and having our community staff make frequent announcements and conversations with potential participants of upcoming ‘clinics’ (assessment visits).
Unfortunately, we did not have any demographic information about them (our only contact with them was via phone or brief visits during a health fair) to run a sensitivity analysis to see if this may have played a role in biasing the findings from this study. Finally, we had indirect evidence of a lack of compliance to treatment assignment in the control group (we found significant weight loss in more than 50% of our control group). This misclassification would most likely bias our findings toward the null.
Despite its weaknesses, our study had several strengths to consider. Our study was conducted among a racially under-represented and geographically diverse group of overweight, AA women who demonstrated significant biological need for intervention to improve health outcomes. Furthermore, this study represented a community-driven health initiative with some of the highest recruitment rates noted in the literature, i.e., 84% were enrolled into the study out of the potential participants assessed for eligibility in the first three waves (Adams et al. 2015, Greiner et al. 2014, Heiney et al. 2006b). This recruitment rate dropped somewhat as the trial progressed, most likely due to a move from the rural to the more urban area. We hypothesized that word of mouth and a relationship with the intervention staff (who resided in the rural area) greatly enhanced our recruitment in that area. Nevertheless, our overall recruitment rate of 62% represents a significant improvement over traditional clinical trial participation (Heiney et al. 2006a). Finally, the prospective cohort study design and standard evaluation protocols ensured the least amount of bias possible in study estimates.
Overall, we found that DII scores significantly decreased at three months, an indicator of positive dietary behavior change, but was not sustained at the 12-month assessment. What remains unknown is whether the primary outcomes would have been different if significant contamination in the control group, as evidenced by a higher frequency of any weight loss during the study, had not occurred. We also do not know what motivated the control group participants to lose weight, despite not receiving the intervention, compared with the intervention group. The baseline values of the inflammatory markers in this sample were markedly higher than those evidenced in most other study populations. We hypothesize that this may provide evidence for underlying biologic pathways leading to increased incidences of diabetes, cardiovascular disease, and other chronic diseases noted among this population in comparison to other racial groups. Also, physical activity was self-reported by the subjects which is a relatively unobjective measure. Finally, the complex interaction between the intervention and the neighborhood environments of participants in both the intervention and control arms is relatively unexplored in this cohort and could have influenced the study findings.
Conclusion
In conclusion, we demonstrated a significant inflammation and obesity burden among AA women who were obese, which may well have long-term health implications. We had only limited success in modifying this profile to a more favorable state. Our study provides promising preliminary evidence that efforts aimed at individually tailoring interventions to each participant’s built environment characteristics may result in improved outcomes. As evidenced by contamination in our control arm, future work also should be focused on consideration of alternative intervention designs that do not minimize the enthusiasm of individuals who volunteer for research studies because they are interested in behavior change. This need is particularly acute in community-based participatory research studies.
ACKNOWLEDGMENTS
The South Carolina Cancer Disparities Community Network Project is supported by the National Institutes of Health, National Center on Minority Health and Health Disparities, GRANT # (1U54CA153461–01). Principal Investigator James R. Hebert, MSPH, ScD, also was supported by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute (K05 CA136975). Both James R. Hébert, Sc.D. and Michael D Wirth, PhD, also were supported by grant R44 DK 103377 from the National Institute of Diabetes, Digestive and Kidney Diseases. Oluwole A. Babatunde was supported by a National Cancer Institute’s F99 Fellowship grant (1F99CA22272201) as principal investigator.
Footnotes
DISCLOSURE: Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the DII™ from the University of South Carolina in order to develop computer and smart applications for patient counselling and dietary intervention in clinical settings. Dr. Michael Wirth is an employee of CHI.
