Abstract
The coronavirus disease-2019 (COVID-19) has been designated as a highly contagious infectious disease caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) since December 2019, when an outbreak of pneumonia cases emerged in Wuhan, China. The COVID-19 pandemic has led to a global health crisis, devastating the social, economic and political aspects of life. Many clinicians, health professionals, scientists, organizations, and governments have actively defeated COVID-19 and shared their experiences of the SARS-CoV2.
Diabetes is one of the major risk factors for fatal outcomes from COVID-19. Patients with diabetes are vulnerable to infection because of hyperglycemia; impaired immune function; vascular complications; and comorbidities such as hypertension, dyslipidemia, and cardiovascular disease. In addition, angiotensin-converting enzyme 2 (ACE2) is a receptor for SARS-CoV-2 in the human body. Hence, the use of angiotensin-directed medications in patients with diabetes requires attention. The severity and mortality from COVID-19 was significantly higher in patients with diabetes than in those without. Thus, the patients with diabetes should take precautions during the COVID-19 pandemic.
Therefore, we review the current knowledge of COVID-19 including the global and regional epidemiology, virology, impact of diabetes on COVID-19, treatment of COVID-19, and standard of care in the management of diabetes during this critical period.
Keywords: Severe acute respiratory syndrome coronavirus-2, Coronavirus disease-2019 (COVID-19), Diabetes mellitus, Angiotensin-converting enzyme 2
1. Introduction
The coronavirus disease-2019 (COVID-19) has been designated as a highly contagious infectious disease caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) since December 2019, when an outbreak of pneumonia cases emerged in Wuhan, Hubei, China[1].
Due to rapid and sustained human-to-human transmission, the COVID-19 pandemic has led to a global health crisis, devastating the social, economic and political aspects of life. Many clinicians, health professionals, scientists, organizations, and governments have actively defeated COVID-19 and shared their experiences and knowledge of the SARS-CoV2.
Diabetes is one of the major risk factors for fatal outcomes from COVID-19 [2]. Patients with diabetes are vulnerable to infection because of hyperglycemia; impaired immune function; and comorbidities such as hypertension, dyslipidemia, and cardiovascular disease. In addition, angiotensin-converting enzyme 2 (ACE2) is a receptor for SARS-CoV-2 in the human body[3]. Hence, the use of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blockers (ARB) and high level of angiotensin II in patients with diabetes requires attention. In fact, it was reported that the overall proportion of diabetics with COVID-19 was 5.3–33.9% in China[4], Italy[5], and the USA[6]. The severity and mortality from COVID-19 was significantly higher in patients with diabetes than in those without [7]. Thus, the patients with diabetes should take precautions during the COVID-19 pandemic. In addition, knowledge of the molecular mechanism of viral entry and replication can direct the treatment strategies and future research on targeted antiviral drugs and vaccines.
Therefore, we review the current knowledge of COVID-19 including the global and western-Pacific regional epidemiology, virology, impact of diabetes on COVID-19, treatment of COVID-19, and standard of care in the management of diabetes during this critical period.
We searched all the articles in PubMed and Google Scholar databases from December 2019 to early May 2020 using keywords: “COVID-19”, “SARS-CoV-2”, “diabetes”, “epidemiology”, “pathophysiology”, “ACE2”, “manifestation”, and “treatment”.
2. Epidemiology
2.1. Global epidemiology
In December 2019, an outbreak of pneumonia cases with unknown cause emerged in Wuhan, Hubei, China. A novel coronavirus was isolated from the patients with pneumonia, and it was named 2019 novel coronavirus (2019-nCoV)[1]; it was relabeled as SARS-CoV-2 and the disease it causes was named coronavirus disease 2019 (COVID-19) in February 2020, by the World Health Organization (WHO)[8].
Due to sustained human-to-human transmission, the rapid spread of SARS-CoV-2 resulted in a formidable outbreak in many cities in China, expanding internationally to South Korea[9], Europe[10], and the United States[11]. The WHO declared the COVID-19 outbreak a pandemic on March 11, 2020[12]. The case fatality rate (CFR) of COVID-19 is lower than that of SARS-CoV1 and MERS-CoV, which were 9.4% and 34.4%, respectively[13]. However, due to the high transmission rate, the absolute number of fatality is strikingly high.
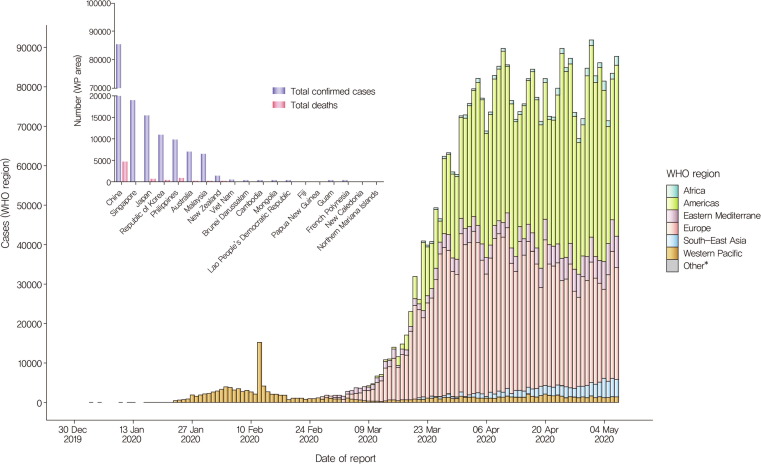
As of May 8, 2020, the WHO reported 3,759,967 confirmed cases including 259,474 deaths (CFR 6.90%). In the Western Pacific Region, 157,447 confirmed cases including 6394 deaths (CFR 4.06%) were reported (https://www.who.int/docs/default-source/coronaviruse/situation-reports)[14]. Recently, as the countries are moving to less restrictive social distancing measures after successful initial defense, the number of COVID-19 patients has been increasing in Japan, Hong Kong, and Singapore. Thus, careful prevention and treatment of COVID-19 is still needed in the western Pacific region (Fig. 1 ).
Fig 1.
Number of confirmed COVID-19 cases in the WHO region and Western pacific (WP) area, from 30 December 2019 through 8 May 2020. As of May 8, 2020, the WHO reported 3,759,967 confirmed cases including 259,474 deaths (CFR 6.90%) in the WHO area. Especially, in the western pacific area, 157,447 confirmed cases including 6394 death cases (CFR 4.06%) were reported[14]. In the WP area, the number of confirmed cases was highest is China, followed by Singapore, Japan, Republic of Korea, Philippines, and Australia. (https://www.who.int/docs/default-source/coronaviruse/situation-reports-109).
2.2. Epidemiology and lessons from the experience of South Korea
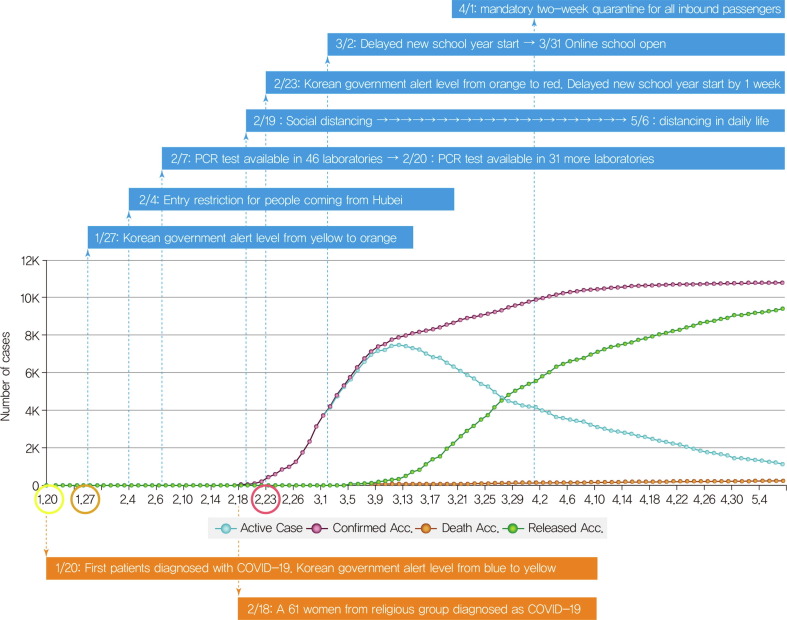
The first confirmed case of COVID-19 in the Republic of Korea (South Korea) was a traveler from Wuhan, China on January 19, 2020[15]. While the spread of COVID-19 was limited before February 20, a huge outbreak occurred in a religious group in the southern part of South Korea, Daegu, and the number of COVID-19 cases in Korea reached 6593 on March 6, 2020. At that time, South Korea had the second-highest number of confirmed cases after China[16]. In the first two months of the COVID-19 pandemic, South Korea was able to lower the incidence of new cases and sustain a low mortality rate by rapid activation of national response protocols led by national leadership. As of May 8, 2020, the Korean Center for Disease Control & Prevention (KCDC) reported 10,822 confirmed cases including 256 deaths (CFR 2.37%)[17] (Fig. 2 ).
