Abstract
Electron microscopy has been a valuable tool for the study of platelet biology and thrombosis for more than 70 years. Early studies using conventional transmission and scanning electron microscopy (EM) provided a foundation for our initial understanding of platelet structure and how it changes upon platelet activation. EM approaches have since been utilized to study platelets and thrombi in the context of basic, translational and clinical research, and they are instrumental in the diagnosis of multiple platelet function disorders. In this brief review, we provide a sampling of the many contributions EM based studies have made to the field, including both historical highlights and contemporary applications. We will also discuss exciting new imaging modalities based on EM and their utility for the study of platelets, hemostasis and thrombosis into the future.
Introduction
The study of platelet biology using electron microcopy methods has a long and rich history. It was not long after the introduction of the electron microscope that the first studies of platelet ultrastructure using transmission electron microscopy were published [1]. Conventional electron microscopy is divided into transmission electron microscopy (TEM) and scanning electron microscopy (SEM). In TEM, a beam of electrons is transmitted through a thin section in order to visualize the internal structures. By contrast in SEM, a focused beam of electrons is used to scan the surface of a specimen. A considerable amount of work went into developing the protocols to fix soft tissues so that they could be imaged by electron microscopy, work strongly motivated by the need to visualize tissues and their internal structures in ways that were not possible with any other microscopy technique before. Once the proper fixation protocols had been developed, electron microscopy became instrumental to study platelets, both for basic research [2] and as a diagnostic tool [3]. Today, conventional TEM and SEM approaches remain valuable tools in platelet and thrombosis research, even as EM approaches continue to evolve and new imaging modalities are developed.
A separate review in this series, by Scandola et al [4], focuses on the use of EM approaches for the study of megakaryocytes. The reader is referred to that review for an outstanding general description of the various EM methods that are in use today for the study of both megakaryocytes and platelets. In this brief review we summarize some of the contributions made using electron microscopy methods for the study of platelets and thrombus formation. Given the plethora of studies that have utilized EM approaches for the study of platelet biology, the goal of this review is not to be exhaustive, but only to provide examples that highlight the utility of EM approaches across a variety of experimental and clinical applications. We will also discuss recent advances in EM methods for visualization of platelets and thrombi, including the use of electron tomography, cryo-EM, serial block face-SEM, focused ion beam-SEM, and correlative light and electron microscopy (CLEM).
Transmission electron microscopy
Historical TEM studies of basic platelet biology
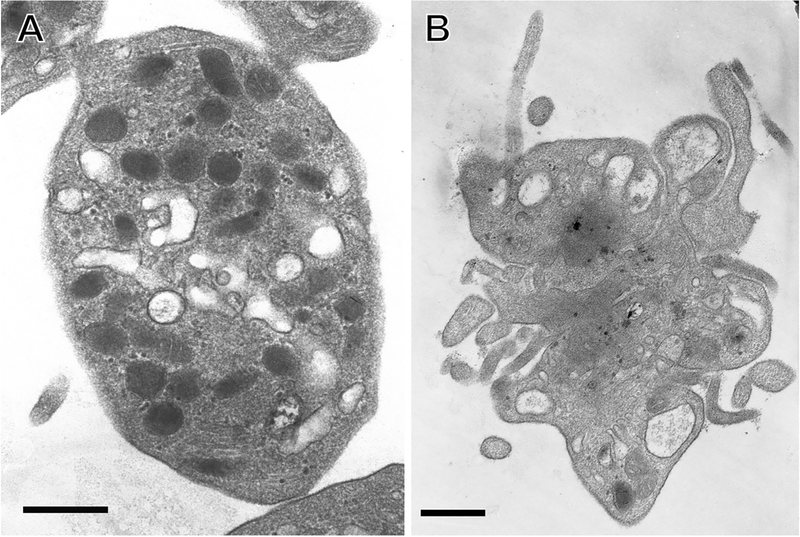
From the time that electron microscopes became available for the study of biological specimens, investigators have utilized EM approaches to study platelets. Using primitive fixation techniques, Bessis first showed crude platelet ultrastructure in the late 1940s and early 1950s [5]. These initial studies paved the way for a much deeper understanding and characterization of the subcellular components of platelets, and soon studies had described the presence of multiple granule subtypes, intracellular membrane systems and cytoskeletal features (Figure 1) [6–8]. The identification of the microtubule coil that is responsible for the discoid shape of resting platelets was an advance in the early understanding of platelet physiology made possible by EM [9–12]. EM studies also showed that the microtubule coil contracts following platelet activation, resulting in centralization of platelet organelles [13]. In the late 1980s and early 90s, fixation and permeabilization protocols were adapted to visualize multiple components of the platelet cytoskeleton using EM. These studies from Hartwig and others were foundational to our current understanding of the platelet cytoskeletal network. They identified the presence of a spectrin-based membrane skeleton in platelets, and characterized how the membrane, actin and microtubule skeletons interact with each other [14,15]. They also further characterized how changes in the platelet cytoskeleton drive platelet shape change [16]. For comprehensive reviews of the platelet cytoskeleton including outstanding EM images, the reader is referred to chapters devoted to the subject [17,18].
Figure 1: Use of TEM to study platelet structure.
TEM images show A) isolated resting human platelet; B) isolated thrombin-activated human platelet. Scale bars = 500 nm. Image credit: from the laboratory of John W. Weisel: Anastasia A. Ponomareva and Rustem I. Litvinov.
TEM studies were also critical to the early understanding of platelet intracellular granules. Reports from the 1960s determined that dense granules (dense bodies, δ-granules) were the storage compartment for serotonin, ADP and ATP and established the lack of dense granules as the basis for several platelet function disorders, including Hermansky-Pudlak syndrome (HPS). Indeed, White showed that electron opaque platelet dense granules were best visualized by TEM imaging of whole mount platelets [6]. Quantification of dense granules using such imaging remains the gold standard for identifying dense granule deficiencies, including HPS and Chediak-Higashi syndrome [19]. TEM studies demonstrated abnormal α-granules in multiple platelet disorders as well, such as gray platelet syndrome and Paris-Trousseau-Jacobsen syndrome [20,21]. Cytoskeletal defects that impact overall platelet morphology and granule number, such as Wiskott-Aldrich syndrome [22], may also be readily identified by TEM. For additional information on the use of TEM to identify platelet disorders, the reader is referred to many excellent reviews on the subject [3,19,23–25].
Continuing use of TEM for basic science studies of platelet biology
The wealth of knowledge on platelet ultrastructure derived from early TEM studies continues to inform basic science research on platelets today. For example, investigators recently used TEM to investigate the effects of human aging on platelets, and reported that platelets from older patients have reduced α-granule content compared to young and middle age patients [26]. TEM-based measurements of mitochondrial content differences in platelets helped establish a link between aging-associated production of the proinflammatory cytokine TNF-α and the development of platelet hyper-reactivity [27]. The intracellular origin and structure of platelet-derived microparticles was also studied using TEM to provide a structural basis for the qualitative differences in microparticle formation induced by various platelet activators [28]. Multiple groups have utilized TEM to detail the molecular mechanisms and kinetics underlying platelet granule release [29–31]. All of these studies serve as examples of how TEM imaging of isolated platelets continues to inform our understanding of basic platelet biology in both health and disease.
