Abstract
There is an urgent need for vaccines and therapeutics to prevent and treat COVID-19. Rapid SARS-CoV-2 countermeasure development is contingent on the availability of robust, scalable, and readily deployable surrogate viral assays to screen antiviral humoral responses, define correlates of immune protection, and down-select candidate antivirals. Here, we generate a highly infectious recombinant vesicular stomatitis virus (VSV) bearing the SARS-CoV-2 spike glycoprotein S as its sole entry glycoprotein and show that this recombinant virus, rVSV-SARS-CoV-2 S, closely resembles SARS-CoV-2 in its entry-related properties. The neutralizing activities of a large panel of COVID-19 convalescent sera can be assessed in a high-throughput fluorescent reporter assay with rVSV-SARS-CoV-2 S, and neutralization of rVSV-SARS-CoV-2 S and authentic SARS-CoV-2 by spike-specific antibodies in these antisera is highly correlated. Our findings underscore the utility of rVSV-SARS-CoV-2 S for the development of spike-specific therapeutics and for mechanistic studies of viral entry and its inhibition.
Keywords: VSV, surrogate, COVID-19, SARS-CoV-2, neutralization assay, ACE2, neutralizing antibody, serology, antiviral drugs, convalescent plasma
Graphical Abstract
Highlights
-
•
Highly infectious recombinant VSV expressing SARS-CoV-2 spike (S) was generated
-
•
rVSV-SARS-CoV-2 S resembles SARS-CoV-2 in entry and inhibitor or antibody sensitivity
-
•
rVSV-SARS-CoV-2 S affords rapid screens and forward-genetic analyses of antivirals
Surrogate systems are needed to evaluate COVID-19 vaccines and therapeutics rapidly and at scale. Dieterle & Haslwanter et al. describe a highly infectious recombinant vesicular stomatitis virus encoding the SARS-CoV-2 spike protein that is suitable for screening and mechanistic studies of small molecule inhibitors, recombinant biologics, and convalescent plasma.
Introduction
A member of the family Coronaviridae, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of the ongoing coronavirus disease 2019 (COVID-19) pandemic that emerged in Wuhan City, China in late 2019 (Wu et al., 2020a). With more than 8 million confirmed cases and at least 435,000 deaths in over 216 countries, areas or territories as of June 15th, 2020, the global scale and impact of COVID-19 is unparalleled in living memory (Dong et al., 2020; World Health Organization, 2020). To date, mitigation strategies have relied largely on physical distancing and other public health measures. Although treatments with some small molecule inhibitors and with convalescent plasma have received approvals for emergency use and vaccines, antivirals, and monoclonal antibodies are being rapidly developed, no FDA-approved countermeasures are currently available.
The membrane-enveloped virions of SARS-CoV-2 are studded with homotrimers of the spike glycoprotein (S), which mediate viral entry into the host cell (Bosch et al., 2003; Walls et al., 2020; Wrapp et al., 2020). S trimers are post-translationally cleaved in the secretory pathway by the proprotein convertase furin to yield N– and C–terminal S1 and S2 subunits, respectively. S1 is organized into an N–terminal domain (NTD), a central receptor-binding domain (RBD), and a C–terminal domain (CTD). S2 bears the hallmarks of a “class I” membrane fusion subunit with an N–terminal hydrophobic fusion peptide, N– and C–terminal heptad repeat sequences, a transmembrane domain, and a cytoplasmic tail (Walls et al., 2020; Wrapp et al., 2020).
The S1 RBD engages the viral receptor, human angiotensin-converting enzyme 2 (hACE2), at the host cell surface (Hoffmann et al., 2020a; Wang et al., 2020; Zhou et al., 2020). Receptor binding is proposed to prime further S protein cleavage at the S2′ site by the transmembrane protease serine protease-2 (TMPRSS2) at the cell surface and/or by host cysteine cathepsin(s) in endosomes. S2′ cleavage activates S2 conformational rearrangements that catalyze the fusion of viral and cellular membranes and escape of the viral genome into the cytoplasm (Hoffmann et al., 2020a).