Fig 2.
Accumulated number of confirmed cases, active cases, released cases, and deaths from COVID-19 and responses of Korean government from 20 January 2020 through 8 May 2020 Reports from Korean Center for disease Control & Prevention[17] and figure from Coronaboard.kr.
South Korea's strategies for favorable outcomes are summarized as five components[18], [19]. (1) Early recognition of the problem and transparent and prompt sharing of relevant information: The KCDC performed careful contact tracing and effective surveillance with rapid feedback. This early response is related to the unfortunate experience with the fatal MERS-CoV in South Korea in 2015. Because SARS-CoV-2 can be transmitted during the asymptomatic period[20], active surveillance with announcement of tracing of confirmed cases and the code of conduct for prevention of COVID-19 through media and mobile text messages has been executed. The Ministry of Education postponed school opening from March 2 to mid-May. These non-pharmaceutical interventions could reduce the possibility of massive transmission[21]. (2) Rapid establishment of diagnostic capacity and extensive testing facilities: The KCDC rapidly developed and established novel diagnostic tests using real-time polymerase chain reaction (PCR) technology through public-private partnership and expedited approval by the Korean Food and Drug Administration and deployment in whole country. Individuals who had respiratory symptoms, identified as having contact with either confirmed or suspected cases, had recently traveled to China, or highly affected local areas were tested by PCR. The Korean government lowered the threshold for COVID-19 screening test and heath care professionals were allowed to perform diagnostic testing on any individual suspected of having COVID-19 free of charge. In addition, fast screening systems such as drive-through and walk-through has been set up[22]. (3) Implementing innovative and aggressive measures for control and preventing community transmission: Government officers meticulously handled contact tracing, quarantining in smaller clusters, and isolation. The Korean government has utilized information and communication technology (ICT) and state-of-the-art devices including the Self-Diagnosis App, Self-Isolation Safety Protection App, and Epidemiological Investigation Support System designed to promptly track the routes of confirmed cases by GPS data, credit card transactions, and CCTV recordings. Instead of declaring an entry ban, the government instituted a mandatory two-week quarantine for all inbound travelers from April 1 and sustained the open border policy to prevent global and domestic economic downturn. (4) Redesigning the triage and treatment system: The South Korean government redesigned health services and divided them into two systems – COVID-19 system for COVID-19 patients and non-COVID-19 system (National Safe Hospital) for general patients. The COVID-19 health system included public quarantine, primary health care triage, admission for observation at primary-care accommodation support centers with health care professionals, and transfer to secondary or tertiary hospitals according to severity of illness. (5) Mobilizing the necessary resources for clinical care and high-caliber healthcare professionals: Nonclinical facilities such as training centers were transformed into clinical facilities staffed with health professionals for mild-to-moderate cases[23]. For severe cases, referral systems beyond the boundary of the city or province was set up with coordination at the national level. In addition, supply of protective equipment and therapeutic agents was managed to resolve frontline difficulties via the Health Insurance Review & Assessment Service. These strategies can be adopted by the global community coping with COVID-19.
3. The prevalence, severity, and mortality of diabetics with COVID-19
Even though there are differences in the pattern and intensity of transmission ranges between different countries, the similar finding was that the elderly; those with comorbidities including hypertension, diabetes, cardiovascular disease, chronic lung disease, immunocompromised state, severe obesity, chronic kidney disease treated with dialysis, and liver disease; and people in nursing homes or long-term care facilities were at a high risk of fatal outcomes from COVID-19[2]. Especially, people with diabetes are vulnerable to infection and adverse outcomes because of hyperglycemia, impaired immune function, vascular complications, and comorbidities such as hypertension, obesity, and cardiovascular disease.
The prevalence of diabetes among people with COVID-19 and severity of COVID-19 in patients with diabetes varies, depending on the location and age of the patients and severity of the pandemic. We summarized the prevalence, severity, and mortality of diabetes among patients infected with COVID-19 (Table 1 ). The overall proportion of diabetics infected with COVID-19 was reported from 5.3% to 33.9% in China, Korea, Italy, and USA[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47]. In China, the prevalence of diabetes in patients with COVID-19 is similar to the nationwide prevalence of diabetes mellitus in China (approximately 10.9%)[7]. Therefore, patients with diabetes do not seem to be more susceptible to COVID-19.
Table 1.
Prevalence of diabetes according to severity among COVID-19 infected patients.
| Author | Date | Area | DM Prevalence | DM Prevalence |
DM Prevalence |
Mortality |
|||
|---|---|---|---|---|---|---|---|---|---|
| DM/total COVID-19 patients | Non-severe cases | Severe cases | Survivors | Non-survivors | nonDM | DM | |||
| Chen N[24] | 2020.1.1–2020.1.20 | Wuhan, China | 12/99 (12%) |
||||||
| Liu K[25] | 2019.12.30–2020.1.24 | Wuhan, China |
14/137 (10.2%) |
||||||
| Song F[26] | 2020.1.20–2020.1.27 | Shanghai, China | 3/51 (6%) |
||||||
| Yang W[27] | 2020.1.17–2020.2–10 | Wenzhou, China | 9/149 (6%) |
||||||
| Chen Q[28] | 2020.1.1.-2020.3.11 | Zhejiang China |
14/145 (9.7%) |
7/102 (6.9%) |
7/43 (16.3%) |
||||
| Huang C[29] | 2019.12.16–2020.1.2 | Wuhan, China |
8/41 (20%) |
No ICU 7/28 (25%) |
ICU 1/13 (8%) |
||||
| Liu W[30] | (2020.1.15) | Wuhan China |
5/78 (6.4%) |
3/67 (4.5%) |
2/11 (18.2%) |
||||
| Wang D[31] | 2020.1.1–2020.1.28 | Wuhan China |
14/138 (10.1%) |
6/102 (5.9%) |
8/36 (22.2%) |
||||
| Xu X[32] | 2020.1.10–2020.1.26 | Hangzhou,China | 1/62 (2%) |
0/29 (0%) |
1/33 (3%) |
||||
| Zhang JJ[33] | 2020.1.16∼ 2020.2.3 |
Wuhan, china | 17/140 (12.1%) |
9/82 (11%) |
8/58 (13.8%) | ||||
| Guan WJ[34] | 2020.1.29 | 31 provinces, China | 81/1099 (7.4%) | 53/926 (5.7%) | 28/173 (16.2%) | 63/1032 (6.1%) | 18/67 (26.9%) | ||
| Wu C[35] | 2019.12.25–2020.1.26 | Wuhan China |
22/201 (10.9%) |
6/117 (5.1%) | 16/84 (19%) | 5/40 (12.5%) | 11/44 (25%) | ||
| Deng Y[36] | 2020.1.1–2020.1.21 | Wuhan, China | 26/225 (11.5%) |
9/116 (7.8%) | 17/109 (15.6%) | ||||
| Yang X[37] | 2019.12–2020.1.26 | Wuhan, china | 9/52 (17%) |
2/20 (10%) | 7/32 (22%) | ||||
| Zhou F[38] | 2019.12.29–2020.1.31 | Wuhan, china | 36/191 (19%) | 19/137 (14%) |
17/54 (31%) | ||||
| Wu Z[39] | CDC china | 1102/44672 (5.3%) |
133/15536 (0.9%) |
80/1102 (7.3%) |
|||||
| Guo W[40] | 2020.02.10.-2020.02.29 | Wuhan, China | 37/174 (21.2%) | 5/137 (3.6%) | 4/37 (10.8%) |
||||
| Yan Y[41] | 2020.1.10–2020.2.24 | Wuhan, China | 48/193 (24.9%) |
69/145 (47.6%) |
39/48 (81.3%) | ||||
| Zhu L[42] | 2019.12.30–2020.03.20 | Hubei, China |
952/7337 (13.0%) | 172/6385 (2.7%) |
74/952 (7.8%) |
||||
| KNCCMC[43] | 2020.1.19–2020.2.17 | Korea | 2/28 (7.1%) |
||||||
| KSI& Korea CDC[44] | 2020.03.10 | Korea | 16/54 (29.6%) |
||||||
| Korea CDC[45] | 2020.03.12 | Korea | 23/66 (36.5%) |
||||||
| Korea [46] |
2020. | Korea | 29/110 (26.4%) |
1/81 (1.2%) | 5/29 (17.2%) |
||||
| Gentile S[5] | 2020.03.27 | Italia | 86,433 (33.9%) |
||||||
| Richardson[6] | 2020.3.1–2020.4.4 | New York USA | 1808/5700 (33.8%) |
||||||
| USA CDC[45], [47] | 2020.2.12–2020.3.28 | USA | 784/7162 (10.9%) | Not hospital 331/5143 (6%) Non-ICU 251/1037 (24%) |
ICU 148/457 (32%) |
||||
CDC, Centers for Disease Control and Prevention; KNCCMC, Korea National Committee for Clinical Management of COVID-19.
