Abstract
Introduction
Management of Type 1 Diabetes (T1D) poses numerous challenges, especially for young children and their families. Parental care positively influences the outcomes of children with T1D, while there are often criticisms in school environment. The COVID-19 pandemic has forced children and parents to spend many hours at home and diabetes care has returned mainly in the hands of parents.
Aim of the study
To evaluate the effectiveness of exclusive return to parental care in pre-school and school children with T1D treated with Tandem Basal IQ system during the COVID-19 pandemic.
Patients and methods
22 children (M:F = 14:8) with T1D have been evaluated. We compared insulin and CGM data (TIR, TBR and TAR) of two periods: PRE-COV and IN-COV, in which children have transitioned from normal school attendance to the exclusive care of their parents.
Results
During the IN-COV period a significantly (p < 0.001) higher median value of TIR (66,41%) was observed as compared to PRE-COV period (61,45%). Patients also showed a statistically significant difference (p < 0.002) between the IN-COV period and the PRE-COV period as concerning the TAR metric: respectively 29,86 ± 10,6% vs 34,73 ± 12,8%. The difference between the bolus insulin doses was statistically significant (PRE-COV 5,3 IU/day, IN-COV 7,9 IU/day – p < 0.05).
Conclusion
Our observational real-life study confirms the positive effect of parental care in T1D very young children and demonstrates that during the COVID-19 pandemic it was possible to obtain a good glycometabolic compensation despite the significant change in lifestyle.
1. Introduction
Type 1 diabetes (T1D) is one of the most common chronic diseases in infancy [1] and the most frequent endocrinopathy in childhood. It is estimated that about 20,000 children are affected by T1D in Italy [2]. Correct management of T1D involves frequent blood glucose monitoring, insulin therapy, dietary indications and structured physical activity, representing a high burden on young children and their families. Because of these daily challenges, effective diabetes treatment requires -in principle- complete parental dedication and involvement [3]. Moreover, parents and teachers of kindergarteners and of children in primary school usually experience the additional challenges of a critical management in school hours, due in particular to the fear of hypoglycemia, the extreme glycemic variability of this age group and the difficulties in correcting the hyperglycemic peaks.
Parents of kids diagnosed with T1D early in life tend to be proactive in the care of diabetes of their children during school and pre-school periods with a strong parental involvement in disease management and a positive influence on the metabolic and psychosocial outcomes [4], [5]. Conversely, parents of patients diagnosed in late childhood or in adolescence are less involved in care, and usually this is associated with less than optimal glycemic control [6]. Nevertheless, parental care remains important throughout childhood into young adulthood and a progressive sharing of responsibilities is considered an important step towards a therapeutic approach well balanced between self-monitoring and quality of life [7].
The gap between family and school care capacities can be bridged by the use of technologies in the treatment of diabetes in children. In the last two decades, new tools for the management of children with T1D have been proven to be safe and useful. Continuous Subcutaneous Insulin Infusion (CSII), Continuous Glucose Monitoring (CGM) and remote monitoring in case of Multiple Daily Injections (MDI) seem to be valid options to manage children with diabetes. In particular, CGM systems and remote control access have improved the treatment and management of diabetes during school hours. Parents of kindergartner (pre-school) and school children reported that the use of remote monitoring and CGM was effective in control glucose excursions [8]. In particular, the use of technologies capable of reducing hypoglycemic risk, such as the Tandem Basal IQ system, have proven to be helpful in reducing parental burden. This system is able to prevent/reduce hypoglycemia thanks to CGM real-time data and was introduced in Italy about 6 months ago.
COVID-19 pandemic has forced children and parents to spend many hours together at home, reducing structured physical activity while bringing back diabetes care in the hands of parents. We aimed at investigating whether this unusual situation lead to an improvement or a worsening of the glucose control.
2. Aim of the study
This is a real-life, retrospective, observational study aimed at evaluating how constant parental care compared to spending time outside home affected glycemic control in pre-school and school children with T1D utilizing Tandem Basal IQ system before and during the quarantine period due to pandemic COVID-19 infection.
