Abstract
The COVID-19 epidemic represents an unprecedented global health emergency, further aggravated by the lack of effective therapies. For this reason, several clinical trials are testing different off-label drugs, already approved for other pathologies. Mesenchymal stem/stromal cells (MSCs) have been tested during the last two decades for the treatment of various pathologic conditions, including acute and chronic lung diseases, both in animal models and in patients. In particular, promising results have been obtained in the experimental therapy of acute respiratory distress syndrome, which represents the most threatening complication of COVID-19 infection. Furthermore, more recently, great interest has been devoted to the possible clinical applications of extracellular vesicles secreted by MSCs, nanoparticles that convey much of the biological effects and of the therapeutic efficacy of their cells of origin. This review summarizes the experimental evidence underlying the possible use of MSCs and of MSC-EVs in severe COVID-19 infection and underlines the need to evaluate the possible efficacy of these therapeutic approaches through controlled studies under the supervision of the Regulatory Authorities.
Graphical abstract
1. Introduction
The ongoing health emergency related to the COVID-19 epidemic is mainly due to the pulmonary complications of the disease, for which there are no proven effective therapies. According to recent studies, the cell entry receptors of the virus are represented by the angiotensin converting enzyme II ACE2 [1] and by the serine protease TMPRSS2 [2]. Both types of proteins are highly expressed on alveolar type II cells (AT2), while the ACE2 receptor is also widely distributed on several human cells, including cardiac and kidney cells, as well as endothelial and smooth muscle cells in several organs, explaining the ability of this virus to generate a systemic disease [3,4]. The binding of the SARS-CoV-2 spike protein to ACE2 has been suggested to cause the downregulation of ACE2 from the cell membrane [5], resulting in an imbalance between ACE and ACE2 activity and contributing to acute lung injury [6]. Indeed, ACE2 has opposite effects to ACE [7]. ACE-catalyzed conversion of Angiotensin I to Angiotensin II promotes vasoconstriction, inflammation and oxidative stress, while ACE2 converts angiotensin II into angiotensin 1–7, a peptide inducing vasodilatation and exhibiting anti-oxidant and anti-inflammatory properties [6,8,9]. Acute respiratory distress syndrome (ARDS) and an exuberant inflammatory response characterized by high blood cytokine levels have been associated with critical and fatal illnesses [10]. ARDS is a devastating hypoxemic respiratory failure, characterized by disruption of the alveolar-capillary membrane barrier [11]. Despite decades of research, current management for ARDS remains supportive [11]. Several clinical trials are currently underway in a collective effort to fight COVID-19 pneumonia, including both new drugs and “off label” drugs, i.e. drugs that can be used in diseases other than those for which they have been authorized [12]. This is the case of some antivirals and some biological anti-cytokine drugs (such as anti-interleukin 6 and anti-TNF), tested on these patients based on their established mechanisms of action.
2. Mesenchymal stromal cells for the treatment of acute inflammatory lung diseases
A recent report described the possible therapeutic efficacy of mesenchymal stem/stromal cells (MSCs) in patients with severe COVID-19 pneumonia [13]. MSCs are a heterogeneous population containing stromal cells, progenitor cells, fibroblasts and stem cells [14,15]. They can be isolated from different tissues including bone marrow, adipose tissue, cord blood, Wharton jelly and placenta, and are currently being used to treat a number of clinical conditions, as well as being tested in several clinical trials around the world due to their immunomodulatory and tissue regenerative properties [[16], [17], [18], [19], [20], [21]] together with their considerable safety [22].
The study of Leng et al. [13] enrolled seven confirmed COVID-19 patients, including one critically severe type, four severe types and two common types in the MSC-treated group, while three severe type patients were enrolled in the placebo control group. Of note, “standard” treatment in the placebo group was not specified. Treated patients received 1 × 106 MSCs per Kg body wt. intravenously. No adverse effects were observed. The pulmonary function and symptoms of treated patients were significantly improved within 2 days after MSC transplantation. Chest CT imaging also showed significant improvement. In this group, three patients (two common and one severe type) recovered and were discharged within 10 days. In the placebo group, one patient died and two were reported to worsen, although their outcome is not described. MSC infusion was associated with increased peripheral lymphocyte count and with decreased systemic markers of inflammation compared to the placebo group.
Clearly, it is premature to draw any conclusion based on a single study with a limited number of patients likely receiving multiple treatments, as warned by scientific societies in the field [[23], [24], [25]]. On the other hand, more rigorous studies excluding possible confounding treatments in the placebo group could raise serious ethical concerns. Due to the complexity of the disease, a large sample size will probably be required to reach statistical significance, and it could be difficult to meet such a requirement with a decreasing prevalence of the infection. However, it is reasonable to put forward some considerations based on the knowledge accumulated over twenty years of experience with the use of these cells in various autoimmune, inflammatory and degenerative diseases. Indeed, the rationale for the use of MSCs in COVID-19 associated pneumonia is manifold.