The severity and mortality of patients with diabetes was higher than those of patients without diabetes infected COVID-19 (Table 1). A previous early large observational study found that the incidence of diabetes was higher in patients with severe illness (16.3%) than in patients with non-severe illness (6.9%)[28]. Other single-center observational studies showed similar results, i.e., higher (13.8–22.2%) proportion of diabetes in severe COVID-19 cases than in non-severe cases (4.5–11%)[29], [30], [31], [32], [33], [34], [35]. Several early single-center retrospective studies in Wuhan showed significantly higher prevalence (15.6–31%) of type 2 diabetes among non-survivors than among survivors (7.8–14%)[34], [35], [36], [37], [38]. The COVID-19 mortality in the presence of diabetes rose up to 7.3% (80/1102), which is significantly higher than the COVID-19 mortality without any comorbidities (0.9%, 133/15,536) and an overall fatality rate of 2.3% in the largest epidemiological investigation from the Chinese Center for Disease Control (CDC) [39]. This result was very similar to those of Guo et al. (10.8% vs. 3.6%)[40] and Yan et al. (81.3% vs. 47.6%)[41]. Recently, a large dataset of Chinese patients with COVID-19 showed that diabetes status increased the mortality risk of COVID-19 (7.8% vs. 2.7%)[42].
In South Korea, as of March 12, 2020, a total of 7755 laboratory-confirmed cases of COVID-19 and 66 deaths were recorded. Case fatality proportion was the highest among people aged ≥80 years. Of 63 cases, 96.8% were reported to have comorbidities: 47.6%, hypertension, 36.5%, diabetes, 16%, neurodegenerative disorders, and 17.5%, pulmonary diseases.[43], [44], [45]. A single-center study conducted in Korea reported that 28-day mortality was significantly higher in patients with diabetes than in those without diabetes (17.2% vs. 1.2%). Patients with diabetes showed a higher severity score and inflammation marker levels[46].
In USA, as of March 28, 2020, among a total of 122,653 laboratory-confirmed COVID-19 cases, diabetes mellitus (784, 10.9%) was the most frequently reported condition among 7162 cases whose data on underlying health conditions were available. The percentage of diabetics among cases that resulted in an ICU admission was higher (32%) than that among non-hospitalized cases (6%) or among cases of non-ICU admissions(24%) [47]. Several meta-analyses also found that the incidence of diabetes was two-fold higher in those who developed severe disease than in patients who experienced non-severe disease [7], [48], [49], [50], [51] (Supplementary table 1). These results suggest an association between diabetes and poor prognosis and increased mortality. Therefore, intensive attention should be paid to patients with diabetes.
The reason for the high morbidity and mortality in patients with diabetes should be investigated. Hyperglycemia, low immune function, vascular complications, and comorbidities are contributing factors to fatal outcomes in patients with diabetes. However, few studies have analyzed the effect of hyperglycemia on prognosis. A retrospective single-center study conducted in Korea reported that age was an independent risk factor for severe outcomes among patients with diabetes. Baseline HbA1c and anti-diabetic medication did not affect COVID-19 outcomes[46]. Because baseline HbA1c does not take into account fluctuations in blood glucose levels, glycemic variability is important for evaluating overall glycemic control. Zhu L et al. analyzed the effect of glycemic variability over a 28-day period from hospital admission on the severity and mortality of COVID-19 in a cohort with COVID-19 and type 2 diabetes[42]. Patients with a well-controlled glycemic state (glycemic variability within 3.9–10 mmol/L) had less severity and lower mortality than patients with poorly controlled hyperglycemia (glycemic variability more than 10 mmol/L) after adjusting for age, gender, COVID-19 severity, comorbidities, and side effect. This study suggested that a well-controlled glycemic status was associated with better outcomes in patients with COVID-19 and diabetes. Owing to the retrospective nature of the study, it was not possible to determine whether intensive management of hyperglycemia ameliorates COVID-19 severity or whether a less severe COVID-19 state leads to good glycemic variability. Therefore, further analysis of the influence of clinical parameters such as duration of diabetes, degree of hyperglycemia, medication, coexisting diabetic complications, or comorbidities on the outcomes of COVID-19 in an ethnically and geographically diverse population, as part of a prospective large cohort study is needed.
4. Pathogenesis
4.1. Characteristics of SARS-CoV-2
Coronaviruses (CoVs) are enveloped RNA viruses able to infect mammals including birds and humans. CoVs are divided into four genera: α−/β−/γ−/δ-CoV. Only α- and β-CoV are able to infect mammals. Humans are susceptible to six CoVs, among which HCoV-229E, HCoV-NL63, HCoV-HKU1, and HCoV-OC43 with low pathogenicity, cause mild respiratory symptoms in immunocompetent hosts[52]. The other two SARS-CoV [53], which caused a major outbreak in 2002–2003[54] and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) lead to fatal outbreak in 2012[55], 2015[56], and 2017[57]. The latest isolated SARS-CoV-2 is the seventh corona virus that causes severe and fatal disease in humans (Table 2 ).
Table 2.
Biological characteristics of seven species of human-susceptible coronaviruses.
| Name | Genus | Natural Host | Intermediate host | receptor | Patho genecity | Outbreak |
|---|---|---|---|---|---|---|
| HCoV-229E | alpha | bats | camelids | APN | Low | – |
| HCoV-NL63 | alpha | bats | ? | ACE2 | Low | – |
| HCoV-OC43 | beta | rodents | cows | 9-O-acetylated sialic acid | Low | – |
| HCoV-HKU1 | beta | rodents | ? | 9-O-acetylated sialic acid | Low | – |
| MERS-CoV | beta | bats | camels | DPP4 or CD26 | Severe, Mortality 35% |
2012, 2015, 2017 |
| SARS-CoV | beta | bats | civets | ACE2 | Severe Mortality 9.6% |
2002–2003 |
| SARS-CoV-2 | beta | bats | ? | ACE2 | Severe Mortality 3.4∼% |
2019∼ |
APN: Aminopeptidase N; ACE2: angiotensin-converting enzyme 2; MERS-CoV: Middle East respiratory syndrome-coronavirus; SARS-CoV: Severe acute respiratory syndrome-coronavirus; DDP-4: dipeptidyl peptidase-4.
