Objective
Pregnant women with coronavirus disease 2019 (COVID-19) show overall similar clinical features as that of nonpregnant adults with COVID-19, except perhaps for higher risk of admission to the intensive care unit (ICU) and mechanical ventilation.1 Among pregnant women with COVID-19, more than 85% to 90% have no or mild symptoms, 5% to 10% have symptoms severe enough to warrant hospitalization and at times oxygen therapy but no mechanical ventilation, and 1% to 2% develop critical disease requiring mechanical ventilation and at times leading even to death.1 Most promising therapeutic possibilities for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pregnancy include currently remdesivir and convalescent plasma (CP). In this study, we present a case of a pregnant woman treated with CP at the city hospital of Mantova in Italy.
Study Design
This study involved a 29-year-old gravida 2, para 1 patient (previous preterm birth at 36 4/7 weeks of gestation) with a body mass index of 31 and with a singleton gestation who presented at the emergency room on April 9, 2020 at 24 2/7 weeks of gestation with worsening cough and fever, which started approximately 7 days before.
At presentation, she was febrile (38.0°C) and normotensive, with a respiratory rate of 20 bpm and an O2 saturation (SpO2) of 95%. Laboratory tests showed normal white blood cell and procalcitonin values, a C-reactive protein concentration of 58.6 mg/L, and normal arterial blood gas values. The polymerase chain reaction (PCR) nasopharyngeal (NP) test for SARS-CoV-2 returned positive. Initial chest X-ray revealed a parenchymal thickening of the upper right lobe. Fetal well-being was assessed by obstetrical ultrasound and nonstress test, the latter not detecting uterine contractions. Antibiotic therapy (ceftriaxone and azithromycin) and prophylactic low-molecular-weight heparin (LMWH) were started. The following day, the patient showed a mild clinical worsening, with persistent dry cough, fatigue, dyspnea, fever (38.5°C), and lymphopenia; her SpO2 was 95% on room air. Following a pneumological evaluation, considering gestational age, the patient was transfused with 300 mL of CP, with no adverse effects. Antepartum testing for fetal well-being was reassuring both before and after the transfusion. The following day, a clinical worsening was observed, with persistently high fever (39.5°C), tachypnoea (30 bpm), hypotension (90/60 mm Hg), and an SpO2 of 91% on room air. Lymphopenia, elevated interleukin-6, and serum ferritin level were reported as well. However, it should be noted that her PCR NP test for SARS-CoV-2 returned negative. Chest ultrasound revealed bilateral pleural thickening with nodulations and several B lines (>4/field). Obstetrical ultrasound showed regular fetal biometry with normal umbilical arterial Doppler assessment. Because of the rapid worsening of her clinical condition and laboratory values (Figure ), the patient was transferred to the ICU were she was given oxygen therapy via a nasal cannula (4 L/min). She was initiated on hydroxychloroquine, LMWH at a therapeutic dose, and methylprednisolone. The peak of severity was reached 2 days after admission to the ICU, when she developed acute respiratory distress syndrome. The ratio of the partial pressure of arterial oxygen to the percentage of inspired oxygen (PaO2/FiO2) fell to a minimum value of 223. She was supported with oxygen therapy via nasal cannula over noninvasive ventilation (FiO2, 36%) without the need for intubation. Obstetrical ultrasound showed a normal umbilical artery pulsatility index (UA-PI) and fetal ductus venosus with an observed a wave. The middle cerebral artery peak systolic velocity (MCA-PSV) was >1.5 multiples of median, with diastolic reverse flow. As a result, the patient was again transfused with 300 mL of CP on day 12 from onset of symptoms, with no adverse reactions. The patient’s clinical condition rapidly improved as shown by normalization of laboratory tests and vital signs within 3 days of the second CP transfusion (Figure). In particular, we observed a rapid normalization in body temperature and SpO2 and a prompt resolution of dyspnea. The PCR NP test for SARS-CoV-2 was repeated on days 15 and 17 with both tests returning with negative results. The patient was discharged 13 days after admission. The mother’s chest ultrasound showed near-complete resolution of bilateral pneumonia. Fetal Doppler assessment was normal, with UA-PI and MCA-PSV within the normal range for gestational age. The outpatient examinations, performed every week for 1 month, showed a complete recovery of pulmonary function. Pregnancy continued to be monitored after hospital discharge by fetal ultrasonography with Doppler assessment every 2 weeks. In the study, fetal biometry results were consistent with gestational age (34 weeks of gestation).
Figure.
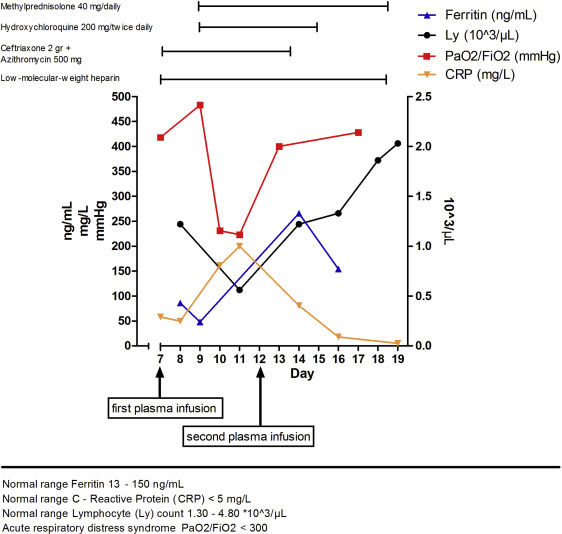
Case report: graphical course of laboratory and clinical parameters
Grisolia. Convalescent plasma for coronavirus disease 2019 in pregnancy. AJOG MFM 2020.