The anti-inflammatory and immune modulatory properties of MSCs are well established and have been exploited in a large number of both preclinical and clinical studies [16,26,27]. Moreover, MSCs behave as tissue-protecting agents, inhibiting apoptosis, limiting oxidative injury and enhancing regeneration [18]. MSCs are massively retained in the lung following intravenous infusion [28] and they have been successfully tested in several animal models of acute and chronic lung injury such as idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, obstructive bronchiolitis, and bronchopulmonary dysplasia [[29], [30], [31], [32], [33], [34], [35]].
Documented biological activities supporting the use of MSCs/MSC-EVs as therapeutic agents in COVID-19 pneumonia include immune modulation, tissue protection and anti-bacterial/antiviral activity.
As stated above, the cytokine release syndrome (CRS) characterized by fever and multiple organ dysfunction is a major cause of death in COVID-19 patients. Data from recent studies suggest that SARS-CoV-2 infection can lead to a complex immune dysregulation affecting different subsets of immune cells [11]. This is probably the reason why targeting specific immune pathways has so far brought only partially beneficial effects to severe COVID-19 patient [36]. For instance, although Interleukin 6 (IL-6) seems to hold a key role in CRS pathophysiology, treatment with selective inhibitors such as Tocilizumab, a blocker of IL-6R that can effectively block IL-6 signal transduction pathway, did not reduce mortality in COVID-19 patients. The beneficial effects of MSCs in different models of lung injury and fibrosis are associated with a reduction in proinflammatory cytokines such as tumor necrosis factor-α and IL-6 and an increase in anti-inflammatory cytokines such as IL-10. Alveolar macrophages are crucial in orchestrating the initiation and resolution of lung inflammation, first by polarizing toward the M1 phenotype releasing pro-inflammatory cytokines and then by switching to the alternative M2 phenotype, releasing IL-10 and promoting the resolution of inflammation [37]. MSCs (activated by LPS or TNF-a via Toll-like receptor 4) can reprogram macrophages to an alternative phenotype by releasing prostaglandin E2 [38]. More recently, Lv et al. have demonstrated in an animal model of acute lung injury that the stress response protein stanniocalcin-2 can have a central role in MSC immunomodulation [39]. These results were obtained in animal models of toxic or LPS-mediated lung injury that could elicit different responses by MSCs when compared to SARS-CoV-2 infection. Interestingly, however, the clinical study by Leng et al. reported that the levels of TNF-α was significantly decreased, while IL-10 increased in COVID patients treated with MSCs compared to the placebo control group. These findings suggest that MSCs might help re-equilibrating the dysregulated immune response observed in these patients.
Lung pathology of COVID-19 pneumonia in critically ill patients include exudative and proliferative phases of diffuse alveolar damage and microvessel thrombosis suggestive of early ARDS [40,41], whose pathogenesis include altered alveolar permeability and neutrophil infiltration [42]. Administration of MSC-EVs was found to reduce protein permeability and to increase alveolar fluid clearance in an ex vivo model of human perfused lung injured with severe E. coli pneumonia [28,43,44]. These functional improvements were associated with decreased neutrophil infiltration. The MSC therapeutic potential was correlated with the secretion of cytoprotective agents such as keratinocyte growth factor (KGF), anti-inflammatory products such as PGE2 or lipoxin A4, antipermeability factors such as angiopoietin-1 (Ang1). Interestingly, culture media of bacteria-stimulated MSCs were found to contain antimicrobial products [45], and in murine sepsis models treatment with MSCs increased bacterial clearance, in part due to enhanced phagocytotic activity of the host immune cells [46]. Moreover, MSC-EVs exhibited antiviral activity, both by suppressing influenza virus replication after virus entry in lung epithelial cells in vitro and by decreasing viral load in a pig model of influenza virus-induced lung injury [47].
Currently, there are several ongoing clinical trials on the use of MSCs in the treatment of several pulmonary diseases including idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, obstructive bronchiolitis and bronchopneumodysplasia [19] as well as for the treatment of ARDS [48] and of septic shock [49]. Interestingly, Leng et al. also showed that MSCs do not express ACE2 and TMPRSS2 receptors, suggesting that the virus should not infect this cell population. Moreover, it was shown that MSC administration to a rat model of hyperoxia-induced lung injury reduced to normal level the hyperoxia-induced overexpression of angiotensin II, angiotensin II type 1 receptor, and of angiotensin-converting enzyme [50]. Very recently, Simonson et al. [51] reported a long-term follow up of two patients with the most severe form of ARDS that in the acute phase needed ECMO support in combination with mechanical ventilation and at the same time were treated with a single systemic infusion of allogeneic MSCs. Remarkably, 5 years after the treatment both patients had fully recovered their physical and mental capacities, which is unusual for ARDS survivors. Moreover, the patient that had the most severe form of ARDS and was on ECMO support for 28 days before the MSC-infusion, had no signs of pulmonary fibrosis five years after the MSC treatment as demonstrated using CT scan with dual energy.
As mentioned above, no firm conclusion can be drawn by the study of Leng et al., but both the reported preliminary results and the scientific background should encourage further investigation on MSC treatment for COVID-19 pneumonia with well-designed clinical trials under the control of Regulatory Authorities.