The SARS-CoV-2 is a novel β-coronavirus, which is an enveloped, non-segmented positive-sense, single-strand RNA virus identified by complete genome sequences of samples from the bronchoalveolar lavage fluid of patients[1]. The SARS-CoV-2 shares 79% sequence identity with SARS-CoV[58], and 50% similarity with MERS-CoV[59]. It is a spherical particle of 100–160 nm in diameter, which contains a 27–32-kb ssRNA genome. The 5′ two-thirds of the genome encodes a polyprotein, pp1ab, which is further cleaved into 16 nonstructural proteins that are involved in genome transcription and replication. The 3′ terminus encodes structural proteins, including envelope glycoproteins spike (S), envelope (E), membrane (M), and nucleocapsid (N)[59]. The genome is packaged inside a capsid formed by nucleocapsid protein (N). Membrane protein (M) and envelope protein (E) are both involved in virion assembly, and spike protein (S) mediates entry into host cells[60]. The S protein is characterized by a receptor-binding domain (RBD) S1 subunit that facilitates binding to the host angiotensin-converting enzyme 2 (ACE2) receptor for both SARS-CoV and SARS-CoV-2 and an S2 subunit that is responsible for membrane fusion[3]. The RBD of MERS-CoV attaches to the host cells via dipeptidyl peptidase 4 (DPP4) rather than ACE2[61]. A recent study demonstrated that the RBD unique to SARS-CoV-2 enhanced ACE2 receptor-binding affinity as compared to SARS-CoV[62]. After binding of ACE2, host cell factors further mediate viral entry through two serine proteases, transmembrane protease serine 2 (TMPRSS2) and furin, which cleave the S protein for membrane fusion and assist in viral processing, respectively [56], [57], [58], [59], [60]. Upon membrane fusion, the viral RNA is translated, replicated, and amplified. Upon amplification of the viral RNA, viral structural and nonstructural proteins are generated, which interact with viral RNA on the membrane of the ER and Golgi apparatus and finally result in viral budding and exocytosis[63].
4.2. Angiotensin-converting enzyme 2 (ACE2) protein
The reason why the ACE2 molecule is the subject of much attention is because it is the receptor for SARS-CoV-2 viral entry as well as an important element in the cardiovascular system, use of ACEI or ARB medication for cardiovascular disease, and severe clinical course of patients with cardiovascular comorbidity and SARS-CoV-2 infection.
ACE2 is a single-pass transmembrane protein with protease activity and is found in the type II pneumocytes of the lower respiratory tract, myocardium, endothelium, gastrointestinal tract, pancreatic islets, bone marrow, kidney, and spleen[64]. This broad expression of ACE2 may explain the multi-organ injury observed with COVID-19.
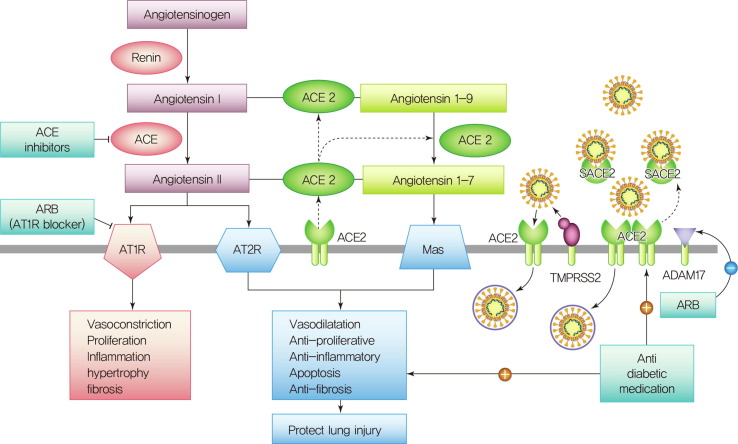
The role of ACE2 in the cardiovascular system is that ACE2 converts angiotensin I (Ang I) into angiotensin 1–9 (Ang 1–9) and also converts angiotensin II (Ang II) into angiotensin (Ang 1–7), which acts on the Mas receptor to modestly lower blood pressure through vasodilation and by promoting sodium and water excretion through the kidney and to attenuate inflammation through the production of nitric oxide[65]. ACE2 is a counter-regulatory enzyme to ACE1. ACE1 converts Ang I into Ang II, which acts at the angiotensin II type-1 receptor (AT1R) to increase blood pressure by inducing vasoconstriction, increasing reabsorption of sodium and water in the kidney, and increasing oxidative stress to promote inflammation and fibrosis[66] (Fig. 3 ). Thus, the balance between ACE–Ang II and ACE2–Ang-(1–7) determines whether acute lung injury will be aggravated or alleviated.
Fig 3.
The role of angiotensin-converting enzyme 2(ACE2) on the cardiovascular system and entry of SARS-CoV-2. ACE2 converts Ang I to Ang-(1–9) and Ang II (angiotensin II) to Ang-(1–7), which acts on the Mas receptor (MasR) to lower blood pressure through vasodilation but also to attenuate inflammation and fibrosis. ACE1 converts Ang I into Ang II, which acts at the angiotensin II type 1 receptor (AT1R) to increase blood pressure by inducing vasoconstriction, increasing kidney reabsorption of sodium and water, and increasing oxidative stress to promote inflammation and fibrosis. ACE2 also binds to and internalizes SARS-CoV-2 after priming by the serine protease TMPRSS2 (transmembrane protease, serine-2). Shedding of membrane-bound ACE2 by a disintegrin and metalloprotease 17 (ADAM17) results in the occurrence of soluble ACE2, which can no longer mediate SARS-Cov-2 entry and might even prevent such entry by keeping the virus in solution. AT1R (Ang II type 1 receptor) upregulates ADAM17, and AT1R blockers (ARBs) would prevent this. However, diminishing production of Ang II with an ACE inhibitor or blocking Ang II–AT1R actions with an ARB can enhance the ACE2-Ang-(1–7)-MasR pathway, which attenuates inflammation, fibrosis, and lung injury. Anti-diabetic medication increased the expression of ACE2 in animal study. However, anti-diabetic medications that enhance immune modulation and effectively control hyperglycemia may have a beneficial effect on the outcomes of patients with COVID-19.
There are controversies regarding whether renin-angiotensin system (RAS) inhibition is harmful or protective in COVID-19[67]. Even though ACE inhibitors and angiotensin-receptor blockers (ARB) do not directly inhibit ACE2[68], ACE inhibitors affect the expression of ACE2 in the heart and kidney, and ARB could increase ACE2 abundance and enhance viral entry. However, there are no data to support the notion that ACE inhibitors or AT1R blockers facilitate coronavirus entry by increasing ACE2 expression. Regarding the protective view, diminishing production of Ang II with an ACE inhibitor or blocking Ang II–AT1R actions with an ARB enhances the generation of Ang-(1–7) by ACE2 and activation of the Mas receptor (MasR), which attenuates inflammation, fibrosis, and lung injury. Interestingly, the administration of losartan, an AT1R blocker alleviated the exacerbating effects of SARS-CoV spike protein in an animal model of acute respiratory distress syndrome (ARDS)[69]. Losartan also ameliorated influenza virus-induced acute lung injury in mice[70]. Fortunately, a retrospective single-center study conducted in Korea showed that ARB or ACEI medication was associated with a protective effect against cardiac injury, and it was an independent factor of favorable outcomes in patients with COVID-19[46]. Angiotensin inhibitors improve blood pressure and might help restore pulmonary function through the MAS receptor pathway.
Soluble ACE2 is released into the blood through cleavage by the membrane-bound protease, A disintegrin and metalloprotease 17 (ADAM17)[71]. The effects of soluble ACE2 are unclear in humans. However, it appears to have favorable effects on lung function. Soluble ACE2 has been studied in a phase II trial of ARDS. In addition, AT1R upregulates ADAM17, thus increasing soluble ACE2 levels. In fact, soluble ACE2 increased in the cerebrospinal fluid level of hypertensive patients[72]. However, the majority of ACE2 is membrane-bound, even a doubling is unlikely to significantly influence the amount of membrane-bound ACE2. The severity of COVID-19 among patients taking ACEi or ARB is confounded by cardiovascular comorbidities, which may alter ACE2 and angiotensin II expression[73]. At this time, it is not clear whether ACEi or ARB medication influences ACE2 expression and severity of COVID-19. Therefore, it is strongly recommend that patients who are taking ACE inhibitors or ARBs for high blood pressure, heart failure, or other medical indications should not withdraw their current treatment regimens unless they are specifically advised to do so by their physician or healthcare provider[74].