TEM examination of hemostatic plugs and thrombi
Studies on ‘single’ platelets are key to define the building blocks of platelet biology. These studies are complemented by studies on platelet aggregates formed under various conditions in vitro and in vivo to understand aspects of hemostasis and thrombosis that emerge from multiple intercellular interactions. TEM observations were pivotal in describing the contents, structure, and temporal evolution of hemostatic plugs formed in vivo in both animal models and humans [32–36]. Similar studies also characterized defective hemostatic plug formation in various pathologic settings, such as hemophilia [35,37]. These studies were in large part the basis for our current models of hemostatic system function, including the initiation of platelet adhesion and activation by binding to collagen exposed at the site of injury. Similarly, thrombosis has been investigated using TEM in conjunction with other laboratory assays or imaging methods. Early studies from Mustard and others provided the initial characterization of platelet-rich thrombi, including the spatiotemporal evolution of platelet activation and fibrin formation [38]. Multiple recent studies highlight the continuing utility of conventional TEM imaging for examination of platelet ultrastructure to elucidate their role in hemostasis and thrombosis. For example, TEM was used to examine how clot contraction induces spatial re-distribution of procoagulant platelets in a murine model of thrombosis [39], and to study how platelet necrosis recruits neutrophils in the setting of ischemic stroke in mice [40].
Immunogold electron microscopy
Immunogold labeling is a staining technique that helps to localize cellular structures and macromolecules by loading antibodies or other specifically targeted proteins with colloidal gold particles of nanometer size. Gold is used for its high electron density that increases electron scatter to give dark spots in the TEM and SEM images. This technique has been applied successfully in many platelet studies. For example, immunogold labeling was used to demonstrate the spatial distribution of the GPIb-IX-V complex and αIIbβ3 on resting and activated platelets (reviewed in [41]). Immunogold labeling in combination with TEM has been used to determine the subcellular localization of many platelet proteins, such as matrix metalloproteinase-9, which was found on the plasma membrane, α-granules, open canalicular system, and within the cytoplasm both in resting and activated platelets [42]. Immunogold labeling of the purine P2Y1 receptor and the thromboxane-prostanoid TP receptor revealed that, while present at the platelet surface, both receptors were also abundantly represented inside the platelet [43]. Immunogold labeling has also been critical for examining platelet α-granule constituents, providing evidence of heterogeneous localization of various protein constituents, such as von Willebrand factor and fibrinogen, within individual α-granules [44,45].
Scanning electron microscopy
SEM of platelets has been an instrumental technology for both basic science and clinical research. In contrast to TEM imaging of intracellular ultrastructure, in conventional SEM the electron beam scans the surface of specimens providing high resolution imaging of morphologic features. Conventional SEM requires different sample preparation than TEM since it involves fixation and dehydration of bulk samples instead of tissue embedding and sectioning. Rather than 2-dimensional information obtained by TEM of thin sections, SEM can give detailed 3-dimensional information of surface topology, making it particularly useful in a variety of situations where the presence and overall structure of blood clots is analyzed. Its applications include characterizing morphologic changes of single platelets following activation (Figure 2A–B) and examination of the composition and structure of ex vivo thrombi (Figure 3). Here, we provide a sampling of how SEM has been utilized to examine platelets in hemostasis and thrombosis, focusing primarily on basic science studies of platelets in vitro and clots or thrombi formed in animal models, as well as in clinical settings. It should be noted that there is an extensive literature utilizing SEM to study fibrin network structure in vitro and in vivo that will not be discussed.
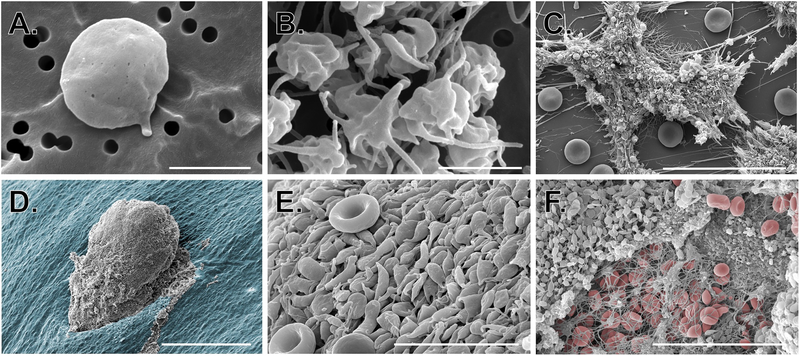
Figure 2: Use of SEM to study platelets in vitro and in vivo.
SEM images show A-B) isolated resting and activated human platelets (scale bars = 2 μm); C) human platelet-rich thrombi formed in a microfluidic flow chamber coated with collagen and tissue factor (scale bar = 30 μm); D-F) platelet-rich hemostatic plugs formed following puncture injury to a mouse jugular vein. D) hemostatic plug imaged from the intraluminal side (scale bar = 150 μm); the endothelium is pseudocolored blue. E) High magnification image of platelets on the luminal surface of a hemostatic plug (red blood cells can be seen for comparison; scale bar = 10 μm). F) Extraluminal component of a hemostatic plug showing platelets, fibrin and a few fibrin entrapped red blood cells (RBCs are pseudocolored red, scale bar = 30 μm). Image credit: M. Tomaiuolo and T.J. Stalker.
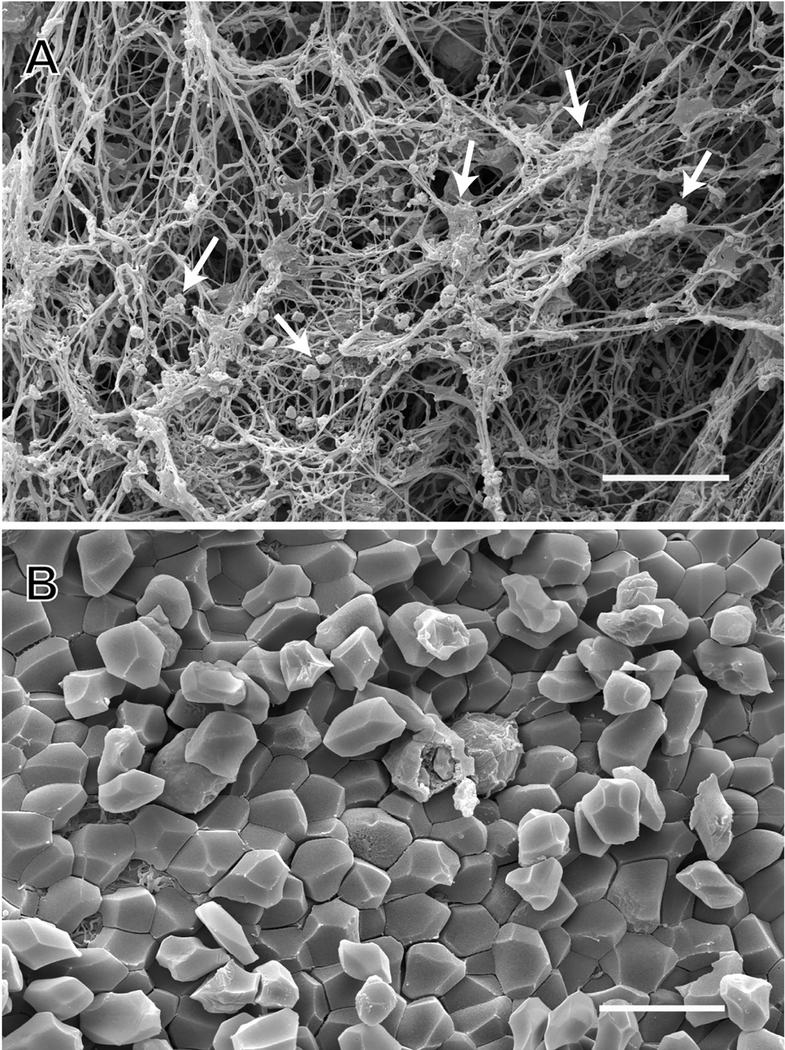
Figure 3: SEM of arterial thrombi taken from patients by thrombectomy.
