The coronavirus disease 2019 pandemic: A role for novel antibody immunotherapy
The rapid emergence and spread of sudden acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), has resulted in a staggering pandemic. Infection with SARS-CoV-2 results in an array of symptoms that range from minor and self-limiting (80% of cases) to severe (15%-20%), with an unacceptably high fatality rate (0.5%-4%).1 Severe disease often leads to an overproduction of proinflammatory mediators that exacerbate disease, impacting progression and clinical outcome (reviewed in Cao2). In addition to the urgent development of vaccines and antiviral drugs, alternative therapeutics that facilitate viral control and limit immunopathology are of interest. mAbs are important biologics that bypass the adaptive immune system and are being deployed for the treatment of various infectious diseases, inflammation-driven autoimmune disorders, and cancers. Limited studies suggest that the passive administration of convalescent SARS-CoV-2 sera may be associated with alleviation of severe disease in some patients, supporting the pursuit of antibody-based approaches for SARS-CoV-2.
Efforts are currently focused on 2 major categories of mAb products—antiviral and anti-inflammatory—to address the major drivers of SARS-CoV-2-related disease (Fig 1 ). However, achieving the rapid production, scalability, and distribution sufficient to combat a pandemic such as COVID-19 using the traditional mAb platform represents a challenge, particularly if re-administration or antibody cocktails are required for optimal efficacy. We have observed how quickly nucleic acid products can be advanced to the clinic with the development of novel vaccines for COVID-19 (Broderick et al3; NCT04283461; NCT04336410). Similarly, nucleic acid approaches represent a potential alternative method for mAb delivery as well as compelling tools for the affordable and rapid in vivo evaluation of mAb-based therapeutic due to their simplicity, ease of production, scalability, conceptual safety, and established potency in preclinical models.
Fig 1.
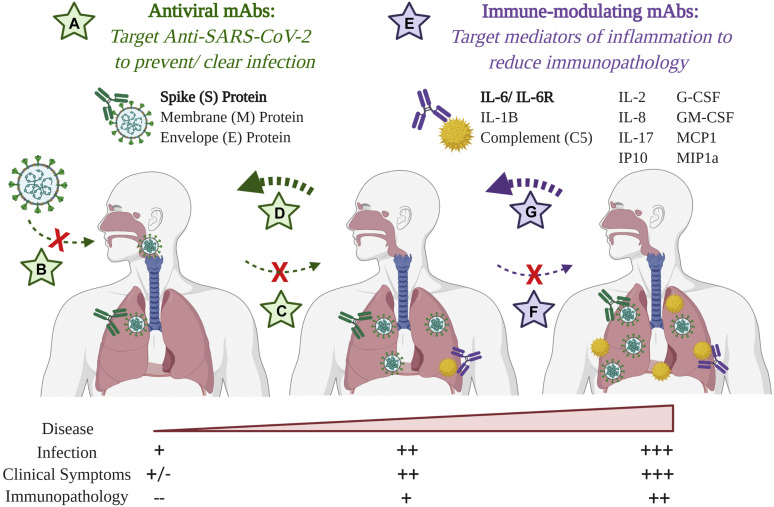
The use of dual-targeting mAb therapy to address both sources of SARS-CoV-2-induced disease. A, Antiviral mAbs target surface-exposed antigens to facilitate pathogen neutralization and clearance. In the case of SARS-CoV-2, efforts are focused on the S protein, which mediates host cell invasion. Depending on the timing of administration, these can (B) prevent infection, (C) prevent viral replication, and/or (D) facilitate viral clearance and disease mitigation via alternate mechanisms. E, To reduce immunopathoglogy, immune-modulating mAbs targeting various inflammatory mediators are under evaluation to (F) prevent and/or (G) alleviate pathogenesis and disease severity. Image created with biorender.com.
In vivo delivery of mAbs using nucleic acid platforms
Because of significant advancement over recent years, nucleic acid–based technologies hold increased potential to provide rapid and consistent antibody-mediated protection while avoiding the technical challenges associated with recombinant mAb production. Selected genetic sequences from antibodies in the form of mRNA or DNA are engineered, formulated, and administered for the in vivo production, assembly, and systemic secretion of encoded antibodies (Fig 2 , A-C) (reviewed in Patel et al4 and Gary and Weiner5) that can reach therapeutic levels in the serum within days of administration based on preclinical models. Numerous reports have described the unique delivery of DNA-encoded mAbs (DMAbs) using optimized expression plasmids that are nonlive, nonreplicating, nonintegrating, and nonimmunogenic. These DMAbs exhibit potent and durable expression kinetics, with functionality and in vivo efficacy comparable to those of their recombinant counterparts. Such biologics have been generated against a diverse set of infectious diseases including drug-resistant Pseudomonas, HIV, influenza virus, Dengue virus, Ebola virus, Chikungunya virus (CHIKV), and Zika virus, among others (reviewed in Patel et al4 and Gary and Weiner5). Phase 1 clinical trials of the anti-Zika DMAb, the first of its class to enter human studies, were initiated (NCT03831503). mRNA-based mAb approaches have been described for HIV, respiratory syncytial virus, and CHIKV,6 with positive outcomes; the CHIKV mRNA mAb has entered phase 1 clinical trials (NCT03829384). The preclinical data from these strategies support their further development as rapid response tools against emerging outbreaks such as COVID-19. Several partnerships have announced the intention to develop such products for SARS-CoV-2 therapy, using both DNA-Lipid Nanoparticles (Sorrento Therapeutics; San Diego, Calif and SmartPharm Therapeutics; Cambridge, Mass) and aerosolized mRNA nanoparticles (Neurimmune; Zurich, Switzerland and Ethris; Planegg, Germany). Efforts using the well-defined DMAb platform are in progress (Fig 2, D-G).