4.3. Pathophysiology
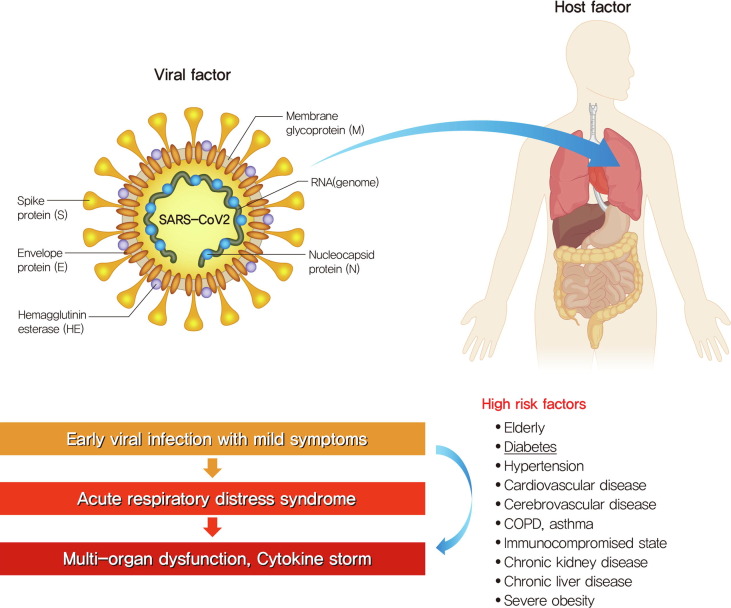
The clinical course of COVID-19 is theoretically divided into three stages[75]. Stage I involves early viral infection with constitutional symptoms. Stage II involves ARDS and hypoxia, which result from direct viral cytotoxicity of the pulmonary system due to inflammatory activation. If the host cannot remove the virus via a protective immune response, COVID-19 progresses to stage III which is a severe status with multi-organ dysfunction and cytokine storm with immune dysregulation (Fig. 4 ). Accumulating evidence suggests that a subgroup of patients with severe COVID-19 might have a cytokine storm syndrome and strong inflammatory response could be linked with uncontrolled pulmonary inflammation and consequent COVID-19 lethality[76]. ICU patients in the severe stage of COVID-19 had high plasma levels of interleukin (IL)-2, IL-7, granulocyte colony-stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor-α than non-ICU patients, suggesting the presence of a hyper-inflammatory condition also known as a cytokine storm[77]. In particular, diabetes was identified as a risk factor for poor prognosis of COVID-19 based on the higher level of inflammation-related biomarkers such as IL-6, C-reactive protein, serum ferritin, coagulation index, and D-dimer[40], [42], [46]. Thus, the identification and treatment of hyperinflammation is recommended in order to reduce mortality.
Fig 4.
High-risk factors for fatal outcomes from COVID-19 and severe clinical course. The elderly, those with comorbidities including hypertension, diabetes, cardiovascular disease, chronic obstructive pulmonary disease (COPD), asthma, severe obesity, chronic kidney disease treated with dialysis, and liver disease, and people in nursing homes or long-term care facilities were at a high risk of a fatal outcome from COVID-19. Clinical course of COVID-19 is divided into three stages. Stage I is early viral infection with constitutional symptoms. Stage II is acute respiratory distress syndrome (ARDS) and hypoxia. Stage III is severe status with multi-organ dysfunction and cytokine storm with immune dysregulating.
4.4. Mode of transmission
SARS-CoV2 spreads primarily through small respiratory droplets that are expelled from infected individuals and can reach approximately 1–2 m. The virus can exist in nature on surfaces and can last for up to 4 h on copper, 24 h on cardboard, and up to 72 h on plastic and stainless steel surfaces leading to fomite transmission[78]. In fact, the Japanese National Institute of Infectious Disease detected SARS-CoV2 RNA on surfaces in cabins of symptomatic and asymptomatic passengers on the Diamond Princess Cruise ships up to 17 days after they were vacated and before disinfection procedure[79]. Live virus has also been isolated and cultured from fecal specimens raising the possibility of oro-fecal transmission, but corroborating clinical evidence of this transmission is lacking[80]. Therefore, it is recommended that precautions to reduce airborne transmission, with the use of N-95 masks, should be implemented in these aerosol-producing settings[81].
Human-to-human transmission is now well established for COVID-19 with an R0 (the expected number of secondary cases produced by a single [typical] infection in a completely susceptible population) of 1.4–2.5, as estimated by the WHO[82]. For comparison, seasonal flu has a median R0 of 1.28 (IQR 1.19–1.37)[83], while measles has an R0 of 12–18[84]. However, the COVID-19 R0 was calculated from incomplete data and may change as further information becomes available.
5. Clinical manifestations
The clinical spectrum of SARS-CoV-2 infection appears to be wide, encompassing asymptomatic infection, mild upper respiratory tract illness, and severe viral pneumonia with respiratory failure and even death.
The prevalence of asymptomatic patients from exposure to admission was as much as one-fifth among participants from a community facility designated for isolation of patients without moderate-to-severe symptoms in South Korea[85]. Most cases are reported to experience a mild course[24]. The most common symptoms of mild COVID-19 patients were cough, hyposmia, and sputum, and not fever. However, the most common symptoms of hospitalized patients were fever, cough, fatigue, dyspnea or chest tightness, sore throat, and headache [4], [9]. In addition, some patients manifested gastrointestinal symptoms, with diarrhea and vomiting. The average length of hospital stay was 12.7 days (range: 8–19 days)[9]. Based on the current information, the elderly and those with chronic underlying diseases were in critical condition and their hospital stay was long.
Chest computed tomography showed ground-glass opacities and patchy bilateral shadowing [4], [9]. However, clinicians should be aware that some patients with COVID-19 also had normal computed tomography findings.
Complications of COVID-19 are ARDS, arrhythmia, shock, acute kidney injury, acute cardiac injury, liver dysfunction, secondary infection, and multiple organ dysfunction. The disease can quickly progress to a severe condition in the elderly or people with comorbidities such as diabetes, hypertension, cardiovascular disease, cerebrovascular disease, chronic obstructive pulmonary disease, asthma, immunocompromised state, chronic kidney disease, chronic liver disease, and severe obesity (Fig. 4).
6. Diagnosis
Samples from nasal and throat swab or other respiratory tract are used to test for the virus. Real-time reverse transcription-polymerase chain reaction (RT-PCR) is the most reliable clinical diagnostic method for COVID-19 around the world[86]. If the test result is positive, the diagnosis of SARSCoV-2 is confirmed. The WHO currently recommends that negative results in the presence of clinical symptoms or exposure to infected patients must be treated with suspicion; the test can be repeated using additional samples from the lower respiratory tract[87]. Also, it would be interesting to examine the cases of positive converters after initial successful negative conversion in some Asian countries[88]. This may be caused by incomplete viral clearance, inappropriate immune response development, incorrect sampling, false-negative and/or false-positive RT-PCR results, or re-infection. Therefore, it needs to be clarified whether they were cases of infective status, reactivation, re-infection, or just a marker of past infection.
7. Conservative management of COVID-19 patients with diabetes
At present, there is no specific vaccine or efficient antiviral therapy against COVID-19. Therefore, current treatments mainly consist of conservative management including symptomatic respiratory support. Nearly all patients accepted oxygen therapy, and the WHO recommends extracorporeal membrane oxygenation (ECMO) to patients with refractory hypoxemia[89]. Because the elderly and those with underlying disorders (i.e., hypertension, diabetes, chronic obstructive pulmonary disease, cardiovascular disease) progressed rapidly into ARDS, identification of high-risk factors and management of comorbidity are very important.