SEM images show A) single and aggregated platelets (white arrows) and fibrin in an arterial thrombus; an example of the appearance of the outside of a contracted blood clot or thrombus; B) polyhedrocytes (compressed polyhedral erythrocytes) in an arterial thrombus; an example of the interior of a contracted blood clot. Scale bars = 10 μm. Image credit: from the laboratory of John W. Weisel: Rafael R. Khismatullin and Rustem I. Litvinov.
SEM for basic science studies of platelet biology
In the 1950s, early studies of platelet biology using SEM detailed the time-dependent changes in platelet shape following exposure to thrombin [46] and began classifying morphologically normal and abnormal platelets [47]. Not long after, early SEM studies examined the structure and composition of in vivo hemostatic plugs and thrombi, such as in artificially induced atherosclerotic lesions formed following endothelial denudation in primates [48]. In more recent years, SEM has been used to facilitate the understanding of thrombus formation in vivo in mouse models. For example, SEM was used to help understand the mechanism responsible for thrombus formation following ferric-chloride application in mouse carotid arteries, highlighting a previously unrecognized role for red blood cell adhesion [49]. In hemostasis models, SEM has been used to characterize the structure and composition of hemostatic platelet plugs following puncture injury (Figure 2D–F) [50]. In vitro models of platelet adhesion under flow using whole blood in microfluidic devices have become increasingly important to study hemostasis and thrombosis using human and mouse blood. Unsurprisingly, SEM has been a valuable imaging technique to examine the outcome of some of these experiments (Figure 2C). One group, for instance, investigated the interaction between fibrinogen patch size and spacing with platelet deposition under flow [51,52], while our group used SEM to study physical attributes, such as porosity and interplatelet gap size distribution, of platelet aggregates under flow [53]. Other studies have utilized SEM to examine the spatiotemporal regulation of platelet procoagulant activity, membrane ballooning and microvesiculation [54,55]. Recent reports have uncovered important roles for platelets beyond hemostasis and thrombosis. The cross-talk between inflammation, platelets, and coagulation has important clinical implications, and multiple studies have detailed the effects of various cytokines on platelet activation and thrombin generation via SEM imaging [56–58].
SEM for translational research
Besides its use in primarily basic science, numerous studies have employed SEM imaging to address clinically related questions. For example, various animal models have been developed to investigate how a particular anticoagulant or antiplatelet therapy is able to inhibit excessive thrombin generation and/or platelet activation. In many of these models SEM was utilized, in combination with laboratory assays, to investigate the presence and structural characteristics of thrombi following experimental injury to study vascular remodeling [59], venous stasis [60], arterial crush injury [61], and thrombolysis [62], to provide just a few examples. One group used SEM to evaluate the effectiveness of a hemostatic powder against gastrointestinal bleeding, examining clots formed in trauma models [63]. Likewise, SEM was used in animal models to investigate the roles of thrombin and platelet integrins [64], and to assess the effectiveness of nitric oxide to reduce undesired platelet activation following balloon angioplasty [65].
SEM examination of human ex vivo thrombi
Many studies have employed SEM imaging to analyze the ultrastructural characteristics and cellular composition of thrombi formed in humans. In the past, these thrombi needed to be examined post mortem, potentially leading to artifacts. More recently, the advent of mechanical thrombectomy as a clinical intervention has provided freshly extracted intravital thrombi for analysis via a variety of imaging modalities that may then be related to clinical patient data. The structure and composition of thrombi from both myocardial infarction and ischemic stroke patients have been analyzed using SEM in multiple studies [66–71]. The results of those studies highlight the heterogeneity of clot composition in both settings. Arterial thrombi contained a surprisingly large amount of fibrin, in addition to platelets [72]. The composition of pulmonary emboli mirrored the most distal part of venous thrombi from which they originated, which differed from the structure of the body and head of the same thrombi. SEM studies of arterial and venous intravital thrombi as well as thrombotic emboli also revealed the abundance of polyhedral-shaped red blood cells named polyhedrocytes (Figure 3), a morphological sign of intravital platelet-driven contraction [72]. Polyhedrocytes and intermediate forms comprised the major constituents of venous thrombi and pulmonary emboli. The structures within all of the thrombi and emboli were very tightly packed, in contrast to clots formed in vitro. Clot contraction both in blood clots in vitro and in thrombi in vivo leads to a characteristic morphology in which platelets and fibrin are on the exterior (Figure 3A) and polyhedrocytes are on the interior (Figure 3B). SEM was also used to examine the interaction of thrombi with retrieval devices [73], and the relationship of thrombus composition to resistance to different fibrinolytic regimes [74].
SEM in clinical device development
During their development, a multitude of clinical devices for cardiovascular intervention are evaluated and inspected for the presence of clots and their composition to help improve design. Stents for percutaneous coronary intervention are one category of such devices with the goal of preventing restenosis. Many designs with different materials and the ability of eluting a drug over time have been invented and are clinically available, but for each new iteration assessing the presence/absence of clots is validated via SEM imaging [75–80]. SEM imaging is also utilized to evaluate artificial heart valves to scan the valve leaflets for microscopic mechanical defects, such as tears, or to inspect platelet deposition and fibrin formation on different mechanical valve replacement materials [81–85]. Another example is provided by left ventricular assist devices, vital devices for the heart failure patients receiving them that also come in a variety of designs. Understanding which combination of factors including geometries, materials, shear rates, etc. is clinically better has direct life and death consequences. SEM imaging is used to visualize the presence or absence of platelets and fibrin clots in these devices, either during the development phase or post mortem [86–88]. Similarly, SEM imaging is used to assess the hemocompatibility and thrombogenicity of bioprosthetic total artificial hearts during the development stage [89]. Finally, SEM remains the obligate imaging choice to examine clot formation in hemodialysis catheters [90,91], as well as to examine the effectiveness of anticoagulant therapies in preventing such clots [92].
Recent advances in electron microscopy for the study of platelets
Newer light microscopy techniques, collectively referred to as super resolution microscopy, have achieved resolutions breaking the limits of light diffraction. These approaches are particularly useful for determining subcellular localization and/or colocalization of proteins within cells and even intracellular organelles such as platelet α-granules [93]. However, with resolution limits in the 20–100 nm XY range [94], super resolution microscopy still does not achieve the single nanometer resolution possible using EM. Thus, rather than supplant EM, super resolution microscopy remains a complementary imaging modality. In fact, fluorescence microscopy (including super resolution techniques) may now be combined with EM, an approach referred to as correlative light and electron microscopy (CLEM). Further, EM approaches continue to advance as new sample preparation techniques and microscope technologies become available. For example, various chemical fixation methods are known to poorly preserve membrane structures and have the potential to introduce artifacts such as membrane blebbing [95]. Application of high-pressure freezing with freeze substitution protocols for fixation/dehydration, as well as vitrification of isolated platelets directly on EM grids, has opened new opportunities to examine platelet internal membranes in near native states (e.g. cryo-EM). Finally, multiple approaches for the examination of cells and tissues at EM resolution in 3-dimensions have been developed, including electron tomography (ET), serial block face SEM (SBF-SEM) and focused ion beam SEM (FIB-SEM). Each of these newer EM approaches overcomes specific limitations of conventional EM approaches, while maintaining EM level resolution. Advantages and limitations of each are described in the sections below.