3. Patients and methods
The Diabetes Unit of the Bambino Gesù Children’s Hospital – Rome, Italy, regularly follows 980 Type 1 Diabetes pediatric patients (age 0–18 years); of these patients, 90 use the Tandem Basal IQ Technology (considered as an inclusion criteria) and 29 are in the school-preschool age range; finally 22 pre-school and school children (M:F = 14:8) with T1D have been retrospectively evaluated (7 patients/parents resulted not reachable during the lockdown period). The mean age was 8,7 ± 1,9 years (range 3,5–10,5 years) and the diabetes duration was at least of 1 year. Enrolled patients were all being intensively insulin treated with the Tandem Basal IQ technology for at least six months. Tandem basal IQ technology consists of an insulin pump integrated with a Dexcom G6 glucose sensor capable of previously suspending insulin delivery in case of hypoglycemia prediction.
A multidisciplinary team (diabetologist, nurse, dietitian and psychologist) dispensed a standardized protocol of education to all patients enrolled and their parents at the time of diabetes diagnosis and at 6 months intervals. All the patients and their families were educated in carbo-counting procedures and were instructed to follow a balanced nutritional program consisting in 55% of carbohydrates, 20% of proteins and 25% of lipids.
The study was conducted according to the Declaration of Helsinki. Participants and their parents provided informed consent to have their CGM data downloaded at regular intervals, as part of routine clinical control and the study was ethically approved. Potential conflict of interest do not exist.
Beginning March 9, 2020, the start of the lockdown in Italy, all patients and families were asked to stay at home due to the COVID-19 pandemic emergency. During the pandemic, the children maintained the same diet with a similar distribution between the different macronutrients and they were unable to carry out any structured physical activity, as often happens also in routine life, considering the young age of the group.
We compared CGM data of the last two weeks of “normal life” (normal school attendance) (PRE-COV period) with the first two weeks of confinement at home (IN-COV period).
The following CGM metrics were evaluated: time in range (TIR – percent of time in the ideal range of glucose between 70 and 180 mg/dl), time above range (TAR – percent of time above 180 mg/dl), time below range (TBR - percent of time below 70 mg/dl). We also compared the insulin requirement in the 2 different periods, in terms of total insulin (IU/day), basal insulin delivery and insulin administered as boluses. All data were extracted from the Dexcom Clarity and Diasend platforms.
4. Statistics
Results are reported as the mean ± SD. Normal distribution was assessed by Shapiro-Wilk test. Mean variations of distributions were evaluated using the Student’s t test for paired data. The entire analyses were performed using SPSS 25.0 (SPSS Inc., Chicago, Ill, United States) with p < 0.05 considered significant.
5. Results
Data from 22 children (mean age 8,7 ± 1,9 years) (M:F 14:8) with T1D and disease duration longer than 1 year were analyzed. The percentage of time of CGM wearing was 98%.
No significant differences between the two evaluated periods (IN-COV vs PRE-COV) were found in TBR (hypoglycemia): respectively 3,73 ± 3,04% vs 3,95 ± 4,4% (Table 1 ).
Table 1.
Results.
| PRE-COV | IN-COV | P | |
|---|---|---|---|
| TIR (%) | 61,45 (11,7) | 66,41 (9,8) | < 0.001 |
| TBR (%) | 3,95 (4,4) | 3,73 (3) | 0,70 |
| TAR (%) | 34,7 (12,8) | 29,8 (10,6) | 0,002 |
Data are expressed as mean (SD); TIR = Time In Range, TBR = Time Below Range, TAR = Time Above Range
In contrast, during the IN-COV period a significantly (p < 0.001) higher median value of TIR (66,41%) was observed as compared to PRE-COV period (61,45%) (Tab 1 and Fig. 1 ). Conversely,
Fig. 1.