7.1. Optimal glucose control for the COVID-19 patients with diabetes
Infection with SARS-CoV-2 might trigger stress conditions and increased secretion of hyperglycemic hormones, such as glucocorticoid and catecholamines, which result in elevated blood glucose, abnormal glucose variability, and diabetic complications. Recently, acute hyperglycemic crisis such as diabetic ketoacidosis (DKA) or hyperosmolar hyperglycemic state (HHS) were reported in patients with diabetes[90], [91]. As ACE2 is expressed in the human endocrine pancreas, SARS-CoV-2 might enter the islets cells and cause acute beta-cell dysfunction[64]. Acute hyperglycemic crises can be precipitated by COVID-19 and result in catastrophic outcomes in patients with diabetes and poor glycemic control. Therefore, timely adequate strategies for glucose control should be emphasized in patients with diabetes during the COVID-19 pandemic. Active cooperation of diabetologists and protocols for glucose control are needed. Even after discharge, adequate blood glucose level should be maintained continuously and patients need to be cautious of infectious diseases due to their low immune response. Long-term follow-up is very important for patients with diabetes to reduce diabetes-related complications and mortality.
7.2. Anti-diabetic medication for COVID-19 patients with diabetes
Many antidiabetic drugs have been studied to evaluate the ACE2 activity or expression in kidney, cardiac, vascular, and pulmonary tissues. The increase in ACE2 activity due to these medications has been proposed as a plausible mechanism of the protective effect on tissue injury. However, ACE2 is a receptor for SARS-CoV-2. Taken together, these findings may raise the question on a role for anti-diabetic medication in COVID-19 immunopathogenesis. Pal and Bhadada[92] reviewed the potential effect of anti-diabetic drugs in novel COVID-19. Metformin[93], pioglitazone[94], liraglutide[95], sodium-glucose cotransporter-2 inhibitor (SGLT2) inhibitor[96], and insulin[97] increased ACE2 expression in animal study. However, metformin showed immune-modulating activity[98]. Pioglitazone reduced the lung fibrotic reaction to silica in rats, which is normally characterized by overproduction of TNFa[99]. DPP4 inhibitor demonstrated a possible protective role of soluble DPP4 in MERS. Since there is a lack of evidence of SARS-CoV2 binding to DPP4, DPP4 inhibitor does not represent a plausible approach to mitigate COVID-19. DPP4 inhibitors and GLP-1R agonists may exert anti-inflammatory actions in human subjects and have been successfully used to control glucose in hospitalized patients[100]. Dapagliflozin, an SGLT2 inhibitor, has been reported to reduce lactate levels by various mechanisms such as activation of lactate/Hþ symporter and direct inhibition of natrium/hydrogen exchanger (NHE)[101]. It was suggested that dapagliflozin can prevent the severe course of COVID-19 infection by lowering cytosolic pH and reducing the viral load. Although anti-diabetic medication has been found to have an effect on immune-modulation, there was no strong evidence on the effect of anti-diabetic medication on the outcome of COVID-19 patients. Good glycemic control is important to improve the outcomes of patients with COVID-19. Therefore, anti-diabetic medications that effectively control the blood glucose and have immune-modulating properties may have a beneficial effect on COVID-19 patients' outcome.
However, adverse reactions and contraindications of anti-diabetic medications should be considered when hyperglycemia is treated in critically ill patients with diabetes. Metformin is contraindicated in people with concomitant sepsis or severe impairment of hepatic and renal function. SGLT2 inhibitors should be discontinued in unstable patients with severe SARS-CoV-2 infection who are dehydrated and have a risk of ketoacidosis. Exenatide-based GLP-1 receptor agonists should be stopped in patients with impaired renal function. TZD is contraindicated in people with severe heart failure. Insulin is adequate for control glucose in severe ill patients, but the risk of hypoglycemia should be monitored.
7.3. Anti-hypertensive medication
Inappropriate discontinuation of an ARB or an ACE inhibitor should be avoided according to the recommendation of American College of Cardiology[75] and The European Society of Cardiology[102]. Calcium-channel blockers (CCBs) have no effect on ACE2 expression. Since CCB has been shown to reduce severity and mortality in patients with pneumonia, its use has been proposed for patients with COVID19 and hypertension[103].
8. Antiviral treatments
Because SARS-CoV-2 has rapidly spread globally, there are no approved and evidence-based treatments for COVID-19. However, there are several potential treatments for COVID-19 that are undergoing clinical trials. (1) Convalescent patients’ plasma and immunoglobulin G showed favorable outcome in COVID-19 patients with ARDS[104] through inactivation of virus particles. (2) Recombinant soluble ACE2 protein showed anti-inflammatory effects that prevent pulmonary damage in non-COVID-19-related ARDS in pilot human clinical trials[105]. (3) (Hydroxy-)chloroquine, (potentially in combination with azithromycin) accelerated viral clearance in small French pilot study[106]. (4) Nucleoside analogues (remdesivir, favipiravir, geldesivir, and ribavirin etc.) can limit virus replication. Remdesivir was originally developed for the treatment of Ebola virus. Remdesivir successfully treated the first US case of COVID-19 in January 2020[107]. (5) Protease inhibitors (lopinavir, ritonavir, etc.) have been used to treat infection with human immunodeficiency virus (HIV)[108] and could improve the outcomes of MERS-CoV[109] and SARS-CoV[110] patients. SARS-CoV-2 viral loads of patients in South Korea significantly decreased after lopinavir/ritonavir treatment[111]. (6) Type 1 interferons induce anti-viral cellular programs through immune modulation[112].(7) The recombinant IL-6 receptor antagonist tocilizumab has been used successfully in patients with COVID-19[113]. The recombinant IL-1 receptor antagonist anakinra is being trialed in children and adults with COVID-19 associated cytokine storm syndrome in China (NCT02780583)[114].(8) Inhibitors of janus kinases (JAK) modulate cytokine receptor signaling, including the IL-6 receptor as well as type 1 and type 2 IFN receptors[115]. Clinical trials on severe COVID-19 are ongoing (ChiCTR2000030170, ChiCTR2000029580)[116]. (9) Corticosteroids can control inflammation in ARDS. However, preliminary data on COVID-19 showed that high-dose steroids did not have beneficial effects on lung injury, but were associated with complications. Thus, high-dose corticosteroids cannot be generally recommended for the treatment of COVID-19[117].
9. Management of glycemic control during the pandemic period
Many countries including China, South Korea[118], Australia[119], England[120], and the USA[121] have announced guidelines for managing diabetes in response to COVID-19. Many countries now use telemedicine programs that allow clinicians to see patients at their home. Telemedicine has been very helpful and useful the during the COVID-19 pandemic[122]. With the aim of preventing person-to-person transmission, a variety of online services of glucose management have been implemented widely for diabetic patients and the general population during COVID-19 pandemic around the world. The popularization of internet and smartphones and emerging fifth-generation networks have enabled endocrinologists to provide remote medical consultation for patients who are not advised to go to the hospital during the COVID-19 outbreak. Furthermore, free educational videos and e-books on diabetes self-management and COVID-19 prevention have been provided to the public via mobile applications[123]. Wearable health systems to monitor blood glucose, blood pressure, physical activity, caloric intake, eating behavior, sleep pattern, and adherence to medication will be helpful for comprehensive management for patients with diabetes.
10. Conclusion
SARS-CoV-2 infection has rapidly spread across the world, resulting in a socioeconomic crisis as well as health crisis. Since diabetes is one of the high-risk factors of COVID-19 with high morbidity and mortality, diabetics require attention and timely adequate care. We reviewed the current status of the COVID-19, impact of diabetes on COVID-19, virology, and management of patients with diabetes during this critical period. Sharing our experience, expanding our knowledge about COVID-19, developing drugs for SARS-CoV-2, and innovative technology to monitor and care for the patients are needed to defeat the COVID-19 pandemic.
Acknowledgments
Acknowledgement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2020.108303.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention People who need extra precautions/people who are at higher risk. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html.
- 3.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The novel coronavirus pneumonia emergency response epidemiology team. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) China, 2020. China CDC Weekly. 2020;2:113-22. Available from http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51. [PMC free article] [PubMed]
- 5.Gentile S., Strollo F., Ceriello A. COVID-19 infection in Italian people with diabetes: lessons learned for our future (an experience to be used) Diabetes Res Clin Pract. 2020 Apr;162:108137. doi: 10.1016/j.diabres.2020.108137. Epub 2020 Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020; 22:e206775. doi: 10.1001/jama.2020.6775 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 7.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Coronavirus Disease 2019 (COVID-19) Situational Report - 22; [cited 2020 Feb 11] Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=fb6d49b1_2.