3. The new therapeutic potential of extracellular vesicles
The provision of large amounts of MSCs at affordable cost is however an issue. Industrial GMP production of clinical-grade MSCs is both cumbersome and expensive [52]. To date, MSCs have been authorized by Regulatory Authorities for diseases involving a limited number of patients, such as GVHD in pediatric patients or anal fistulas resistant to conventional therapy in Crohn's disease. Clearly, the use of MSCs in a large number of patients such as the one encountered in a pandemic disease would require a significant cost reduction, also considering the relatively high dose used in the study of Leng et al.
MSCs exert most of their therapeutic effect via paracrine mechanisms [[53], [54], [55], [56]], including the secretion of extracellular vesicles (EVs). EVs, including exosomes and microvesicles, are complex biological machines secreted by all cell types and ranging from 0.03 to 1 μm in size. EVs carry.
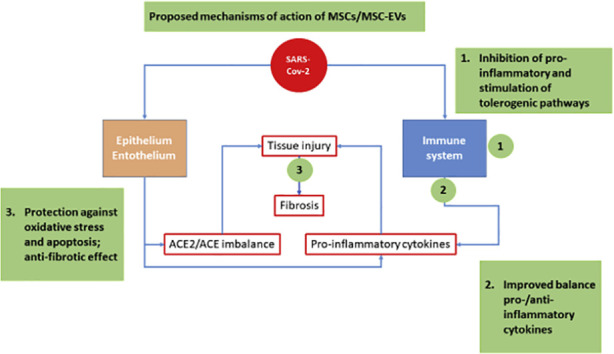
a variety of proteins, lipids and nucleic acids, with profound effects on target cells [[57], [58], [59]]. In the case of MSC-derived EVs, both others and we have demonstrated their immunomodulatory effects in vitro [[60], [61], [62], [63], [64]] and revealed a remarkable anti-inflammatory and pro-regenerative capacity in several animal models of disease [65,66]. More specifically, both others and we demonstrated that MSC-EV administration shows therapeutic effects in animal models of lung injury, including hyperoxia [[67], [68], [69]], severe bacterial pneumonia [70] and viral pneumoni [47]. Moreover, administration of MSC-EVs showed beneficial effects in ex vivo perfused human lungs injured with severe E. coli pneumonia [71]. Finally, MSC-EVs were effective in rehabilitating marginal donor human lungs, by increasing alveolar fluid clearance in a dose-dependent manner, decreasing lung weight gain following perfusion and ventilation, and improving both airway and hemodynamic parameter [59]. Based on these promising data, the role of MSC-EVs in mitigation and repair of lung injury in ARDS is being increasingly recognized [54,70,72,73]. Finally yet importantly, MSC-EVs can prevent the development of fibrosis following experimental lung injury, similarly to their cells of origin [34,74]. Of note, anecdotic descriptions of fibrotic sequelae with reduced lung function in patients recovered from COVID-19 pneumonia are being reported [75]. Indeed, the risk of developing idiopathic pulmonary fibrosis in increased following viral infections [76], and this long term complication was also reported in some patients following SARS infection [77]. Actually, there is growing interest in the potential use of EVs as therapeutic tools (see graphical abstract for a proposed effect of MSC/ MSC-EVs on COVID-19 mediated tissue injury). EVs are considered safer than their cells of origin, and are easier and cheaper to produce, isolate, store and administer [78], which should result in reduced cost and larger availability of the product. It should be mentioned that EVs seem to be extremely versatile products that can be engineered by various techniques, such as manipulation of their parent cells through genetic engineering, by introducing exogenous material that is subsequently incorporated into secreted EV [[79], [80], [81]]. A similar strategy has been used to deliver exogenous microRNA-let7c via MSC-EVs to attenuate renal fibrosis in mice with unilateral ureteral obstruction [82]. EVs can also be loaded with therapeutic molecules to improve targeting to the desired site of action [81,[83], [84], [85]].
So far, clinical experience is limited to a few trials on the use of EVs derived from dendritic cells in adoptive immune therapies for cancer [86] and to a single patient successfully treated with MSC-derived EVs for steroid-resistant GVHD [87]. A clinical trial on the use of MSC-EVs in premature neonates at high risk for bronchopulmonary dysplasia is currently recruiting [88].
However, there are still significant barriers in the development of MSC-EVs as therapeutic tool [89]. Some of these challenges are shared with their parent cells, including the variability in tissues of origin and culture conditions, [90,91]. Although MSCs from different sources exhibit different immune suppressive and differentiation capacity, the optimal source(s) of MSCs for immunomodulation have not been conclusively determined [92] and no comparative studies on MSC-EVs from different tissues are available. Large scale production of MSC-EVs suitable for clinical applications remains a major challenge. Refined isolation methods yielding EVs with a high degree of purity may not be applicable at industrial level [93]. Moreover, some “contaminants” co-isolating with EVs may contribute to their therapeutic efficacy [94]. The heterogeneity of EV subpopulations represents an additional challenge [95]. Indeed, so far all published studies have used a heterogeneous population of EVs, even if in some cases a partial size selection (“small” EVs <200 nm) is performed. Of note, it was shown that EV populations of different sizes secreted by dendritic cells can induced different patterns of polarization of activated T cells [96]. Quantifying EV preparations is also an unresolved problem, since accurate EV counting is hampered both by lack of standardization and by the inability of currently used devices to distinguish membrane- enclosed vesicles from non-vesicular particles [95]. The above issues highlight the need for reliable potency assays, which according to regulatory authorities should measure the biological activity of the product that mediates the therapeutic effect of a given drug [97]. Unfortunately, as stated above, the mechanism of action of MSC-EVs is complex and poorly understood, and potency assays for this therapeutic product are not validated and are still experimental [87]. It should be noticed that it took about two decades of preclinical and clinical tests before some MSC-based treatments were approved by Regulatory Authorities. Even if knowledge in the field has progressed fast, additional improvements in EV production and better understanding on their mechanisms of action will be required for EV-based treatments to become more clinically applicable. On the clinical side, little is known regarding optimal therapeutic doses and optimal route(s) of administration [89]. Regarding safety, MSC-EVs have been shown to exhibit procoagulant activity, similarly to their cells of origin [98], a property that could cause concern in patients prone to thrombotic events such as those with SARS-CoV-2 infection.