Correlative light and electron microscopy (CLEM)
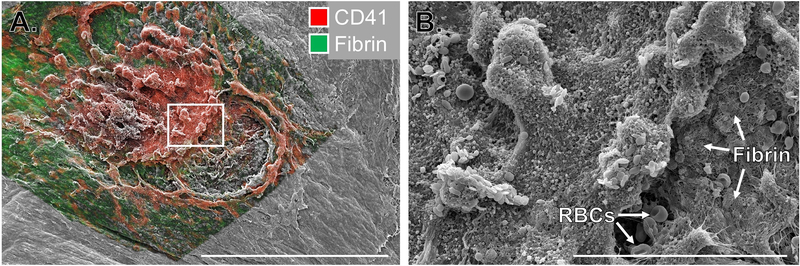
Correlative light and electron microscopy (CLEM) combines the best features of both fluorescence imaging and EM techniques while overcoming some of their limitations. In CLEM, a fluorescently labeled sample is first analyzed with light microscopy followed by high-resolution EM of the same site. CLEM is usually applied for the following reasons: to locate a rare fluorescently labelled event followed by focusing on that signal for high-resolution EM imaging; to complement information from live imaging with ultrastructural information; and to identify the location of a fluorescently labelled molecule within the ultrastructure [96]. There are only a few examples of CLEM application to platelet studies [97,98]. While CLEM typically combines fluorescence microscopy with TEM or 3D-EM approaches (discussed below), a new frontier is combining fluorescence microscopy with SEM. We recently performed such a study examining hemostatic plug architecture utilizing fluorescence and SEM imaging (Figure 4). We were able to combine biochemical and morphologic information using these two imaging modalities to draw conclusions about the spatial regulation of platelet activation during hemostasis [52]. The fluorescence imaging also provided 3-dimensional information that was not obtainable with the conventional SEM alone, while the SEM yielded more detailed surface topology.
Figure 4: Correlative light and electron microscopy to study hemostatic plug architecture.
Images show the extravascular surface of a mouse jugular vein following puncture injury. A) Multiphoton fluorescence image of platelets (CD41, red) and fibrin (green) overlaid on an SEM image of the same region. Scale bar is 300 μm. B) SEM image of the region indicated by the white rectangle in A. Scale bar is 50 μm. Images adapted from Tomaiuolo et al [52].
Electron tomography
A major limitation of conventional TEM is the lack of 3-dimensional information when imaging thin sections. Besides advances in tissue preservation protocols, advances in microscope technology coupled with computational tools and image processing techniques now provide for 3-dimensional reconstruction of images obtained from serial tissue sections. Moreover, electron tomography (ET) is an approach whereby a single relatively thick tissue section is imaged by TEM at multiple angles (see Engberts et al for a methodological review [97]). The resulting images may then be computationally reconstructed into a 3-dimensional volume. ET is characterized by outstanding axial resolution (as low as 2 nm), making it particularly useful for imaging of fine membranous structures, including membrane pores, and individual macromolecules can even be localized and identified. The thickness of the tissue section to be imaged is limited to 200–300 nm. ET may be coupled with cryopreservation of cells (cryo-ET) to achieve outstanding resolution of structures in near-native states, which is discussed in the next section.
Cryo-electron microscopy
Cryogenic electron microscopy (Cryo-EM) is a relatively new and rapidly developing type of TEM of hydrated biological samples that are instantaneously cooled to cryogenic temperatures (liquid ethane) without fixation and dehydration. The cooling procedure is so fast that water molecules do not form crystals that damage the structures but acquire a state of amorphous or vitreous ice that preserves the native biological organization of macromolecules, supramolecular structures, and cellular organelles. The cryogenically prepared samples can be analyzed using TEM, including tomography that involves acquisition of multiple images of the tilted specimen followed by 3D reconstruction [99]. Cryo-electron tomography (cryo-ET) of platelets combined with immunogold labeling was used to characterize the 3-dimensional organization of the platelet open canalicular system and its relationship to the dense tubular system, as well as to define α-granule subtypes [100]. Cryo-ET of intact platelets allowed visualization of the cytoskeletal architecture of spread platelets on extracellular matrix correlated with stiffness maps of the platelets determined with atomic force microscopy [101]. Using cryo-ET to examine frozen-hydrated platelets from patients with ovarian cancer revealed significant morphological differences between the cancer and control platelets, including disruption of the microtubule marginal band as well as differences in mitochondria and other subcellular structures [102]. These and other papers suggest the potential of cryo-EM as a powerful technology to study the ultrastructure of platelets in their native hydrated state at nanometer to subnanometer resolution [103].
SBF-SEM and FIB-SEM
To provide an even greater depth of 3-dimensional imaging at EM level resolution, newer techniques incorporate serial tissue sectioning within scanning electron microscopes. Serial block face-scanning electron microscopy (SBF-SEM) incorporates an ultramicrotome into an SEM. The surface of the tissue block is imaged with the electron beam after each section of tissue is removed to collect serial z-plane images that can subsequently be reconstructed in 3-D. Focused ion beam-SEM (FIB-SEM) is a similar application that utilizes a beam of gallium ions to mill the surface of a tissue block with high precision [104]. Both techniques can be used to obtain serial images with nanometer z plane spacing (~20–30 nm for SBF and ~5 nm for FIB), while overcoming the limitation of tissue thickness in tilt series electron tomography. The application of these approaches has been pioneered in the neuroscience community, where they have been used to map individual neurons and neural connections in brain tissue. In platelet biology, they have been used primarily to obtain 3D structures of whole, isolated platelets, in order to examine subcellular structures and changes that occur during the activation process. Pokrovskaya et al recently used SBF-SEM to provide a detailed characterization of platelet size, volume, granule number, OCS volume and other parameters obtained from 3D reconstructions of whole human platelets [105]. FIB-SEM was coupled with immunogold labeling to examine the distribution of clotting factors on procoagulant platelets under flow, highlighting the importance of platelet ‘cap’-like structures capable of increasing the local concentration of clotting factors [106]. Multiple studies have examined the membrane fusion events occurring during α-granule secretion [30,107]. SBF-SEM and FIB-SEM have also been used to examine additional platelet membrane components, such as Golgi [108], and to analyze granule repertoires in the presence of various molecular perturbations [109–111].
Given the large datasets generated when performing ET, SBF-SEM and FIB-SEM, newer computational tools, including machine learning approaches, are now being applied to EM imaging to permit analysis of complex 2- and 3-dimensional data sets. Such approaches allow semi-automated segmentation and analysis of cells within a multicellular tissue (such as a platelet aggregate), as well as intracellular constituents such as granules, other organelles and even microtubules. We anticipate that in time, it will be possible using these approaches to image and reconstruct in 3-dimensions entire hemostatic plugs and thrombi formed in vivo at nanometer resolution. Such studies would allow observation and analysis of platelet subcellular events as they occur in (patho)physiologic settings at an unprecedented level of detail.