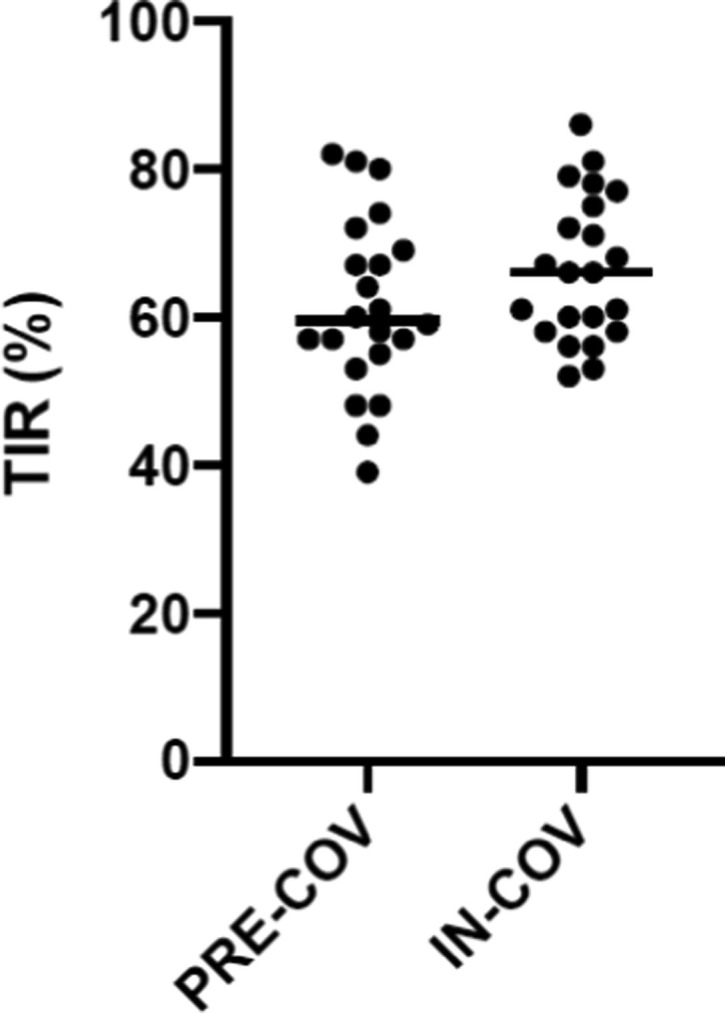
TIR in the PRE-COV and IN-COV periods.
patients showed a lower TAR during IN-COV period than PRE-COV period 29,86 ± 10,6% vs 34,73 ± 12,8%, p < 0.002).
Interestingly, no differences between IN-COV and PRE-COV periods were observed regarding the total insulin dose (20,7 vs 18,2 IU/day) and the basal insulin delivery (13,3 vs 11,9 IU/day), while a statistically significant difference (p < 0.05) was found between the mean bolus doses (7,9 vs 5,3 IU/day) and the daily number of correction boluses (3,1 vs 1,9 IU/day).
6. Discussion
In this group of pre-school and school T1D children using CGM and semi-automated insulin delivery systems (TANDEM BASAL IQ), the forced and exclusive return to parental care due to the “stay at home” rule decided by the Italian government was associated with a better metabolic performance with higher percentage values of TIR and lower mean values of TAR. This result seems to be due to a greater use of correction boluses; in fact, our evaluation shows a significant increase in insulin boluses during the IN-COV period as compared to PRE-COV one.
We speculate that similar results will be hardly seen in adolescents and middle school teens, because the management of the disease in these age groups remains in the hands of the patients even during confinement the and physical activity has no significant impact in younger children. Nonetheless, we think that it would be worthwhile to investigate older age groups in Countries where the lockdown is still in force, though we recognize that other variables -say good or bad interaction with parents- are probably at work in adolescents with T1D. We predict that a better outcome in terms of TBR could be likely in this group because of increased regularity of meals’ content and stringent control on alcohol consumption [9]. The study has some limitations: first, patients selection limited only to preschool/school children who used Tandem Basal IQ technology can represent a selection bias; secondly, the small size of the sample may not make the results obtained generalizable.