When this manuscript was under review, a first-in-man trial on 24 patients with severe COVID-19 pneumonia treated with MSC-EVs was published [99]. The study included both outpatients (cohort A) and hospitalized patients without (cohort B) or with (cohort C) artificial ventilation and a follow-up period of 14 days following MSC-EV administration. Associated treatments included hydroxychloroquine and azythromycin. The patients received a single IV dose of MSC-EVs over 60 min. Unfortunately, neither the origin of MSCs nor the EV dose were specified. The study met its primary safety endpoints, with no reported adverse events in the immediate (<24 h), intermediate (<72 h) or delayed (>72 h) period following EV infusion. Overall survival rate was 83% (4 deaths/24 patients, 2 in cohort B and 2 in cohort C), and 13% of the patients (3/24) remained critically ill, still requiring mechanical ventilation and intensive care at the end of the follow up period. Eighty percent of patients (20/24) exhibited improved PaO2/FiO2 ratio within 3 days of treatment. Again, no conclusion on efficacy can be driven from a small phase I study. Interestingly, however, inflammatory markers and absolute neutrophil count significantly decreased while total lymphocyte and CD8+ count significantly increased within 5 days following EV treatment. Moreover, D-dimer was significantly decreased, a reassuring finding in view both of the thromboembolic syndrome often associated with serious SARS-CoV-2 infection and of the above-cited potential procoagulant activity of MSC-EVs.
In conclusion, MSC-EVs should also be considered in parallel with MSCs as an experimental therapeutic tool in seriously compromised patients at the risk of life and/or for the prevention of fibrotic complications after the acute phase, following the current regulations of the phase I / II clinical trials or for compassionate use.
Authors contributions
Each author has approved the submitted version and agrees to be personally accountable for the author's own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature.
Funding
This work received no external funding.
Declaration of Competing Interest
None.
Footnotes
This manuscript represents a consensus paper issued by the Scientific Board of STEMNET, a federation gathering the following four scientific societies devoted to stem cell research. • Italian Group for the Study of Mesenchymal Stem Cells (GISM): A. Pessina (President), E. Lucarelli, M. Muraca, M. Pozzobon. • Forum of Italian Researchers on Mesenchymal Stromal and Stem Cells (FIRST): L. Lazzari (President), M. Dominici. • Stem Cell Research Italy (SCRI): U. Galderisi (President) • International Placenta Stem Cell Society (IPLASS): O. Parolini (Past President).
References
- 1.Zhou P., Lou Yang X., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Di Jiang R., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020 doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., Niemeyer D., Schneider H., Drosten C., Pohlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011 doi: 10.1128/jvi.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004 doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., Simmons G., Hofmann H., Kuri T., Weber F., Eichler J., Drosten C., Pöhlmann S. Differential Downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010 doi: 10.1128/jvi.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholls J., Peiris M. Good ACE, bad ACE do battle in lung injury, SARS. Nat. Med. 2005 doi: 10.1038/nm0805-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005 doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Hashim A.Z., Renno W.M., Raghupathy R., Abduo H.T., Akhtar S., Benter I.F. Angiotensin-(1-7) inhibits allergic inflammation, via the MAS1 receptor, through suppression of ERK1/2- and NF-kB-dependent pathways. Br. J. Pharmacol. 2012 doi: 10.1111/j.1476-5381.2012.01905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tufan A., Avanoğlu Güler A., Matucci-Cerinic M. Covid-19, immune system response, hyperinflammation and repurposinantirheumatic drugs. Turkish J. Med. Sci. 2020;50:620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jee Y. WHO international health regulations emergency committee for the COVID-19 outbreak. Epidemiol. Health. 2020 doi: 10.4178/epih.e2020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L.W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K.J., Ellison-Hughes G., Caruso C., Jin K., Zhao R.C. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with covid-19 pneumonia. Aging Dis. 2020 doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 15.Galderisi U., Giordano A. The gap between the physiological and therapeutic roles of mesenchymal stem cells. Med. Res. Rev. 2014 doi: 10.1002/med.21322. [DOI] [PubMed] [Google Scholar]