Fig 2.
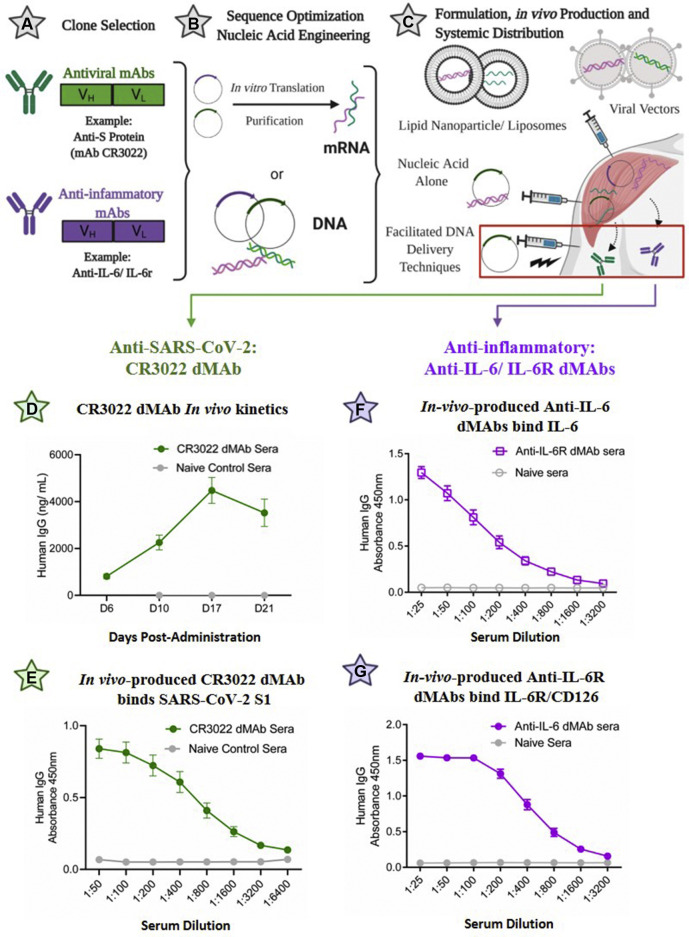
Nucleic acid strategies for the in vivo production of mAbs against COVID-19. A-C, Gene-delivery approaches to antibody delivery. A, mAbs with suitable specificity and potency are identified. B, For DNA delivery, optimized sequences are directly subcloned into the preferred expression or viral vector for in vivo delivery; for mRNA approaches, sequences are subcloned into DNA expression vectors, amplified, transcribed in vitro, and purified to yield short mRNA transcripts for delivery. C, Various delivery approaches—including electroporation (DNA) and lipid nanoparticle formulations (mRNA)—facilitate gene uptake, leading to mAb production and secretion into systemic circulation. D-G, Expression of DMAbs against COVID using the synthetic DNA platform. DNA sequences encoding the heavy and light chain of the indicated antibodies were engineered for in vivo expression (human IgG1) and administered to mice via electroporation (CELLECTRA-EP technology); D, Serum levels of the CR3022 DMAb were quantified via ELISA using anti-human reagents; E, Binding of CR3022 to SARS-CoV-2 S protein was demonstrated via ELISA; similarly, DMAbs targeting IL-6 (F) and IL6-R (G) were expressed in vivo and demonstrated strong binding to their respective targets via ELISA (day 7 sera). Data in E and F courtesy of Elliott S., manuscript in preparation (2020). Images in Fig 2, A-C, were created with Biorender.com.