Conclusions
In conclusion, for over 70 years electron microscopy has proven to be a staple for clinical and basic research studies in hemostasis and thrombosis. Early studies using conventional EM techniques provided a foundation for our understanding of basic platelet cell biology, including identification of granule subtypes, morphologic changes upon platelet activation and the important role of platelet cytoskeletal components. Electron microscopy remains the gold standard for identifying various platelet abnormalities and storage pool deficiencies. Similarly, EM imaging continues to be a valuable tool for analyzing platelet and thrombus ultrastructure in both humans and animal models of disease, as well as in settings of clinical device development. Recent advances in EM technology coupled with computationally-based image analysis are now starting to open new possibilities for imaging of platelets and thrombi in 3-dimensions at nanometer level resolution. Such approaches hold promise to lead to new discoveries and address outstanding questions in hemostasis and thrombosis. These include a 3-dimensional analysis of α-granule cargoes to resolve once and for all questions regarding α-granule heterogeneity and regulated cargo release; analysis of the spatiotemporal regulation of secretion and other activation events during hemostasis and thrombosis in vivo; comparisons of the architecture of hemostatic plugs with thrombi of various etiologies; and alterations in platelets following non-traditional stimulation, e.g. in response to inflammatory stimuli, just to name a few. Further, the increasing inclusion of electron microscopes within dedicated core facilities that provide both hardware and technical expertise makes the use if EM more accessible to investigators than ever before. There is no doubt that electron microscopy will continue to be a valuable tool for the study of platelets and thrombosis into the foreseeable future.
Acknowledgements
The authors gratefully acknowledge research funding from the National Heart, Lung and Blood Institute (P01-HL139420 to TJS and R01-HL135254 to JWW), the American Heart Association (19TPA34880016 to TJS) and Bayer Healthcare (Bayer Hemophilia Awards Program to MT). The authors have no conflicting financial interests.
References
- 1.Bessis M *Ultra-Structure Du Protoplasma Des Thrombocytes Au Microscope Electronique. Comptes Rendus Des Seances De La Societe De Biologie Et De Ses Filiales. 1948;142(9–10):647–649. [PubMed] [Google Scholar]
- 2.White JG, Krumwiede M. Some contributions of electron microscopy to knowledge of human platelets. Thromb Haemost. 2007;98(1):69–72. [PubMed] [Google Scholar]
- 3.White JG. Use of the electron microscope for diagnosis of platelet disorders. Semin Thromb Hemost. 1998;24(2):163–8. [DOI] [PubMed] [Google Scholar]
- 4.Scandola C, Erhardt M, Rinckel JY, Proamer F, Gachet C, Eckly A. Use of electron microscopy to study megakaryocytes. Platelets. 2020:1–10. [DOI] [PubMed] [Google Scholar]
- 5.Bessis M Studies in electron microscopy of blood cells. Blood. 1950;5(12):1083–98. [PubMed] [Google Scholar]
- 6.White JG. The dense bodies of human platelets: inherent electron opacity of the serotonin storage particles. Blood. 1969;33(4):598–606. [PubMed] [Google Scholar]
- 7.Siegel A, Luscher EF. Non-identity of the alpha-granules of human blood platelets with typical lysosomes. Nature. 1967;215(5102):745–7. [DOI] [PubMed] [Google Scholar]
- 8.Day HJ, Holmsen H, Hovig T. Subcellular particles of human platelets. A biochemical and electron microscopic study with particular reference to the influence of fractionation techniques. Scand J Haematol Suppl. 1969;7:3–35. [PubMed] [Google Scholar]
- 9.Behnke O Further studies on microtubules. A marginal bundle in human and rat thrombocytes. J Ultrastruct Res. 1965;13(5):469–77. [DOI] [PubMed] [Google Scholar]
- 10.Behnke O, Zelander T. Substructure in negatively stained microtubules of mammalian blood platelets. Exp Cell Res. 1966;43(1):236–9. [DOI] [PubMed] [Google Scholar]
- 11.White JG. The substructure of human platelet microtubules. Blood. 1968;32(4):638–48. [PubMed] [Google Scholar]
- 12.Nachmias VT. Cytoskeleton of human platelets at rest and after spreading. J Cell Biol. 1980;86(3):795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White JG, Krivit W. Changes in platelet microtubules and granules during early clot development. Thromb Diath Haemorrh Suppl. 1967;26:29–42. [PubMed] [Google Scholar]
- 14.Hartwig JH, DeSisto M. The cytoskeleton of the resting human blood platelet: structure of the membrane skeleton and its attachment to actin filaments. J Cell Biol. 1991;112(3):407–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox JE, Boyles JK, Berndt MC, Steffen PK, Anderson LK. Identification of a membrane skeleton in platelets. J Cell Biol. 1988;106(5):1525–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol. 1992;118(6):1421–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartwig JH. The platelet cytoskeleton In: Michelson AD, editor. Platelets. 3rd ed. London: Elsevier; 2013. p. 145–168. [Google Scholar]
- 18.Thomas SG. The structure of resting and activated platelets In: Michelson AD, editor. Platelets. 4th ed. London: Elsevier; 2019. p. 47–77. [Google Scholar]
- 19.Rand ML, Reddy EC, Israels SJ. Laboratory diagnosis of inherited platelet function disorders. Transfus Apher Sci. 2018;57(4):485–493. [DOI] [PubMed] [Google Scholar]
- 20.White JG. Ultrastructural studies of the gray platelet syndrome. Am J Pathol. 1979;95(2):445–62. [PMC free article] [PubMed] [Google Scholar]
- 21.Breton-Gorius J, Favier R, Guichard J, Cherif D, Berger R, Debili N, Vainchenker W, Douay L. A new congenital dysmegakaryopoietic thrombocytopenia (Paris-Trousseau) associated with giant platelet alpha-granules and chromosome 11 deletion at 11q23. Blood. 1995;85(7):1805–14. [PubMed] [Google Scholar]
- 22.Grottum KA, Hovig T, Holmsen H, Abrahamsen AF, Jeremic M, Seip M. Wiskott-Aldrich syndrome: qualitative platelet defects and short platelet survival. Br J Haematol. 1969;17(4):373–88. [DOI] [PubMed] [Google Scholar]
- 23.White JG. Platelet granule disorders. Crit Rev Oncol Hematol. 1986;4(4):337–77. [DOI] [PubMed] [Google Scholar]
- 24.Hughes M, Webert K, Kelton JG. The use of electron microscopy in the investigation of the ultrastructural morphology of immune thrombocytopenic purpura platelets. Semin Hematol. 2000;37(3):222–8. [DOI] [PubMed] [Google Scholar]
- 25.Clauser S, Cramer-Borde E. Role of platelet electron microscopy in the diagnosis of platelet disorders. Semin Thromb Hemost. 2009;35(2):213–23. [DOI] [PubMed] [Google Scholar]