- 16.Gao F., Chiu S.M., Motan D.A.L., Zhang Z., Chen L., Ji H.L., Tse H.F., Fu Q.L., Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016 doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Blanc K., Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J. Intern. Med. 2007 doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 18.Brown C., McKee C., Bakshi S., Walker K., Hakman E., Halassy S., Svinarich D., Dodds R., Govind C.K., Chaudhry G.R. Mesenchymal stem cells: cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019 doi: 10.1002/term.2914. [DOI] [PubMed] [Google Scholar]
- 19.Wecht S., Rojas M. Mesenchymal stem cells in the treatment of chronic lung disease. Respirology. 2016 doi: 10.1111/resp.12911. [DOI] [PubMed] [Google Scholar]
- 20.Winkler T., Perka C., von Roth P., Agres A.N., Plage H., Preininger B., Pumberger M., Geissler S., Hagai E.L., Ofir R., Pinzur L., Eyal E., Stoltenburg-Didinger G., Meisel C., Consentius C., Streitz M., Reinke P., Duda G.N., Volk H.D. Immunomodulatory placental-expanded, mesenchymal stromal cells improve muscle function following hip arthroplasty. J. Cachexia. Sarcopenia Muscle. 2018 doi: 10.1002/jcsm.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norgren L., Weiss N., Nikol S., Hinchliffe R.J., Lantis J.C., Patel M.R., Reinecke H., Ofir R., Rosen Y., Peres D., Aberman Z. PLX-PAD cell treatment of critical limb Ischaemia: rationale and design of the PACE trial. Eur. J. Vasc. Endovasc. Surg. 2019 doi: 10.1016/j.ejvs.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Thompson M., Mei S.H.J., Wolfe D., Champagne J., Fergusson D., Stewart D.J., Sullivan K.J., Doxtator E., Lalu M., English S.W., Granton J., Hutton B., Marshall J., Maybee A., Walley K.R., Dos Santos C., Winston B., McIntyre L. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: an updated systematic review and meta-analysis. EClinicalMedicine. 2020;19 doi: 10.1016/j.eclinm.2019.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ISCT press release . 2020. ISCT Releases Statement on Unproven Stem Cell Treatments for COVID-19 - ISCT. https://isctglobal.org/news/494824/ISCT-Releases-Statement-on-Unproven-Stem-Cell-Treatments-for-COVID-19.htm (accessed March 28, 2020)., (n.d.) [Google Scholar]
- 24.ISSCR ISSCR Statement Regarding the Marketing of Unproven Stem Cell Treatments for COVID-19. 2020. https://www.isscr.org/news-publicationsss/isscr-news-articles/article-listing/2020/03/06/isscr-statement-regarding-the-marketing-of-unproven-stem-cell-tr
- 25.STEMNET Scientific Board STEMNET position statement about the use of cell therapies for Covid-19 infections - March 16, 2020. 2020. https://www.isscr.org/news-publicationsss/isscr-news- (accessed April 2, 2020)., (n.d.)
- 26.Ringden O., Le Blanc K. Mesenchymal stem cells for treatment of acute and chronic graft-versus-host disease, tissue toxicity and hemorrhages. Best Pract. Res. Clin. Haematol. 2011 doi: 10.1016/j.beha.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 27.de Girolamo L., Lucarelli E., Alessandri G., Antonietta Avanzini M., Ester Bernardo M., Biagi E., Teresa Brini A., D’Amico G., Fagioli F., Ferrero I., Locatelli F., Maccario R., Marazzi M., Parolini O., Pessina A. Italian mesenchymal stem cell group (GISM), mesenchymal stem/stromal cells: a new ''cells as drugs'' paradigm. Efficacy and critical aspects in cell therapy. Cell Therap. Curr. Pharm. Des. 2013 doi: 10.2174/1381612811319130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., Semprun-Prieto L., Delafontaine P., Prockop D.J. Intravenous hMSCs improve myocardial infarction in mice because cells Embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simonson O.E., Mougiakakos D., Heldring N., Bassi G., Johansson H.J., Dalén M., Jitschin R., Rodin S., Corbascio M., El Andaloussi S., Wiklander O.P.B., Nordin J.Z., Skog J., Romain C., Koestler T., Hellgren-Johansson L., Schiller P., Joachimsson P.-O., Hägglund H., Mattsson M., Lehtiö J., Faridani O.R., Sandberg R., Korsgren O., Krampera M., Weiss D.J., Grinnemo K.-H., Le Blanc K. In vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. Stem Cells Transl. Med. 2015 doi: 10.5966/sctm.2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthay M.A. Therapeutic potential of mesenchymal stromal cells for acute respiratory distress syndrome. Ann. Am. Thorac. Soc. 2015 doi: 10.1513/AnnalsATS.201406-254MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laffey J.G., Matthay M.A. Cell-based therapy for acute respiratory distress syndrome: biology and potential therapeutic value. Am. J. Respir. Crit. Care Med. 2017 doi: 10.1164/rccm.201701-0107CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthay M.A., Goolaerts A., Howard J.P., Lee J.W. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit. Care Med. 2010 doi: 10.1097/CCM.0b013e3181f1ff1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gotts J.E., Matthay M.A. Mesenchymal stem cells and acute lung injury. Crit. Care Clin. 2011 doi: 10.1016/j.ccc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cargnoni A., Gibelli L., Tosini A., Signoroni P.B., Nassuato C., Arienti D., Lombardi G., Albertini A., Wengler G.S., Parolini O. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplant. 2009 doi: 10.3727/096368909788809857. [DOI] [PubMed] [Google Scholar]