Our observational real-life study confirms the positive effect of parental care in T1D very young children and that, though new technologies can potentially improve diabetes outcomes also in this sub-population, maintenance of a good glucose control remains largely dependent on family competence and education [10].
Usually the majority of young people with T1D spend many hours at school. Trained school-staff is therefore essential to provide a safe environment for children with diabetes. Teachers, along with the school's auxiliary staff, play a key role in reducing strong glycemic oscillations typical of younger children [11].
Our study shows also a no significant differences in TBR, despite the shift to predominant parental care. This can be explained by the fact that all the evaluated children were already using a technology (Tandem Basal IQ System) that effectively reduces the risk of hypoglycemia and therefore for this specific metric it can be assumed that parental intervention does not change the outcomes.
In the past 2 decades, technological innovations have revolutionized the treatment of T1D and the real-world data highlighted that patients using insulin pump therapy have a better short and long‐term glycemic control relative to the matched injection therapy groups [12]. However, despite the use of technology, parental intervention still seems to be more effective and probably the difference in the near future could be made by the spread of Hybrid Closed Loop (HCL) systems. In the real-world experiences HCL use is associated with improved glycemic control and no change in psychosocial outcomes [13].
Why children with T1D attending school are often not adequately managed? Recent studies showed that a limited availability of glucagon kits, the shortage of trained personnel able to manage daily diabetes-specific emergencies and a reduced ability to perform an adequate carbohydrate counting, considered an effective means to provide good glycemic control, are the main causes [14], [15]. Consequently, family-based interventions for youth with T1D are believed to be effective at improving diabetes outcomes [16].
Since young patients with T1D spend most of daytime at school, teachers and assisting personnel should receive appropriate training in order to provide a safe environment. However, it is likely that teachers of kindergartens, preschools and primary schools have not enough knowledge to appropriately assist children with T1D [17], [18]. This may negatively reverberate on trust in school personnel of parents of very young children with T1D, whose perception of the burden of care is very strong. Thus, health care providers, parents, teachers, and school assistants of youngsters with T1D should team up to improve, by specific educational programs [19], [20], the skills to handle the disease and guarantee a safe attendance at school.
Funding
The authors received no funding from an external source.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redazione ANSA 13 febbraio 2017 19:44
- 3.Noser A.E., Patton S.R., Van Allen J., et al. Evaluating Parents' Self-Efficacy for Diabetes Management in Pediatric Type 1 Diabetes. J Pediatr Psychol. 2017;42(3):296–303. doi: 10.1093/jpepsy/jsw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wysocki T., Nansel T.R., Holmbeck G.N., Chen R., Laffel L., Anderson B.J. Weissberg-Benchell J; Steering Committee of the Family Management of Childhood Diabetes Study. Collaborative involvement of primary and secondary caregivers: associations with youths’ diabetes outcomes. J Pediatr Psychol. 2009;34(8):869–881. doi: 10.1093/jpepsy/jsn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson A.C., DeCourcey W.M., Freeman K.A. The Impact of Managing School-Aged Children's Diabetes: The Role of Child Behavior Problems and Parental Discipline Strategies. J Clin Psychol Med Settings. 2009;16(3):216–222. doi: 10.1007/s10880-009-9163-x. [DOI] [PubMed] [Google Scholar]