- 9.COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention. Early Epidemiological and Clinical Characteristics of 28 Cases of Coronavirus Disease in South Korea. Osong Public Health Res Perspect. 2020;11(1):8–14. doi: 10.24171. [DOI] [PMC free article] [PubMed]
- 10.Pullano G., Pinotti F., Valdano E., Boëlle P.Y., Poletto C., Colizza V. Novel coronavirus (2019-nCoV) early-stage importation risk to Europe, January 2020. Euro Surveill. 2020;25:2000057. doi: 10.2807/1560-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. Novel Coronavirus in the United States. N Engl J Med. 2019;2020(382):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization [Internet] Coronavirus disease 2019 (COVID-19) Situation Report – 52 [cited 2020 Mar 11]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 13.Ruan S. Likelihood of survival of coronavirus disease 2019. Lancet Infect Dis 2020. Mar 30. pii: S1473-3099(20)30257-7. doi: 10.1016/S1473-3099(20)30257-7. [DOI] [PMC free article] [PubMed]
- 14.World Health Organization [Internet] Coronavirus disease 2019 (COVID-19) Situation Report – 109. [cited 2020 May 8] Available from https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200508covid-19-sitrep-109.pdf?sfvrsn=68f2c632_6.
- 15.Kim J.Y., Choe P.G., Oh Y., Oh K.J., Kim J., Park S.J., et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases; Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention. Report on the Epidemiological Features of Coronavirus Disease 2019 (COVID-19) Outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35:e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed]
- 17.Korean Centers for Disease Control and Prevention situation reports: http://ncov.mohw.go.kr/en/bdBoardList.do?brdId=16&brdGubun=161&dataGubun=&ncvContSeq=&contSeq=&board_id=.
- 18.Oh J., Lee J.K., Schwarz D., Ratcliffe H.L., Markuns J.F., Hirschhorn L.R. National Response to COVID-19 in the Republic of Korea and Lessons Learned for Other Countries. Health Syst Reform. 2020;6 doi: 10.1080/23288604.2020.1753464. [DOI] [PubMed] [Google Scholar]
- 19.Korean Centers for Disease Control and Prevention : Publications and Briefing: Korea’s Response to COVID-19 and Future Direction: http://ncov.mohw.go.kr/en/infoBoardView.do?brdId=15&brdGubun=151&dataGubun=&ncvContSeq=2187&contSeq=2187&board_id=&gubun=#.
- 20.Song JY, Yun JG, Noh JY, Cheong HJ, Kim WJ. Covid-19 in South Korea -Challenges of Subclinical Manifestations. N Engl J Med. 2020 Apr 6. doi:10.1056/NEJMc2001801. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 21.Kim S., Kim Y.J., Peck K.R., Jung E. School Opening Delay Effect on Transmission Dynamics of Coronavirus Disease 2019 in Korea: Based on Mathematical Modeling and Simulation Study. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kym S. Fast Screening Systems for COVID-19. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park P.G., Kim C.H., Heo Y., Kim T.S., Park C.W., Kim C.H. Out-of-Hospital Cohort Treatment of Coronavirus Disease 2019 Patients with Mild Symptoms in Korea: an Experience from a Single Community Treatment Center. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020 Feb 7 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song F., Shi N., Shan F., Zhang Z., Shen J., Lu H., et al. Emerging 2019 novel coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A., et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang. China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Q, Zheng Z, Zhang C, Zhang X, Wu H, Wang J,et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020 Apr 28. doi:10.1007/s15010-020-01432-5. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 29.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W, Tao ZW, Lei W, Ming-Li Y, Kui L, Ling Z, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl).2020 Feb 28. doi: 10.1097/CM9.0000000000000775. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 31.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368 doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ,et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 Feb 19. doi:10.1111/all.14238 [Epub ahead of print]. [DOI] [PubMed]
- 34.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Medical treatment expert group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. 2020 Mar 13 [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng Y., Liu W., Liu K., Fang Y.Y., Shang J., Zhou L., et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000824. 2020 Mar 20 [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-62.https://doi.org/10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed]
- 39.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648. [DOI] [PubMed]
- 40.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., et al. Clinical characteristics and outcomes of severe COVID-19 patients with diabetes. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu L., She Z., Cheng X., Guo J., Zhang B., Li H., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1–10. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim E.S., Chin B.S., Kang C.K., Kim N.J., Kang Y.M., Choi J.P., et al. Korea National Committee for Clinical Management of COVID-19. Clinical Course and Outcomes of Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection: a Preliminary Report of the First 28 Patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention. Analysis on 54 Mortality Cases of Coronavirus Disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. 2020;35(12):e132. doi: 10.3346/jkms.2020.35.e132. [DOI] [PMC free article] [PubMed]
- 45.COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention. Coronavirus disease-19: the first 7,755 cases in the Republic of Korea. Osong Public Health Res Perspect 2020;11:85-90. [DOI] [PMC free article] [PubMed]
- 46.Chung SM, Lee YY, Ha E, Yoon JS, Won KC, Lee HW et al. The risk of diabetes on clinical outcomes in patients with COVID-19: a retrospective cohort study. Diab Meta J 2020;44:e27 doi:10.4093/dmj.2020.01050 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 47.Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019: United States, February 12-March 28, 2020. CDC COVID-19 Response Team. MMWR Morb Mortal Wkly Rep 2020;69:382-6. [DOI] [PMC free article] [PubMed]
- 48.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020 Mar 11:1-8.doi:10.1007/s00392-020-01626-9 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 49.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12:6049–6057. doi: 10.18632/aging.103000. Epub 2020 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu Y., Sun J., Dai Z., Deng H., Li X., Huang Q., et al. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su S., Wong G., Shi W., Liu J., Lai A.C.K., Zhou J., et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 54.Stockman L.J., Bellamy R., Garner P. SARS: Systematic review of treatment effects. PLoS Med. 2006;3:1525–1531. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 56.Kang C.K., Song K.H., Choe P.G., Park W.B., Bang J.H., Kim E.S., et al. Clinical and epidemiologic characteristics of spreaders of Middle East respiratory syndrome coronavirus during the 2015 Outbreak in Korea. J Korean Med Sci. 2017;32:744–749. doi: 10.3346/jkms.2017.32.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.World Health Organization. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Fact Sheet. [Accessed 28 Aug 2017.] Available from URL: http://www.who.int/mediacentre/factsheets/mers-cov/en/.