CONFLICTS: No Author declares a conflict of interest
References
- Adams Swann Arp, Hebert James R., Susan Bolick-Aldrich Virginie G. Daguise, Mosley Catishia M., Modayil Mary V., Berger Sondra H., Teas Jane, Mitas Michael, Cunningham Joan E., Steck Susan E., Burch James, Butler William M., Horner Marie-Josephe D., and Brandt Heather M.. 2006. “Breast Cancer Disparities in South Carolina: Early Detection, Special Programs, and Descriptive Epidemiology.” Journal of the South Carolina Medical Association (1975) 102 (7):231–239. [PMC free article] [PubMed] [Google Scholar]
- Adams SwannArp, Heiney SueP, Brandt HeatherM, Wirth MichaelD, Khan Samira, Johnson Hiluv, Davis Lisa, Wineglass CassandraM, TatianaY Warren-Jones TishaM Felder, Drayton RubyF, Davis Briana, Farr DeeonnaE, and Hébert JamesR. 2015. “A Comparison of a Centralized Versus De-centralized Recruitment Schema in Two Community-Based Participatory Research Studies for Cancer Prevention.” Journal of Community Health 40 (2):251–259. doi: 10.1007/s10900-014-9924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen T, Vanhala M, Kautiainen H, Kumpusalo E, and Saltevo J.. 2012. “Sex differences in the association of adiponectin and low-grade inflammation with changes in the body mass index from youth to middle age.” Gend Med 9 (1):1–8. doi: 10.1016/j.genm.2012.01.002. [DOI] [PubMed] [Google Scholar]
- An G, and Kulkarni S.. 2015. “An agent-based modeling framework linking inflammation and cancer using evolutionary principles: description of a generative hierarchy for the hallmarks of cancer and developing a bridge between mechanism and epidemiological data.” Math Biosci 260:16–24. doi: 10.1016/j.mbs.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babatunde Oluwole A, Adams Swann A, Wirth Michael D, Eberth Jan M, Sofge Jameson, Harmon Brook, Davis Lisa, Drayton Ruby, Hurley Tom, and Brandt Heather M. 2016. “Predictors of participants’ retention among African Americans in the Healthy Eating and Living in The Spirit (HEALS) trial.” Ethnicity & Diseases 76 (14 Supplement):1764–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk SS, and Manson JE. “(2009) ‘Physical Activity, Fitness, and the Prevention of Cardiovascular Disease’ in Epidemiologic Methods in Physical Activity Studies First Edition Oxford University Press; 198 Madison Avenue, NY 10016: 158–177.” [Google Scholar]
- Bellia A, Garcovich C, D’Adamo M, Lombardo M, Tesauro M, Donadel G, Gentileschi P, Lauro D, Federici M, Lauro R, and Sbraccia P.. 2013. “Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects.” Intern Emerg Med 8 (1):33–40. doi: 10.1007/s11739-011-0559-x. [DOI] [PubMed] [Google Scholar]
- Caspi CE, Sorensen G, Subramanian SV, and Kawachi I.. 2012. “The local food environment and diet: a systematic review.” Health Place 18 (5):1172–87. doi: 10.1016/j.healthplace.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SK, SA Adams H Johnson C Wineglass, Khan S, Wirth MD, Warren-Jones T, Bevel MS, Ingram C, and Hebert JR. 2014. “Food access impacts dietary intervention effect among obese African-American women.” 142nd Annual Meeting of the American Public Health Association. [Google Scholar]
- Chung YC, and Chang YF. 2003. “Serum interleukin-6 levels reflect the disease status of colorectal cancer.” J Surg Oncol 83 (4):222–6. [DOI] [PubMed] [Google Scholar]
- Coussens Lisa M., and Werb Zena. 2002. “Inflammation and cancer.” Nature 420 (6917):860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart LW, Biro DJ, Wasserman T, Stein RF, Reider LR, and Brown B.. 2010. “Designing and pilot-testing a church-based community program to reduce obesity among African Americans.” ABNF J 21 (1):4–10. [PubMed] [Google Scholar]
- Dietze EC, Chavez TA, and Seewaldt VL. 2018. “Obesity and Triple-Negative Breast Cancer: Disparities, Controversies, and Biology.” Am J Pathol 188 (2):280–290. doi: 10.1016/j.ajpath.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J, Ellwood K, Leader NP, Moshfegh AJ, and Johnson CL. 2001. “Integration of the Continuing Survey of Food Intakes by Individuals and the National Health And Nutrition Examination Survey.” J Am Diet Assoc 101 (10):1142–3. [DOI] [PubMed] [Google Scholar]