- 35.Lee J.W., Fang X., Gupta N., Serikov V., Matthay M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. U. S. A. 2009 doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahmati M., Moosavi M.A. Cytokine-targeted therapy in severely ill COVID-19 patients: options and cautions. Eurasian J. Med. Oncol. 2020;4:179–180. doi: 10.14744/ejmo.2020.72142. [DOI] [Google Scholar]
- 37.Herold S., Mayer K., Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front. Immunol. 2011;2 doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Németh K., Leelahavanichkul A., Yuen P.S.T., Mayer B., Parmelee A., Doi K., Robey P.G., Leelahavanichkul K., Koller B.H., Brown J.M., Hu X., Jelinek I., Star R.A., Mezey É. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv H., Liu Q., Sun Y., Yi X., Wei X., Liu W., Zhang Q., Yi H., Chen G. Mesenchymal stromal cells ameliorate acute lung injury induced by LPS mainly through stanniocalcin-2 mediating macrophage polarization. Ann. Transl. Med. 2020;8:334–349. doi: 10.21037/atm.2020.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., Heinrich F., Mushumba H., Kniep I., Schröder A.S., Burdelski C., de Heer G., Nierhaus A., Frings D., Pfefferle S., Becker H., Bredereke-Wiedling H., de Weerth A., Paschen H.-R., Sheikhzadeh-Eggers S., Stang A., Schmiedel S., Bokemeyer C., Addo M.M., Aepfelbacher M., Püschel K., Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020 doi: 10.7326/m20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zemans R.L., Matthay M.A. What drives neutrophils to the alveoli in ARDS? Thorax. 2017;72:1–3. doi: 10.1136/thoraxjnl-2016-209170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J., Kim S., Lim H., Liu A., Hu S., Lee J., Zhuo H., Hao Q., Matthay M.A., Lee J.-W. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019;74:43–50. doi: 10.1136/thoraxjnl-2018-211576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gennai S., Monsel A., Hao Q., Park J., Matthay M.A., Lee J.W. Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am. J. Transplant. 2015;15:2404–2412. doi: 10.1111/ajt.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krasnodembskaya A., Song Y., Fang X., Gupta N., Serikov V., Lee J.W., Matthay M.A. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mei S.H.J., Haitsma J.J., Dos Santos C.C., Deng Y., Lai P.F.H., Slutsky A.S., Liles W.C., Stewart D.J. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am. J. Respir. Crit. Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 47.Khatri M., Richardson L.A., Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9 doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthay M.A., Calfee C.S., Zhuo H., Thompson B.T., Wilson J.G., Levitt J.E., Rogers A.J., Gotts J.E., Wiener-Kronish J.P., Bajwa E.K., Donahoe M.P., McVerry B.J., Ortiz L.A., Exline M., Christman J.W., Abbott J., Delucchi K.L., Caballero L., McMillan M., McKenna D.H., Liu K.D. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir. Med. 2019 doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McIntyre L.A., Stewart D.J., Mei S.H.J., Courtman D., Watpool I., Granton J., Marshall J., Dos Santos C., Walley K.R., Winston B.W., Schlosser K., Fergusson D.A. Cellular immunotherapy for septic shock: a phase I clinical trial. Am. J. Respir. Crit. Care Med. 2018;197:337–347. doi: 10.1164/rccm.201705-1006OC. [DOI] [PubMed] [Google Scholar]
- 50.Chen C.M., Chou H.C. Human mesenchymal stem cells attenuate hyperoxia-induced lung injury through inhibition of the renin-angiotensin system in newborn rats. Am. J. Transl. Res. 2018;10(8):2628–2635. PMID: 30210699. [PMC free article] [PubMed] [Google Scholar]
- 51.Simonson O.E., Ståhle E., Hansen T. 2020. 5-year Follow up After MSC-based Treatment of Severe Acute, in press; pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lloyd-Williams H., Hughes D.A. A systematic review of economic evaluations of advanced therapy medicinal products. Br. J. Clin. Pharmacol. 2020 doi: 10.1111/bcp.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doorn J., Moll G., Le Blanc K., Van Blitterswijk C., De Boer J. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng. - Part B Rev. 2012 doi: 10.1089/ten.teb.2011.0488. [DOI] [PubMed] [Google Scholar]
- 54.Li J., Huang S., Wu Y., Gu C., Gao D., Feng C., Wu X., Fu X. Paracrine factors from mesenchymal stem cells: a proposed therapeutic tool for acute lung injury and acute respiratory distress syndrome. Int. Wound J. 2014 doi: 10.1111/iwj.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gnecchi M., Zhang Z., Ni A., Dzau V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008 doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silini A.R., Magatti M., Cargnoni A., Parolini O. Is immune modulation the mechanism underlying the beneficial effects of amniotic cells and their derivatives in regenerative medicine? Cell Transplant. 2017 doi: 10.3727/096368916X693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kowal J., Tkach M., Théry C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014 doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Vlassov A.V., Magdaleno S., Setterquist R., Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 59.Ragni E., Banfi F., Barilani M., Cherubini A., Parazzi V., Larghi P., Dolo V., Bollati V., Lazzari L. Extracellular vesicle-shuttled mRNA in mesenchymal stem cell communication. Stem Cells. 2017 doi: 10.1002/stem.2557. [DOI] [PubMed] [Google Scholar]