- 58.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyerholz D.K., Lambertz A.M., McCray P.J. Dipeptidyl peptidase 4 distribution in the human respiratory tract: implications for the middle east respiratory syndrome. Am J Pathol. 2016;186:78–86. doi: 10.1016/j.ajpath.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.South A.M., Shaltout H.A., Washburn L.K., Hendricks A.S., Diz D.I., Chappell M.C. Fetal programming and the angiotensin-(1–7) axis: a review of the experimental and clinical data. Clin Sci. 2019;133:55–74. doi: 10.1042/CS20171550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sparks M.A., Crowley S.D., Gurley S.B., Mirotsou M., Coffman T.M. Classical renin- angiotensin system in kidney physiology. Compr Physiol. 2014;4:1201–1208. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.South A.M., Tomlinson L., Edmonston D., Hiremath S., Sparks M.A. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020 Apr;3:1–3. doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tikellis C., Thomas M.C. Angiotensin-Converting Enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int JPept. 2012;2012 doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou Z., Yan Y., Shu Y., Gao R., Sun Y., Li X., et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5:3594. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AIet al. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 2005;280:30113-30119. [DOI] [PMC free article] [PubMed]
- 72.Xu J, Sriramula S, Xia H, Moreno-Walton L, Culicchia F, Domenig O,et al. Clinical Relevance and Role of Neuronal AT(1) Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ Res 2017;121:43-55 [DOI] [PMC free article] [PubMed]
- 73.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med 2020;382:1653-1659. [DOI] [PMC free article] [PubMed]
- 74.Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382-1385 [DOI] [PMC free article] [PubMed]
- 75.Atri D, Siddiqi HK, Lang J, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the Cardiologist: A Current Review of the Virology, Clinical Epidemiology, Cardiac and Other Clinical Manifestations and Potential Therapeutic Strategies. JACC Basic Transl Sci. 2020 Apr 10. doi: 10.1016/j. jacbts.2020.04.002. [DOI] [PMC free article] [PubMed]
- 76.Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020 Apr 28:1-12. doi:10.1038/s41577-020-0311-8 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 77.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID- 19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. NEngl JMed. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moriarty L.F., Plucinski M.M., Marston B.J., Kurbatova E.V., Knust B., Murray E.L., et al. Public health responses to COVID-19 outbreaks on cruise ships - worldwide, February-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:347–352. doi: 10.15585/mmwr.mm6912e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alhazzani W., Moller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E., et al. Surviving sepsis campaign: guidelines on the management of critically Ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T. Importation and human-tohuman transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Biggerstaff M., Cauchemez S., Reed C., Gambhir M., Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis. 2014;14:480. doi: 10.1186/1471-2334-14-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guerra F.M., Bolotin S., Lim G., Heffernan J., Deeks S.L., Li Y., et al. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis. 2017;17:e420–e428. doi: 10.1016/S1473-3099(17)30307-9. [DOI] [PubMed] [Google Scholar]
- 85.Kim G.U., Kim M.J., Ra S.H., Lee J., Bae S., Jung J., et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 2020;S1198–743X(20):30268–30278. doi: 10.1016/j.cmi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hong K.H., Lee S.W., Kim T.S., Huh H.J., Lee J., Kim S.Y., et al. Guidelines for Laboratory Diagnosis of Coronavirus Disease 2019 (COVID-19) in Korea. Ann Lab Med. 2020 Sep;40:351–360. doi: 10.3343/alm.2020.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.WHO Coronavirus disease (COVID-19) technical guidance: Laboratory testing for 2019-nCoV in humans. Available from https://www.who.int/emergencies/diseases/novelcoronavirus-2019/technical guidance/laboratory-guidance [accessed 02 Mar. 2020].
- 88.Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502-1503. doi.10.1001/ jama.2020.2783. [DOI] [PMC free article] [PubMed]
- 89.WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publicationsdetail/ clinical-management-of-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected [accessed 28 Jan 2020].
- 90.Kim N.Y., Ha E., Moon J.S., Lee Y.H., Choi E.Y. Acute Hyperglycemic Crises with Coronavirus Disease-19: Case Reports. Diabetes Metab J. 2020 Apr;44(2):349–353. doi: 10.4093/dmj.2020.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020 Apr 20 doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pal R., Bhadada S.K. Should anti-diabetic medications be reconsidered amid COVID-19 pandemic? Diabetes ResClin Pract. 2020;163 doi: 10.1016/j.diabres.2020.108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang J., Dong J., Martin M., He M., Gongol B., Marin T.L., et al. AMP-activated protein kinase phosphorylation of angiotensin-converting enzyme 2 in endothelium mitigates pulmonary hypertension. Am J Respir Crit Care Med. 2018;198:509–520. doi: 10.1164/rccm.201712-2570OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tripathy D., Daniele G., Fiorentino T.V., Perez-Cadena Z., Chavez-Velasquez A., Kamath S., et al. Pioglitazone improves glucose metabolism and modulates skeletal muscle TIMP-3–TACE dyad in type 2 diabetes mellitus: a randomised, doubleblind, placebo-controlled, mechanistic study. Diabetologia. 2013;56:2153–2163. doi: 10.1007/s00125-013-2976-z. [DOI] [PubMed] [Google Scholar]
- 95.Romaní-Pérez M, Outeiriñ o-Iglesias V, Moya CM, Santisteban P, Gonzá lez-Matías LC, Vigo E, et al. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology 2015;156:3559–3569. [DOI] [PubMed]
- 96.Kawanami D., Matoba K., Takeda Y., Nagai Y., Akamine T., Yokota T., et al. SGLT2 inhibitors as a therapeutic option for diabetic nephropathy. Int J Mol Sci. 2017;18:1083. doi: 10.3390/ijms18051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salem E.S.B., Grobe N., Elased K.M. Insulin treatment attenuates renal ADAM17 and ACE2 shedding in diabetic Akita mice. Am J Physiol-Ren Physiol. 2014;306:F629–F639. doi: 10.1152/ajprenal.00516.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ursini F., Russo E., Pellino G., D'Angelo S., Chiaravalloti A., De Sarro G., et al. Metformin and Autoimmunity: A “New Deal” of an Old Drug. Front Immunol. 2018;9:1236. doi: 10.3389/fimmu.2018.01236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barbarin V., Nihoul A., Misson P., Mohammed A., Delos M., Leclercq I., et al. The role of pro- and anti-inflammatory responses in silica-induced lung fibrosis. Respir Res. 2005;6:112. doi: 10.1186/1465-9921-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Drucker DJ. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev. 2020 Apr 15. pii: bnaa011. doi:10.1210/endrev/bnaa011. [Epub ahead of print] PubMed PMID: 32294179. [DOI] [PMC free article] [PubMed]
- 101.Cure E, Cumhur Cure M. Can dapagliflozin have a protective effect against COVID-19 infection? A hypothesis. Diabetes Metab Syndr. 2020 Apr 21;14(4):405-406. doi: 10.1016/j.dsx.2020.04.024. [Epub ahead of print] PubMed PMID: 32335366. [DOI] [PMC free article] [PubMed]
- 102.European Society of Cardiology. ESC guidance for the Diagnosis and Management of CV disease during COVID-19 pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance.
- 103.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 Apr;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. Epub 2020 Mar 11. [DOI] [PMC free article] [PubMed]
- 104.Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35(14) doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID- 19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cvetkovic R.S., Goa K.L. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63(8):769–802. doi: 10.2165/00003495-200363080-00004. [DOI] [PubMed] [Google Scholar]
- 109.Arabi Y.M., Asiri A.Y., Assiri A.M., Aziz Jokhdar H.A., Alothman A., Balkhy H.H., et al. Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21(1):8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35(6) doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arabi Y.M., Shalhoub S., Mandourah Y., Al-Hameed F., Al-Omari A., Al Qasim E., et al. Ribavirin and interferon therapy for critically ill patients with middle east respiratory syndrome: a multicenter observational study. Clin Infect Dis. 2020;70:1837–1844. doi: 10.1093/cid/ciz544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z., et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4:1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Monteagudo L.A., Boothby A., Gertner E. Continuous intravenous anakinra infusion to calm the cytokine storm in macrophage activation syndrome. ACR Open Rheumatol. 2020 Apr 8 doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seo S.U., Kweon M.N. Virome-host interactions in intestinal health and disease. Curr Opin Virol. 2019;37:63–71. doi: 10.1016/j.coviro.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 116.Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: Immunology and treatment options. Clin Immunol. 2020 Apr;27 doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Won K.C., Yoon K.H. The outbreak of COVID-19 and diabetes in Korea: we will find a way as we have always done. Diabetes Metab J. 2020 Apr;44(2):211–212. doi: 10.4093/dmj.2020.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Austalian Diabetes Society. Updates on Diabetes & COVID-19: COVID-19 resources for diabetes health professionals. https://diabetessociety.com.au/covid-19-updates.asphttps://diabetessociety.com.au/covid-19-updates.asp.
- 120.Rayman G, Lumb A, Kennon B, Cottrell C, Nagi D, Page E, et al; London Inpatient Diabetes Network-COVID-19. Guidelines for the management of diabetes services and patients during the COVID-19 pandemic. Diabet Med 2020 May 4. doi: 10.1111/dme.14316. [DOI] [PubMed]
- 121.American Diabetes Association Diabetes & COVID-19. https://www.diabetes.org/diabetes/treatment-care/planning-sick-days/coronavirus [accessed on March 19, 2020].
- 122.Hollander J.E., Carr B.G. Virtually Perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 123.Wang A., Zhao W., Xu Z., Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.