- 26.Tian J, Cheng LH, Cui X, Lei XX, Tang JB, Cheng B. Investigating the effect of age on platelet ultrastructure using transmission electron microscopy. Int Wound J. 2019;16(6):1457–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davizon-Castillo P, McMahon B, Aguila S, Bark D, Ashworth K, Allawzi A, Campbell RA, Montenont E, Nemkov T, D’Alessandro A, et al. TNF-alpha-driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood. 2019;134(9):727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponomareva AA, Nevzorova TA, Mordakhanova ER, Andrianova IA, Rauova L, Litvinov RI, Weisel JW. Intracellular origin and ultrastructure of platelet-derived microparticles. J Thromb Haemost. 2017;15(8):1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi S, Banerjee M, Zhang J, Kesaraju A, Pokrovskaya ID, Storrie B, Whiteheart SW. Alterations in platelet secretion differentially affect thrombosis and hemostasis. Blood Adv. 2018;2(17):2187–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pokrovskaya ID, Joshi S, Tobin M, Desai R, Aronova MA, Kamykowski JA, Zhang G, Whiteheart SW, Leapman RD, Storrie B. SNARE-dependent membrane fusion initiates alpha-granule matrix decondensation in mouse platelets. Blood Adv. 2018;2(21):2947–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge S, Woo E, Haynes CL. Quantal regulation and exocytosis of platelet dense-body granules. Biophys J. 2011;101(10):2351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sixma JJ, Wester J. The hemostatic plug. Semin Hematol. 1977;14(3):265–99. [PubMed] [Google Scholar]
- 33.Wester J, Sixma JJ, Geuze JJ, Heijnen HF. Morphology of the hemostatic plug in human skin wounds: transformation of the plug. Lab Invest. 1979;41(2):182–92. [PubMed] [Google Scholar]
- 34.Wester J, Sixma JJ, Geuze JJ, van der Veen J. Morphology of the early hemostasis in human skin wounds: influence of acetylsalicylic acid. Lab Invest. 1978;39(3):298–311. [PubMed] [Google Scholar]
- 35.Hovig T, Rowsell HC, Dodds WJ, Jorgensen L, Mustard JF. Experimental hemostasis in normal dogs and dogs with congenital disorders of blood coagulation. Blood. 1967;30(5):636–68. [PubMed] [Google Scholar]
- 36.Warren BA, de Bono AH. The ultrastructure of initial stages of platelet aggregation and adhesion to damaged vessel walls in vivo. Br J Exp Pathol. 1970;51(4):415–22. [PMC free article] [PubMed] [Google Scholar]
- 37.Sixma JJ, van den Berg A. The haemostatic plug in haemophilia A: a morphological study of haemostatic plug formation in bleeding time skin wounds of patients with severe haemophilia A. Br J Haematol. 1984;58(4):741–53. [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen L, Rowsell HC, Hovig T, Mustard JF. Resolution and organization of platelet-rich mural thrombi in carotid arteries of swine. Am J Pathol. 1967;51(5):681–719. [PMC free article] [PubMed] [Google Scholar]
- 39.Nechipurenko DY, Receveur N, Yakimenko AO, Shepelyuk TO, Yakusheva AA, Kerimov RR, Obydennyy SI, Eckly A, Leon C, Gachet C, et al. Clot Contraction Drives the Translocation of Procoagulant Platelets to Thrombus Surface. Arterioscler Thromb Vasc Biol. 2019;39(1):37–47. [DOI] [PubMed] [Google Scholar]
- 40.Denorme F, Manne BK, Portier I, Eustes AS, Kosaka Y, Kile BT, Rondina MT, Campbell RA. Platelet necrosis mediates ischemic stroke outcome in mice. Blood. 2020;135(6):429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White JG. Platelet structure In: Michelson AD, editor. Platelets. 3rd ed. London: Elsevier; 2013. p. 117–144. [Google Scholar]
- 42.Sheu JR, Fong TH, Liu CM, Shen MY, Chen TL, Chang Y, Lu MS, Hsiao G. Expression of matrix metalloproteinase-9 in human platelets: regulation of platelet activation in in vitro and in vivo studies. Br J Pharmacol. 2004;143(1):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nurden P, Poujol C, Winckler J, Combrie R, Pousseau N, Conley PB, Levy-Toledano S, Habib A, Nurden AT. Immunolocalization of P2Y1 and TPalpha receptors in platelets showed a major pool associated with the membranes of alpha -granules and the open canalicular system. Blood. 2003;101(4):1400–8. [DOI] [PubMed] [Google Scholar]
- 44.Sander HJ, Slot JW, Bouma BN, Bolhuis PA, Pepper DS, Sixma JJ. Immunocytochemical localization of fibrinogen, platelet factor 4, and beta thromboglobulin in thin frozen sections of human blood platelets. J Clin Invest. 1983;72(4):1277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cramer EM, Meyer D, le Menn R, Breton-Gorius J. Eccentric localization of von Willebrand factor in an internal structure of platelet alpha-granule resembling that of Weibel-Palade bodies. Blood. 1985;66(3):710–3. [PubMed] [Google Scholar]
- 46.De Robertis E, Paseyro P, Reissig M. Electron microscopic studies of the action of thrombin on blood platelets. Blood. 1953;8(7):587–97. [PubMed] [Google Scholar]
- 47.Braunsteiner H [Morphology, physiology and pathology of the blood platelets]. Klin Wochenschr. 1951;29(19–20):335–8. [DOI] [PubMed] [Google Scholar]
- 48.Stemerman MB, Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972;136(4):769–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barr JD, Chauhan AK, Schaeffer GV, Hansen JK, Motto DG. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood. 2013;121(18):3733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leong L, Chernysh IN, Xu Y, Sim D, Nagaswami C, de Lange Z, Kosolapova S, Cuker A, Kauser K, Weisel JW. Clot stability as a determinant of effective factor VIII replacement in hemophilia A. Res Pract Thromb Haemost. 2017;1(2):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van de Walle AB, Fontenot J, Spain TG, Brunski DB, Sanchez ES, Keay JC, Curtis ME, Johnson MB, Snyder TA, Schmidtke DW. The role of fibrinogen spacing and patch size on platelet adhesion under flow. Acta Biomater. 2012;8(11):4080–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomaiuolo M, Matzko CN, Poventud-Fuentes I, Weisel JW, Brass LF, Stalker TJ. Interrelationships between structure and function during the hemostatic response to injury. Proc Natl Acad Sci U S A. 2019;116(6):2243–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirramezani M, Herbig BA, Stalker TJ, Nettey L, Cooper M, Weisel JW, Diamond SL, Sinno T, Brass LF, Shadden SC, et al. Platelet packing density is an independent regulator of the hemostatic response to injury. J Thromb Haemost. 2018;16(5):973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agbani EO, Hers I, Poole AW. Temporal contribution of the platelet body and balloon to thrombin generation. Haematologica. 2017;102(10):e379–e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agbani EO, van den Bosch MT, Brown E, Williams CM, Mattheij NJ, Cosemans JM, Collins PW, Heemskerk JW, Hers I, Poole AW. Coordinated Membrane Ballooning and Procoagulant Spreading in Human Platelets. Circulation. 2015;132(15):1414–24. [DOI] [PubMed] [Google Scholar]
- 56.Bester J, Pretorius E. Effects of IL-1beta, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep. 2016;6:32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Page MJ, Bester J, Pretorius E. Interleukin-12 and its procoagulant effect on erythrocytes, platelets and fibrin(ogen): the lesser known side of inflammation. Br J Haematol. 2018;180(1):110–117. [DOI] [PubMed] [Google Scholar]