- 60.Budoni M., Fierabracci A., Luciano R., Petrini S., Di Ciommo V., Muraca M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013;22:369–379. doi: 10.3727/096368911X582769. [DOI] [PubMed] [Google Scholar]
- 61.Del Fattore A., Luciano R., Pascucci L., Goffredo B.M., Giorda E., Scapaticci M., Fierabracci A., Muraca M. Immunoregulatory effects of mesenchymal stem cell-derived extracellular vesicles on T lymphocytes. Cell Transplant. 2015;24:2615–2627. doi: 10.3727/096368915X687543. [DOI] [PubMed] [Google Scholar]
- 62.Di Trapani M., Bassi G., Midolo M., Gatti A., Kamga P.T., Cassaro A., Carusone R., Adamo A., Krampera M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henao Agudelo J.S., Braga T.T., Amano M.T., Cenedeze M.A., Cavinato R.A., Peixoto-Santos A.R., Muscará M.N., Teixeira S.A., Cruz M.C., Castoldi A., Sinigaglia-Coimbra R., Pacheco-Silva A., De Almeida D.C., Saraiva Camara N.O. Mesenchymal stromal cell-derived microvesicles regulate an internal pro-inflammatory program in activated macrophages. Front. Immunol. 2017 doi: 10.3389/fimmu.2017.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu L.L.F.-P., Dong J.-J., Sun S.-J., Gao W.-Y., Zhang Z.-W., Zhou X.-J., Yang L., Zhao J.-Y., Yao J.-M., Liu M. Autologous bone marrow stem cell transplantation in critical limb ischemia: a meta-analysis of randomized controlled trials. Chin. Med. 2017;125(23):4296–4300–4300. PMID: 23217403. [PubMed] [Google Scholar]
- 65.Phinney D.G., Pittenger M.F. Concise {review}: {MSC}-{derived} {Exosomes} for {cell}-{free} {therapy} Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 66.Fierabracci A., Del Fattore A., Luciano R., Muraca M., Teti A., Muraca M. Recent advances in mesenchymal stem cell immunomodulation: the role of microvesicles. Cell Transplant. 2015 doi: 10.3727/096368913X675728. [DOI] [PubMed] [Google Scholar]
- 67.Willis G.R., Fernandez-Gonzalez A., Anastas J., Vitali S.H., Liu X., Ericsson M., Kwong A., Mitsialis S.A., Kourembanas S. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am. J. Respir. Crit. Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porzionato A., Zaramella P., Dedja A., Guidolin D., Van Wemmel K., Macchi V., Jurga M., Perilongo G., De Caro R., Baraldi E., Muraca M. Intratracheal administration of mesenchymal stem-cell-derived extracellular vesicles reduces lung injury in a rat model of bronchopulmonary dysplasia. Am. J. Phys. Lung Cell. Mol. Phys. 2018;316(1):L6–L19. doi: 10.1152/ajplung.00109.2018. [DOI] [PubMed] [Google Scholar]
- 69.Braun R.K., Chetty C., Balasubramaniam V., Centanni R., Haraldsdottir K., Hematti P., Eldridge M.W. Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem. Biophys. Res. Commun. 2018 doi: 10.1016/j.bbrc.2018.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monsel A., Zhu Y.G., Gennai S., Hao Q., Hu S., Rouby J.J., Rosenzwajg M., Matthay M.A., Lee J.W. Therapeutic effects of human mesenchymal stem cell-derived microvesicles in severe pneumonia in mice. Am. J. Respir. Crit. Care Med. 2015 doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park J.S., Shim M.-S., Shim S.H., Yang H.N., Jeon S.Y., Woo D.G., Lee D.R., Yoon T.K., Park K.-H. Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGF-β3. Biomaterials. 2011;32:8139–8149. doi: 10.1016/j.biomaterials.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 72.Shah T.G., Predescu D., Predescu S. Mesenchymal stem cells-derived extracellular vesicles in acute respiratory distress syndrome: a review of current literature and potential future treatment options. Clin. Transl. Med. 2019 doi: 10.1186/s40169-019-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee J.W.L.J.H., Park J. Therapeutic use of mesenchymal stem cell–derived extracellular vesicles in acute lung injury. Transfusion. 2019;59:876–883. doi: 10.1111/Trf.14838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mansouri N., Willis G.R., Fernandez-Gonzalez A., Reis M., Nassiri S., Mitsialis S. Alex, Kourembanas S. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 2019 doi: 10.1172/jci.insight.128060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coronavirus: Some Recovered Patients May Have Reduced Lung Function and Are Left Gasping for Air while Walking Briskly, Hong Kong Doctors Find | South China Morning Post. 2020. https://www.scmp.com/news/hong-kong/health-environ