- 6.Friedemann-Sanchez G., Capistrant B.D., Ron J., et al. Caregiving for children with type 1 diabetes and clinical outcomes in central India: The IDREAM study. Pediatr Diabetes. 2018;19(3):527–533. doi: 10.1111/pedi.12567. [DOI] [PubMed] [Google Scholar]
- 7.Marker A.M., Noser A.E., Clements M.A., Patton S.R. Shared responsibility for Type 1 Diabetes care is associated with glycemic variability and risk of glycemic excursions in youth. J Pediatr Psychol. 2018;43(1):61–71. doi: 10.1093/jpepsy/jsx081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burckhardt M.A., Fried L., Bebbington K., et al. Use of remote monitoring with continuous glucose monitoring in young children with Type 1 Diabetes: the parents’ perspective. Diabet Med. 2019;36(11):1453–1459. doi: 10.1111/dme.14061. [DOI] [PubMed] [Google Scholar]
- 9.Ismail D., Gebert R., Vuillermin P.J., Fraser L., McDonnell C.M., Donath S.M., et al. Social Consumption of Alcohol in Adolescents With Type 1 Diabetes Is Associated With Increased Glucose Lability, but Not Hypoglycaemia. Diabet Med. 2006;23(8):830–833. doi: 10.1111/j.1464-5491.2006.01868.x. [DOI] [PubMed] [Google Scholar]
- 10.Garvey K., Wolfsdorf J.I. The Impact of Technology on Current Diabetes Management. Pediatr Clin North Am. 2015;62(4):873–888. doi: 10.1016/j.pcl.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Jackson C.C., Albanese-O’Neill A., Butler K.L., et al. Diabetes care in the school setting: a position statement of the American Diabetes Association. Diabetes care. 2015;38(10):1958–1963. doi: 10.2337/dc15-1418. [DOI] [PubMed] [Google Scholar]
- 12.Burckhardt M.A., Smith G.J., Cooper M.N., et al. Real-world outcomes of insulin pump compared to injection therapy in a population-based sample of children with type 1 diabetes. Pediatr Diabetes. 2018;19(8):1459–1466. doi: 10.1111/pedi.12754. [DOI] [PubMed] [Google Scholar]
- 13.Berget C., Messer L.H., Vigers T., et al. Six months of hybrid closed loop in the real-world: An evaluation of children and young adults using the 670G system. Pediatr Diabetes. 2020;21(2):310–318. doi: 10.1111/pedi.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alageel A.A. Are children and adolescents with type 1 diabetes in Saudi Arabia safe at school? Saudi Med J. 2019;40(10):1019–1026. doi: 10.15537/smj.2019.10.24582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gökşen D., Atik Altınok Y., Ozen S., Demir G., Darcan S. Effects of carbohydrate counting method on metabolic control in children with Type 1 Diabetes Mellitus. J Clin Res Pediatr Endocrinol. 2014;6(2):74–78. doi: 10.4274/Jcrpe.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman M.A., Anderson L.M., Shapiro J.B., et al. Family-Based Interventions Targeting Improvements in Health and Family Outcomes of Children and Adolescents with Type 1 Diabetes: a Systematic. Review. Curr Diab Rep. 2018;19;18(3):15. doi: 10.1007/s11892-018-0981-9. [DOI] [PubMed] [Google Scholar]
- 17.Chatzistougianni P., Tsotridou E., Dimitriadou M., et al. Level of knowledge and evaluation of perceptions regarding pediatric diabetes among Greek teachers. Diabetes Res Clin Pract. 2020;159:1–9. doi: 10.1016/j.diabres.2019.107952. [DOI] [PubMed] [Google Scholar]
- 18.Amillategui B., Mora E., Calle J.R., Giralt P. Special needs of children with type 1 diabetes at primary school: perceptions from parents, children, and teachers. Pediatr Diabetes. 2009;10(1):67–73. doi: 10.1111/j.1399-5448.2008.00457.x. [DOI] [PubMed] [Google Scholar]
- 19.Commissariat P.V., Harrington K.R., Whitehouse A.L., et al. “I'm essentially his pancreas”: Parent perceptions of diabetes burden and opportunities to reduce burden in the care of children <8 years old with type 1 diabetes. Pediatr Diabetes. 2020;21(2):377–383. doi: 10.1111/pedi.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bratina N., Forsander G., Annan F., et al. ISPAD Clinical Practice Consensus Guidelines 2018: Management and Support of Children and Adolescents With Type 1 Diabetes in School. Pediatr Diabetes. 2018;19(Suppl 27):287–301. doi: 10.1111/pedi.12743. [DOI] [PubMed] [Google Scholar]