Results
Comparing our patient with those in a US cohort of pregnant women with severe COVID-19,2 the clinical course was similar, with hospital admission on day 7 after onset of symptoms, initiation of O2 therapy on day 9, peak respiratory support on day 10, hydroxychloroquine treatment started on day 9, and symptom resolution on day 15. The rationales for the use of CP in our patient were to prevent the mother’s condition from worsening, to avoid a possible cesarean delivery at a gestational age of severe prematurity, and to reduce potential maternal and fetal risk associated with the administration of various drugs utilized for COVID-19.
In 1 published randomized controlled trial, there is evidence that CP can be used effectively as a therapy for severe COVID-19 in nonpregnant adults, shortening time to clinical improvement by 5 days, and with trends for improvement in other outcomes, including death, without causing severe adverse events.3 Regarding SARS-CoV-2 infection in pregnancy, pregnancy is not a contraindication to blood component transfusion, and 2 other cases of pregnancies managed using CP have been reported (Table ).4 , 5 In a single report, CP administration was associated with survival of the mother but resulted to death of the newborn.4 In the second case report, CP therapy in association with remdesivir successfully managed a critically ill obstetrical patient.5 The case in this study is the first treated with CP without antiviral drugs with a favorable outcome for both the mother and fetus.
Table.
Clinical course of 3 pregnant women affected by COVID-19 treated with CP
| Author | Gestational age | Severity of disease | Invasive procedures | Comorbidity | CP dose (units) | Other medications | Outcome |
|---|---|---|---|---|---|---|---|
| Zhang et al4 | 35 wk and 2 d | Severe ARDS | Mechanical ventilation | — | 1 | Lopinavir and ritonavir | Maternal well-being |
| Septic shock | CRRT | Ribavirin | Newborn death because of intrauterine asphyxia | ||||
| MOF | ECMO | Imipenem | |||||
| Cesarean delivery | Vancomycin | ||||||
| Anderson et al5 | 22 wk and 2 d | Severe ARDS | Mechanical ventilation | Type 2 DM | 1 | Remdesivir | Maternal well-being |
| Asthma | Ceftriaxone | Normal ongoing pregnancy | |||||
| Class III obesity | Azithromycin | ||||||
| Hydroxychloroquine | |||||||
| Hydrocortisone | |||||||
| LMWH | |||||||
| Grisolia et al (present study) | 24 wk and 2 d | Mild ARDS | — | Class I obesity | 2 | Ceftriaxone | Maternal well-being |
| Azithromycin | Normal ongoing pregnancy | ||||||
| Hydroxychloroquine | |||||||
| Methylprednisolone | |||||||
| LMWH |
ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; CP, convalescent plasma; CRRT, continuous renal replacement therapy; DM, diabetes mellitus; ECMO, extracorporeal membrane oxygenation; LMWH, low-molecular-weight heparin; MOF, multiorgan failure.
Grisolia. Convalescent plasma for coronavirus disease 2019 in pregnancy. AJOG MFM 2020.
Conclusion
This case report has several limitations, including the short follow-up (the patient was at 34 weeks of gestation and well) and the concomitant use of other medications that may confound the evaluation of CP effectiveness. Nevertheless, the close temporal association between CP transfusion and improvement in clinical and laboratory parameters represents an encouraging finding.
There are several ongoing randomized clinical trials regarding the use of CP in patients with COVID-19; in many of those trials, pregnancy is not considered as an exclusion criterion.6
We hope that further studies on plasma administration can be carried out during pregnancy, especially in a gestational age of severe prematurity, with the purpose of prolonging the course of pregnancy as long as possible.
Footnotes
This paper is part of a supplement that represents a collection of COVID-related articles selected for publication by the editors of AJOG MFM without additional financial support.
The authors report no conflict of interest.
References
- 1.Center for Disease Control and Prevention Data on COVID-19 during pregnancy. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19.html Available at: Accessed July 22, 2020.
- 2.Pierce-Williams R.A.M., Burd J., Felder L., et al. Clinical course of severe and critical COVID-19 in hospitalized pregnancies: a US cohort study. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100134. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L., Zhang W., Hu Y., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020 doi: 10.1001/jama.2020.10044. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B., Liu S., Tan T., et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158:e9–e13. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson J., Schauer J., Bryant S., Graves C.R. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Rep Womens Health. 2020 doi: 10.1016/j.crwh.2020.e00221. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valk S.J., Piechotta V., Chai K.L., et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;5:CD013600. doi: 10.1002/14651858.CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]