- Fonseca FA, and Izar MC. 2016. “High-Sensitivity C-Reactive Protein and Cardiovascular Disease Across Countries and Ethnicities.” Clinics (Sao Paulo) 71 (4):235–42. doi: 10.6061/clinics/2016(04)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin MD, Schlundt DG, McClellan LH, Kinebrew T, Sheats J, Belue R, Brown A, Smikes D, Patel K, and Hargreaves M.. 2007. “Religious fatalism and its association with health behaviors and outcomes.” Am J Health Behav 31 (6):563–72. doi: 10.5555/ajhb.2007.31.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, and Kushner I.. 1999. “Acute-phase proteins and other systemic responses to inflammation.” N Engl J Med 340 (6):448–454. [DOI] [PubMed] [Google Scholar]
- Gode F, Karagoz C, Posaci C, Saatli B, Uysal D, Secil M, and Akdeniz B.. 2011. “Alteration of cardiovascular risk parameters in women with polycystic ovary syndrome who were prescribed to ethinyl estradiol-cyproterone acetate.” Arch Gynecol Obstet 284 (4):923–9. doi: 10.1007/s00404-010-1790-9. [DOI] [PubMed] [Google Scholar]
- Greaves Colin J., Sheppard Kate E., Abraham Charles, Hardeman Wendy, Roden Michael, Evans Philip H., and Schwarz Peter. 2011. “Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions.” BMC Public Health 11 (1):119. doi: 10.1186/1471-2458-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner K. Allen, Friedman Daniela B., Adams Swann Arp, Gwede Clement K., Cupertino Paula, Engelman Kimberly K., Meade Cathy D., and Hebert James R.. 2014. “Effective recruitment strategies and community-based participatory research: community networks program centers’ recruitment in cancer prevention studies.” Cancer Epidemiology, Biomarkers & Prevention 23 (3):416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter MJ, Canzian F, Landi S, Chanock SJ, Sinha R, and Rothman N.. 2006. “Inflammation-related gene polymorphisms and colorectal adenoma.” Cancer Epidemiol Biomarkers Prev 15 (6):1126–31. doi: 10.1158/1055-9965.epi-06-0042. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, and Bauman A.. 2007. Physical Activity and Public Health. Updated Recommendation for Adults From the American College of Sports Medicine and the American Heart Association. Reprint, NOT IN FILE. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Gupta PC, Mehta H, Ebbeling CB, Bhonsle RR, and Varghese F.. 2000. “Sources of variability in dietary intake in two distinct regions of rural India: implications for nutrition study design and interpretation.” Eur J Clin Nutr 54 (6):479–86. [DOI] [PubMed] [Google Scholar]
- Hébert James R, Frongillo Edward A, Adams Swann A, Turner-McGrievy Gabrielle M, Hurley Thomas G, Miller Donald R, and Ockene Ira S. 2016. “Perspective: Randomized Controlled Trials Are Not a Panacea for Diet-Related Research.” Advances in Nutrition: An International Review Journal 7 (3):423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert James R., Wirth Michael, Davis Lisa, Davis Briana, Harmon Brook E., Hurley Thomas G., Drayton Ruby, Murphy E. Angela, Shivappa Nitin, Wilcox Sara, Adams Swann A., Brandt Heather M., Blake Christine E., Armstead Cheryl A., Steck Susan E., and Blair Steven N.. 2013. “C-reactive protein levels in African Americans: a diet and lifestyle randomized community trial.” American Journal of Preventive Medicine 45 (4):430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiney SP, Adams SA, Cunningham JE, McKenzie W, Harmon B, Hebert JR, and Modayil M.. 2006a. “Subject recruitment for cancer control studies in an adverse environment.” Cancer Nursing 29 (4):291–299. [DOI] [PubMed] [Google Scholar]
- Heiney Sue P, Adams SA, Cunningham JE, McKenzie W, Harmon B, Hebert J, and Modayil M. 2006b. “Subject recruitment for cancer control studies in an adverse environment.” Cancer Nursing 29 (4):291–301. [DOI] [PubMed] [Google Scholar]