- 58.Page MJ, Bester J, Pretorius E. The inflammatory effects of TNF-alpha and complement component 3 on coagulation. Sci Rep. 2018;8(1):1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Courtman DW, Schwartz SM, Hart CE. Sequential injury of the rabbit abdominal aorta induces intramural coagulation and luminal narrowing independent of intimal mass: extrinsic pathway inhibition eliminates luminal narrowing. Circ Res. 1998;82(9):996–1006. [DOI] [PubMed] [Google Scholar]
- 60.Trabucchi E, Foschi D, Marazzi M, Abelli P, Andriuoli G, Lami F, Scagnol I, Del Soldato P, De Santis F, Montorsi W. Heparin prevents stasis-induced thrombosis through protection of the venous endothelial cells: an electron microscopic study in rabbits. Haemostasis. 1991;21(1):37–44. [DOI] [PubMed] [Google Scholar]
- 61.Savoie FH, Cooley BC, Gould JS. Evaluation of the effect of pharmacologic agents on crush-avulsion arterial injuries: a scanning electron microscopy study. Microsurgery. 1991;12(4):292–300. [DOI] [PubMed] [Google Scholar]
- 62.Cho JS, Martelli E, Mozes G, Miller VM, Gloviczki P. Effects of thrombolysis and venous thrombectomy on valvular competence, thrombogenicity, venous wall morphology, and function. J Vasc Surg. 1998;28(5):787–99. [DOI] [PubMed] [Google Scholar]
- 63.Holster IL, van Beusekom HM, Kuipers EJ, Leebeek FW, de Maat MP, Tjwa ET. Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy. 2015;47(7):638–45. [DOI] [PubMed] [Google Scholar]
- 64.Kaplan AV, Leung LL, Leung WH, Grant GW, McDougall IR, Fischell TA. Roles of thrombin and platelet membrane glycoprotein IIb/IIIa in platelet-subendothelial deposition after angioplasty in an ex vivo whole artery model. Circulation. 1991;84(3):1279–88. [DOI] [PubMed] [Google Scholar]
- 65.Groves PH, Lewis MJ, Cheadle HA, Penny WJ. SIN-1 reduces platelet adhesion and platelet thrombus formation in a porcine model of balloon angioplasty. Circulation. 1993;87(2):590–7. [DOI] [PubMed] [Google Scholar]
- 66.Fitzgerald S, Mereuta OM, Doyle KM, Dai D, Kadirvel R, Kallmes DF, Brinjikji W. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome. J Neurosurg Sci. 2019;63(3):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sadowski M, Zabczyk M, Undas A. Coronary thrombus composition: links with inflammation, platelet and endothelial markers. Atherosclerosis. 2014;237(2):555–61. [DOI] [PubMed] [Google Scholar]
- 68.Silvain J, Collet JP, Guedeney P, Varenne O, Nagaswami C, Maupain C, Empana JP, Boulanger C, Tafflet M, Manzo-Silberman S, et al. Thrombus composition in sudden cardiac death from acute myocardial infarction. Resuscitation. 2017;113:108–114. [DOI] [PubMed] [Google Scholar]
- 69.Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, et al. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57(12):1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kovacs A, Sotonyi P, Nagy AI, Tenekedjiev K, Wohner N, Komorowicz E, Kovacs E, Nikolova N, Szabo L, Kovalszky I, et al. Ultrastructure and composition of thrombi in coronary and peripheral artery disease: correlations with clinical and laboratory findings. Thromb Res. 2015;135(4):760–6. [DOI] [PubMed] [Google Scholar]
- 71.Zalewski J, Bogaert J, Sadowski M, Woznicka O, Doulaptsis K, Ntoumpanaki M, Zabczyk M, Nessler J, Undas A. Plasma fibrin clot phenotype independently affects intracoronary thrombus ultrastructure in patients with acute myocardial infarction. Thromb Haemost. 2015;113(6):1258–69. [DOI] [PubMed] [Google Scholar]
- 72.Chernysh IN, Nagaswami C, Kosolapova S, Peshkova AD, Cuker A, Cines DB, Cambor CL, Litvinov RI, Weisel JW. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Sci Rep. 2020;10(1):5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Autar ASA, Hund HM, Ramlal SA, Hansen D, Lycklama ANGJ, Emmer BJ, de Maat MPM, Dippel DWJ, van der Lugt A, van Es A, et al. High-Resolution Imaging of Interaction Between Thrombus and Stent-Retriever in Patients With Acute Ischemic Stroke. J Am Heart Assoc. 2018;7(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Meglio L, Desilles JP, Ollivier V, Nomenjanahary MS, Di Meglio S, Deschildre C, Loyau S, Olivot JM, Blanc R, Piotin M, et al. Acute ischemic stroke thrombi have an outer shell that impairs fibrinolysis. Neurology. 2019;93(18):e1686–e1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Acharya G, Lee CH, Lee Y. Optimization of Cardiovascular Stent against Restenosis: Factorial Design-Based Statistical Analysis of Polymer Coating Conditions. Plos One. 2012;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao J, Falotico R, Nguyen T, Cheng Y, Parker T, Dave V, Rogers C, Riesenfeld J. A nonelutable low-molecular weight heparin stent coating for improved thromboresistance. J Biomed Mater Res B Appl Biomater. 2012;100(5):1274–82. [DOI] [PubMed] [Google Scholar]
- 77.Halwani DO, Anderson PG, Brott BC, Anayiotos AS, Lemons JE. The role of vascular calcification in inducing fatigue and fracture of coronary stents. J Biomed Mater Res B Appl Biomater. 2012;100(1):292–304. [DOI] [PubMed] [Google Scholar]
- 78.Roopmani P, Satheesh S, Raj DC, Krishnan UM. Development of Dual Drug Eluting Cardiovascular Stent with Ultrathin Flexible Poly(L-lactide-co-caprolactone) Coating. Acs Biomaterials Science & Engineering. 2019;5(6):2899–2915. [DOI] [PubMed] [Google Scholar]
- 79.Piquet P, Rolland PH, Bartoli JM, Tranier P, Moulin G, Mercier C. Tantalum-Dacron coknit stent for endovascular treatment of aortic aneurysms: a preliminary experimental study. J Vasc Surg. 1994;19(4):698–706. [DOI] [PubMed] [Google Scholar]
- 80.van der Giessen WJ, Serruys PW, van Beusekom HM, van Woerkens LJ, van Loon H, Soei LK, Strauss BH, Beatt KJ, Verdouw PD. Coronary stenting with a new, radiopaque, balloon-expandable endoprosthesis in pigs. Circulation. 1991;83(5):1788–98. [DOI] [PubMed] [Google Scholar]
- 81.Okazaki Y, Wika KE, Matsuyoshi T, Fukamachi K, Kunitomo R, Tweden KS, Harasaki H. Platelets are deposited early post-operatively on the leaflet of a mechanical heart valve in sheep without post-operative anticoagulants or antiplatelet agents. A scanning electron microscopic observation of the pyrolytic carbon surface in a mechanical heart valve. ASAIO J. 1996;42(5):M750–4. [DOI] [PubMed] [Google Scholar]
- 82.Chu CL, Ji HL, Yin LH, Pu YP, Lin PH, Chu PK. Microstructure, mechanical properties, and blood compatibility of zirconium nitride deposited on nickel-titanium shape memory alloy. Surface & Coatings Technology. 2010;204(16–17):2841–2845. [Google Scholar]
- 83.Fedel M, Motta A, Maniglio D, Migliaresi C. Carbon Coatings for Cardiovascular Applications: Physico-Chemical Properties and Blood Compatibility. Journal of Biomaterials Applications. 2010;25(1):57–74. [DOI] [PubMed] [Google Scholar]