- 76.Sheng G., Chen P., Wei Y., Yue H., Chu J., Zhao J., Wang Y., Zhang W., Zhang H.L. Viral infection increases the risk of idiopathic pulmonary fibrosis: a meta-analysis. Chest. 2020 doi: 10.1016/j.chest.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang P., Li J., Liu H., Han N., Ju J., Kou Y., Chen L., Jiang M., Pan F., Zheng Y., Gao Z., Jiang B. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020 doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muraca M., Piccoli M., Franzin C., Tolomeo A., Jurga M., Pozzobon M., Perilongo G. Diverging concepts and novel perspectives in regenerative medicine. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18051021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Armstrong J.P.K., Holme M.N., Stevens M.M. Re-engineering extracellular vesicles as smart Nanoscale therapeutics. ACS Nano. 2017;11:69–83. doi: 10.1021/acsnano.6b07607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wiklander O.P.B., Brennan M.Á., Lötvall J., Breakefield X.O., EL Andaloussi S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019;11 doi: 10.1126/SCITRANSLMED.AAV8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016 doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 82.Wang B., Yao K., Huuskes B.M., Shen H.-H., Zhuang J., Godson C., Brennan E.P., Wilkinson-Berka J.L., Wise A.F., Ricardo S.D. Mesenchymal stem cells deliver exogenous MicroRNA-let7c via Exosomes to attenuate renal fibrosis. Mol. Ther. 2016;24:1290–1301. doi: 10.1038/MT.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pascucci L., Coccè V., Bonomi A., Ami D., Ceccarelli P., Ciusani E., Viganò L., Locatelli A., Sisto F., Doglia S.M., Parati E., Bernardo M.E., Muraca M., Alessandri G., Bondiolotti G., Pessina A. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J. Control. Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 84.Cappariello A., Loftus A., Muraca M., Maurizi A., Rucci N., Teti A. Osteoblast-derived extracellular vesicles are biological tools for the delivery of active molecules to bone. J. Bone Miner. Res. 2017 doi: 10.1002/jbmr.3332. [DOI] [PubMed] [Google Scholar]
- 85.Van Der Meel R., Fens M.H.A.M., Vader P., Van Solinge W.W., Eniola-Adefeso O., Schiffelers R.M. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J. Control. Release. 2014;195 doi: 10.1016/j.jconrel.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 86.a Morse M., Garst J., Osada T., Khan S., Hobeika A., Clay T.M., Valente N., Shreeniwas R., Sutton M.A., Delcayre A., Hsu D.-H., Le Pecq J.-B., Lyerly H.K. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kordelas L., Rebmann V., Ludwig A.K., Radtke S., Ruesing J., Doeppner T.R., Epple M., Horn P.A., Beelen D.W., Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 88.A Safety study of IV stem cell-derived extracellular vesicles (UNEX-42) in preterm neonates at high risk for BPD. Case Med. Res. 2019 doi: 10.31525/ct1-nct03857841. [DOI] [Google Scholar]
- 89.Tsiapalis D., O’Driscoll L. Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells. 2020;9:991. doi: 10.3390/cells9040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pittenger M.F., Discher D.E., Péault B.M., Phinney D.G., Hare J.M., Caplan A.I. Mesenchymal stem cell perspective: cell biology to clinical progress. Npj Regen. Med. 2019;4:1–15. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel D.B., Santoro M., Born L.J., Fisher J.P., Jay S.M. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol. Adv. 2018;36:2051–2059. doi: 10.1016/J.BIOTECHADV.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao F., Chiu S.M., Motan D.A.L., Zhang Z., Chen L., Ji H.-L., Tse H.-F., Fu Q.-L., Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muraca M., Zaramella P., Porzionato A., Baraldi E. Exosome treatment of Bronchopulmonary dysplasia: how pure should your exosome preparation be? Am. J. Respir. Crit. Care Med. 2018;197:969–970. doi: 10.1164/rccm.201709-1851LE. [DOI] [PubMed] [Google Scholar]
- 94.Théry C., Witwer K.W., Board I., Edit M., Di Vizio D., Juan M., Gardiner C., Gho Y.S., Hill A.F., Lötvall J., Nieuwland R., Peter J., Sahoo S., Soekmadji C., Tahara H., Torrecilhas A.C., Marca H.M., Weaver A.M., Yin H., Zheng L., Lavieu G., Martin-jaular L., Mathieu M., Tkach M. Vol. 2018. 2018. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): a Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines; pp. 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tkach M., Kowal J., Théry C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2018;373 doi: 10.1098/rstb.2016.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kowal J., Arras G., Colombo M., Jouve M., Morath J.P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.FDA Center for Biologics Evaluation and Research . 2011. Potency Tests for Cellular and Gene Therapy Products | FDA. [Google Scholar]
- 98.Silachev D., Goryunov K., Shpilyuk M., Beznoschenko O., Morozova N., Kraevaya E., Popkov V., Pevzner I., Zorova L., Evtushenko E., Starodubtseva N., Kononikhin A., Bugrova A., Evtushenko E., Plotnikov E., Zorov D., Sukhikh G. Effect of MSCs and MSC-derived extracellular vesicles on human blood coagulation. Cells. 2019;8:258. doi: 10.3390/cells8030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. Epub 2020 May 12.PMID: 32380908. [DOI] [PMC free article] [PubMed] [Google Scholar]