Rapid response tools: In vivo mAb production against COVID-19 using the DMAb platform
Anti–SARS-CoV-2 DMAbs
Antiviral antibodies typically target surface-exposed antigens to inhibit viral infection and/or progression (Fig 1). Although SARS-CoV-2 contains several potential surface targets, the focus remains largely on the highly conserved coronavirus spike (S) protein, which mediates viral attachment and invasion via engagement with the host angiotensin-converting enzyme 2 receptor (ACE-2). Antibodies directed against the S protein of SARS-CoV, a closely related coronavirus that previously caused a deadly outbreak in humans, conferred protection in vivo. One such neutralizing antibody, mAb CR3022, cross-reacts with a unique epitope in the receptor-binding domain of the SARS-CoV-2 S protein.7 , 8 As an exemplative use of this technology against SARS-CoV-2, CR3022 sequences were modified and optimized as previously described9 to generate a novel DMAb encoding human CR3022, which was successfully expressed in mice (Fig 2, D). Sera harvested 7 days post-administration exhibited potent binding to the SARS-CoV-2 S1 protein (Fig 2, E). Studies of additional important SARS-CoV-2 antibodies using nucleic acid platforms will be informative.
Immune-modulating DMAbs
In addition to antiviral tools, there is a need to develop therapies to reduce SARS-CoV-2–mediated immunopathology. Severe disease is associated with significant upregulation of numerous proinflammatory cytokines and chemokines including IL-6, IL-1β, TNF-α, IL-2, IL-8, IL-17, G-CSF, GM-CSF, IP10, MCP1, and MIP1α (reviewed in Cao2). In addition, overactivation of the complement system is thought to contribute to this pathology. Immunotherapy with mAbs that target such inflammatory markers is of interest. The potential benefit of mAbs targeting the IL-6 pathway (anti–IL-6/IL-6R) as a therapy for critically ill patients with COVID-19 is under investigation. Similar to delivery of pathogen-targeting DMAbs, studies have demonstrated the ability to administer immune-modulating antibodies with potent in vivo effects (reviewed in Patel et al4). Accordingly, we optimized inserts encoding anti–IL-6 and anti–IL-6R DMAbs and administered them in vivo. As shown, anti–IL-6 (Fig 2, F) and anti–IL-6R (Fig 2, G) DMAbs were successfully expressed, able to bind their respective targets, and inhibited IL-6 activation in vitro (data in progress).
As rapid response tools, nucleic acid approaches can be used to generate, screen, compare, down-select, and develop potent biologics in vivo. Furthermore, the pursuit of mAbs-based products able to mitigate disease via unique and complementary mechanisms to enhance treatment efficacy is of interest. Gene delivery approaches allow for these types of combination studies in which antibody cocktails are rapidly evaluated and optimized for increased potency over corresponding monoclonal formulations; this has been validated for HIV-targeting antibody combinations using the DMAb platform.10 This represents a tailored, rapid, and possibly important approach to therapeutic development for SARS-CoV-2 and future outbreaks.
Conclusions
The astonishing pace with which the COVID-19 pandemic has spread across the globe emphasizes the need to have strategies in place for the rapid development of novel biologics including vaccines and immunotherapeutics. The speed at which nucleic acid vaccines entered and continue to advance through the clinic supports the use of these approaches in generating additional therapeutics to combat global outbreaks. Advancing such strategies to clinical trials will help define their feasibility to serve as potential alternatives or supplements to traditional mAb therapy. Importantly, these tools could improve the accessibility of such impactful mAb-based biologics to larger global populations.
Footnotes
Disclosure of potential conflict of interest: D. B. Weiner discloses the following paid associations with commercial partners: GeneOne (consultant), Geneos (Advisory Board), Astrazeneca (Advisory Board, speaker), Inovio (BOD, SRA, Stock), Pfizer (speaker), Merck (speaker), Sanofi (Advisory Board), and BBI (Advisory Board). E. Parzych declares no relevant conflicts of interest.
References
- 1.Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith T.R.F., Patel A., Ramos S., Elwood D., Zhu X., Yan J. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11:2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel A., Bah M.A., Weiner D.B. In vivo delivery of nucleic acid-encoded monoclonal antibodies. BioDrugs. 2020;34:273–293. doi: 10.1007/s40259-020-00412-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gary E.N., Weiner D.B. DNA vaccines: prime time is now. Curr Opin Immunol. 2020;65:21–27. doi: 10.1016/j.coi.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kose N., Fox J.M., Sapparapu G., Bombardi R., Tennekoon R.N., de Silva A.D. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aaw6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ter Meulen J., van den Brink E.N., Poon L.L., Marissen W.E., Leung C.S., Cox F. Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants. PLoS Med. 2006;3:e237. doi: 10.1371/journal.pmed.0030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel A., Park D.H., Davis C.W., Smith T.R.F., Leung A., Tierney K. In vivo delivery of synthetic human DNA-encoded monoclonal antibodies protect against Ebolavirus infection in a mouse model. Cell Rep. 2018;25:1982–1993.e4. doi: 10.1016/j.celrep.2018.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wise M.C., Xu Z., Tello-Ruiz E., Beck C., Trautz A., Patel A. In vivo delivery of synthetic DNA-encoded antibodies induces broad HIV-1-neutralizing activity. J Clin Invest. 2020;130:827–837. doi: 10.1172/JCI132779. [DOI] [PMC free article] [PubMed] [Google Scholar]