- Imayama I, Ulrich CM, Alfano CM, Wang C, Xiao L, Wener MH, Campbell KL, Duggan C, Foster-Schubert KE, Kong A, Mason CE, Wang CY, Blackburn GL, Bain CE, Thompson HJ, and McTiernan A.. 2012. “Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial.” Cancer Res 72 (9):2314–26. doi: 10.1158/0008-5472.can-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S, Watkins A, Storey M, Allen SJ, Brooks CJ, Garaiova I, Heaven ML, Jones R, Plummer SF, Russell IT, Thornton CA, and Morgan G.. 2013. “Volunteer bias in recruitment, retention, and blood sample donation in a randomised controlled trial involving mothers and their children at six months and two years: a longitudinal analysis.” PLoS One 8 (7):e67912. doi: 10.1371/journal.pone.0067912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristal AR, Satia JA. 2001. “Evaluation of Nutrition Intervention In: Nutrition in the Prevention and Treatment of Disease, editor. Coulston AM, Rock CL, Monsen ER. San Diego: Academic Press 2001.” [Google Scholar]
- Kubota Y, Moriyama Y, Yamagishi K, Tanigawa T, Noda H, Yokota K, Harada M, Inagawa M, Oshima M, Sato S, and Iso H.. 2010. “Serum vitamin C concentration and hs-CRP level in middle-aged Japanese men and women.” Atherosclerosis 208 (2):496–500. doi: 10.1016/j.atherosclerosis.2009.07.052. [DOI] [PubMed] [Google Scholar]
- Kuczmarski, Fanelli Marie, Mason Marc A., Allegro Deanne, Zonderman Alan B., and Evans Michele K.. 2013. “Diet quality is inversely associated with C-reactive protein levels in urban, low-income African-American and white adults.” Journal of the Academy of Nutrition & Dietetics 113 (12):1620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevel Malcolm, Babatunde Oluwole, Heiney Sue P, Brandt Heather M, Wirth Michael D, Hurley Thomas G, Khan Samira, Johnson Hiluv, Wineglass Cassandra, Warren-Jones Tatiana Y, Murphy E Angela, Sercy Erica, Thomas Amanda, Hebert James R, and Adams Swann Arp. 2018. “Sistas Inspiring Sistas Through Activity and Support (SISTAS): Study Design and Demographics of Participants.” Ethnicity and Disease; In press: Accepted for Publication January 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CE, Keadle SK, Sampson J, Lyden K, Bowles HR, Moore SC, Libertine A, Freedson PS, and Fowke JH. 2013. “Validation of a previous-day recall measure of active and sedentary behaviors.” Med Sci Sports Exerc 45 (8):1629–38. doi: 10.1249/MSS.0b013e3182897690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarac F, Ozgen AG, Yilmaz C, and Tuzun M.. 2007. “Cardiovascular risk factors in obese women and their first-degree relatives.” Anadolu Kardiyol Derg 7 (4):371–7. [PubMed] [Google Scholar]
- Services, U.S. Department of Health and Human. 2001. “The Surgeon General’s call to action to prevent and decrease overweight and obesity. [Rockville, MD: ]: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; [2001]. Available from: U.S. GPO, Washington.”. [PubMed] [Google Scholar]
- Shivappa N, Blair CK, Prizment AE, Jacobs DR, and Hebert JR. 2017. “Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women.” Mol Nutr Food Res 61 (5). doi: 10.1002/mnfr.201600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivappa N, Hebert JR, Zucchetto A, Montella M, Serraino D, La Vecchia C, and Rossi M.. 2016. “Dietary inflammatory index and endometrial cancer risk in an Italian case-control study.” Br J Nutr 115 (1):138–46. doi: 10.1017/s0007114515004171. [DOI] [PubMed] [Google Scholar]
- Shivappa N, Steck SE, Hurley TG, Hussey JR, and Hebert JR. 2014. “Designing and developing a literature-derived, population-based dietary inflammatory index.” Public Health Nutr 17 (8):1689–96. doi: 10.1017/s1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel Rebecca L., Miller Kimberly D., and Jemal Ahmedin. 2016. “Cancer statistics, 2016.” CA: A Cancer Journal for Clinicians 66 (1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Tsai JP, Po HL, Yen CH, Hou CJ, Kuo JY, and Hung CL. 2012. “Body composition, C-reactive protein, carotid artery remodeling and subclinical atherosclerosis in a general Taiwanese population.” J Thromb Thrombolysis 33 (2):185–92. doi: 10.1007/s11239-011-0669-3. [DOI] [PubMed] [Google Scholar]
- Willett W. “(2013) ‘24-Hour Recall and Diet Record Methods’ in Nutritional Epidemiology: Third Edition Monographs in Epidemiology and Biostatistics. Oxford University Press 198 Madison Avenue, NY: 10016: 49–68.” [Google Scholar]