- 84.Tellez A, Dillon KN, Rousselle SD. Comprehensive Preclinical Postmortem Evaluation of Valvular Prosthesis. Toxicol Pathol. 2017;45(8):1077–1090. [DOI] [PubMed] [Google Scholar]
- 85.Goldsmith I, Kumar P, Carter P, Blann AD, Patel RL, Lip GY. Atrial endocardial changes in mitral valve disease: a scanning electron microscopy study. Am Heart J. 2000;140(5):777–84. [DOI] [PubMed] [Google Scholar]
- 86.Yamanaka H, Rosenberg G, Weiss WJ, Snyder AJ, Zapanta CM, Siedlecki CA. Short-term in vivo studies of surface thrombosis in a left ventricular assist system. ASAIO J. 2006;52(3):257–65. [DOI] [PubMed] [Google Scholar]
- 87.Linneweber J, Dohmen PM, Kertzscher U, Affeld K, Konertz W. Local glycoprotein IIb/IIIa receptor inhibitor delivery from the pump surface attenuates platelet adhesion in continuous flow ventricular assist devices. Artif Organs. 2008;32(10):792–9. [DOI] [PubMed] [Google Scholar]
- 88.Topper SR, Navitsky MA, Medvitz RB, Paterson EG, Siedlecki CA, Slattery MJ, Deutsch S, Rosenberg G, Manning KB. The Use of Fluid Mechanics to Predict Regions of Microscopic Thrombus Formation in Pulsatile VADs. Cardiovasc Eng Technol. 2014;5(1):54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jansen P, van Oeveren W, Capel A, Carpentier A. In vitro haemocompatibility of a novel bioprosthetic total artificial heart. Eur J Cardiothorac Surg. 2012;41(6):e166–72. [DOI] [PubMed] [Google Scholar]
- 90.Lucas TC, Tessarolo F, Jakitsch V, Caola I, Brunori G, Nollo G, Huebner R. Blood flow in hemodialysis catheters: a numerical simulation and microscopic analysis of in vivo-formed fibrin. Artif Organs. 2014;38(7):556–65. [DOI] [PubMed] [Google Scholar]
- 91.Lucas TC, Tessarolo F, Veniero P, Caola I, Piccoli F, Haase A, Nollo G, Huebner R, Brunori G. Hemodialysis catheter thrombi: visualization and quantification of microstructures and cellular composition. J Vasc Access. 2013;14(3):257–63. [DOI] [PubMed] [Google Scholar]
- 92.Hofbauer R, Moser D, Frass M, Oberbauer R, Kaye AD, Wagner O, Kapiotis S, Druml W. Effect of anticoagulation on blood membrane interactions during hemodialysis. Kidney Int. 1999;56(4):1578–83. [DOI] [PubMed] [Google Scholar]
- 93.Kamykowski J, Carlton P, Sehgal S, Storrie B. Quantitative immunofluorescence mapping reveals little functional coclustering of proteins within platelet alpha-granules. Blood. 2011;118(5):1370–3. [DOI] [PubMed] [Google Scholar]
- 94.Schermelleh L, Ferrand A, Huser T, Eggeling C, Sauer M, Biehlmaier O, Drummen GPC. Super-resolution microscopy demystified. Nat Cell Biol. 2019;21(1):72–84. [DOI] [PubMed] [Google Scholar]
- 95.Morgenstern E Aldehyde fixation causes membrane vesiculation during platelet exocytosis: a freeze-substitution study. Scanning Microsc Suppl. 1991;5(4):S109–15. [PubMed] [Google Scholar]
- 96.Bykov YS, Cortese M, Briggs JA, Bartenschlager R. Correlative light and electron microscopy methods for the study of virus-cell interactions. FEBS Lett. 2016;590(13):1877–95. [DOI] [PubMed] [Google Scholar]
- 97.Engberts KB, Seinen C, Geerts WJC, Heijnen HFG. Electron Tomography and Correlative Approaches in Platelet Studies. Methods Mol Biol. 2018;1812:55–79. [DOI] [PubMed] [Google Scholar]
- 98.Page MJ, Thomson GJA, Nunes JM, Engelbrecht AM, Nell TA, de Villiers WJS, de Beer MC, Engelbrecht L, Kell DB, Pretorius E. Serum amyloid A binds to fibrin(ogen), promoting fibrin amyloid formation. Sci Rep. 2019;9(1):3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oikonomou CM, Jensen GJ. Cellular Electron Cryotomography: Toward Structural Biology In Situ. Annu Rev Biochem. 2017;86:873–896. [DOI] [PubMed] [Google Scholar]
- 100.van Nispen tot Pannerden H, de Haas F, Geerts W, Posthuma G, van Dijk S, Heijnen HF. The platelet interior revisited: electron tomography reveals tubular alpha-granule subtypes. Blood. 2010;116(7):1147–56. [DOI] [PubMed] [Google Scholar]
- 101.Sorrentino S, Studt JD, Horev MB, Medalia O, Sapra KT. Toward correlating structure and mechanics of platelets. Cell Adh Migr. 2016;10(5):568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang R, Stone RL, Kaelber JT, Rochat RH, Nick AM, Vijayan KV, Afshar-Kharghan V, Schmid MF, Dong JF, Sood AK, et al. Electron cryotomography reveals ultrastructure alterations in platelets from patients with ovarian cancer. Proc Natl Acad Sci U S A. 2015;112(46):14266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Irobalieva RN, Martins B, Medalia O. Cellular structural biology as revealed by cryo-electron tomography. J Cell Sci. 2016;129(3):469–76. [DOI] [PubMed] [Google Scholar]
- 104.Eckly A, Rinckel JY, Proamer F, Gachet C. High-Resolution 3D Imaging of Megakaryocytes Using Focused Ion Beam-Scanning Electron Microscopy. Methods Mol Biol. 2018;1812:217–231. [DOI] [PubMed] [Google Scholar]
- 105.Pokrovskaya ID, Yadav S, Rao A, McBride E, Kamykowski JA, Zhang G, Aronova MA, Leapman RD, Storrie B. 3D ultrastructural analysis of alpha-granule, dense granule, mitochondria, and canalicular system arrangement in resting human platelets. Res Pract Thromb Haemost. 2020;4(1):72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Podoplelova NA, Sveshnikova AN, Kotova YN, Eckly A, Receveur N, Nechipurenko DY, Obydennyi SI, Kireev II, Gachet C, Ataullakhanov FI, et al. Coagulation factors bound to procoagulant platelets concentrate in cap structures to promote clotting. Blood. 2016;128(13):1745–55. [DOI] [PubMed] [Google Scholar]
- 107.Eckly A, Rinckel JY, Proamer F, Ulas N, Joshi S, Whiteheart SW, Gachet C. Respective contributions of single and compound granule fusion to secretion by activated platelets. Blood. 2016;128(21):2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yadav S, Williamson JK, Aronova MA, Prince AA, Pokrovskaya ID, Leapman RD, Storrie B. Golgi proteins in circulating human platelets are distributed across non-stacked, scattered structures. Platelets. 2017;28(4):400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aguilar A, Weber J, Boscher J, Freund M, Ziessel C, Eckly A, Magnenat S, Bourdon C, Hechler B, Mangin PH, et al. Combined deficiency of RAB32 and RAB38 in the mouse mimics Hermansky-Pudlak syndrome and critically impairs thrombosis. Blood Adv. 2019;3(15):2368–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saultier P, Vidal L, Canault M, Bernot D, Falaise C, Pouymayou C, Bordet JC, Saut N, Rostan A, Baccini V, et al. Macrothrombocytopenia and dense granule deficiency associated with FLI1 variants: ultrastructural and pathogenic features. Haematologica. 2017;102(6):1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Selvadurai MV, Brazilek RJ, Moon MJ, Rinckel JY, Eckly A, Gachet C, Meikle PJ, Nandurkar HH, Nesbitt WS, Hamilton JR. The PI3-kinase PI3KC2-alpha regulates mouse platelet membrane structure and function independently of membrane lipid composition. FEBS Letters. 2018;593:88–96. [DOI] [PubMed] [Google Scholar]