The S glycoprotein is the major antigenic target on the virus for protective antibodies (Rogers et al., 2020; Wec et al., 2020; Wu et al., 2020a), and is thus of high significance for the development of vaccines and therapeutic antibodies. Plasma derived from COVID-19 human convalescents and replete with such antibodies has shown early promise as a COVID-19 treatment; it is currently being evaluated in clinical trials of antiviral prophylaxis and therapy (Casadevall and Pirofski, 2020). Considerable efforts are also being aimed at the identification and deployment of S-glycoprotein-specific neutralizing monoclonal antibodies (mAbs) (Cao et al., 2020; Pinto et al., 2020; Rogers et al., 2020; Wec et al., 2020; Wu et al., 2020b; Zost et al., 2020). A key requirement for the rapid development of such vaccines and treatments with convalescent plasma, small-molecule inhibitors, and recombinant biologics is the availability of safe, robust, and faithful platforms to study S-glycoprotein inhibition with high assay throughput. Given the limited access to biosafety level 3 (BSL-3) containment facilities required to safely handle SARS-CoV-2, researchers have turned to surrogate viral systems that afford studies of cell entry at biosafety level 2 (BSL-2) and facilitate rapid inhibitor screening through the use of fluorescence- or luminescence-based reporters. These include retroviruses, lentiviruses, or vesiculoviruses “pseudotyped” with SARS-CoV-2 S and competent for a single round of viral entry and infection (Lei et al., 2020; Nie et al., 2020; Ou et al., 2020; Pu et al., 2020; Tan et al., 2020; Xiong et al., 2020). However, these single-cycle, pseudotyped viruses are typically laborious to produce and challenging to scale up, yield poorly infectious preparations, and suffer background issues in some cases due to contamination with viral particles bearing the orthologous entry glycoprotein (e.g., low levels of vesicular stomatitis virus [VSV] pseudotypes bearing VSV G).
In contrast to the single-cycle pseudotypes, replication-competent recombinant VSVs (rVSVs) encoding the heterologous virus entry glycoprotein gene(s) in cis as their only entry protein(s) are easier to produce at high yields and also afford forward-genetic studies of viral entry. We and others have generated and used such rVSVs to safely and effectively study entry by lethal viruses that require high biocontainment (Caì et al., 2019; Jae et al., 2013; Jangra et al., 2018; Kleinfelter et al., 2015; Maier et al., 2016; Raaben et al., 2017; Whelan et al., 1995; Wong et al., 2010) Although rVSVs bearing the S glycoprotein from SARS-CoV (Fukushi et al., 2006; Kapadia et al., 2005, 2008) and the Middle East respiratory syndrome coronavirus (MERS-CoV) (Liu et al., 2018) have been developed, no such systems have been described to date for SARS-CoV-2.
Here, we generate a rVSV encoding SARS-CoV-2 S and identify key passage-acquired mutations in the S glycoprotein that facilitate robust rVSV replication. We show that the entry-related properties of rVSV-SARS-CoV-2 S closely resemble those of the authentic agent and use a large panel of COVID-19 convalescent sera to demonstrate that the neutralization of the rVSV and authentic SARS-CoV-2 by spike-specific antibodies is highly correlated. Our findings underscore the utility of rVSV-SARS-CoV-2 S for the development of spike-specific antivirals and for mechanistic studies of viral entry and its inhibition.
Results
Identification of S Gene Mutations That Facilitate Robust rVSV-SARS-CoV-2 S Replication
To generate a replication-competent rVSV expressing SARS-CoV-2 S, we replaced the open-reading frame of the native VSV entry glycoprotein gene, G, with that of the SARS-CoV-2 S (Wuhan-Hu-1 isolate) (Figure 1 A). We also introduced a sequence encoding the enhanced green fluorescent protein (eGFP) as an independent transcriptional unit at the first position of the VSV genome. Plasmid-based rescue of rVSV-SARS-CoV-2 S generated a slowly replicating virus bearing the wild-type S sequence. Five serial passages yielded viral populations that displayed enhanced spread. This was associated with a dramatic increase in the formation of syncytia (Figures 1B and S1) driven by S-mediated membrane fusion (Figure S1). Sequencing of this viral population identified nonsense mutations that introduced stop codons in the S glycoprotein gene (amino acid position C1250∗ and C1253∗), causing 24- and 21-amino acid deletions, respectively, in the S cytoplasmic tail. SΔ24 and SΔ21 were maintained in the viral populations upon further passage and SΔ21 in all plaque-purified isolates, highlighting their likely importance as adaptations for viral growth. Viral population sequencing after four more passages identified two additional mutations—L517S and P812R in S1 and S2, respectively—whose emergence coincided with more rapid viral spread and the appearance of non-syncytium-forming infectious centers (Figure 1B, passage 5). Pelleted viral particles from clarified infected-cell supernatants incorporated the S glycoprotein, as determined by an S-specific ELISA (Figure 1C).
Figure 1.
Generation of a Recombinant Vesicular Stomatitis Virus (rVSV) Bearing the SARS-CoV-2 Spike (S) Glycoprotein
(A) Schematic representation of the VSV genome in which its native glycoprotein gene has been replaced by that encoding the SARS-CoV-2 S protein. The VSV genome has been further modified to encode an enhanced green fluorescent protein (eGFP) reporter to easily score for infection.
(B) Infectious center formation assay on Vero cells at 24 h post-infection showing growth of the rVSV-SARS-CoV-2 S after the indicated number of rounds of serial passage of the passage #1 virus (carrying wild-type [WT] S sequences) on Huh7.5.1 cell line (scale bar, 100 μm). Two representative images for each virus passage, showing infected cells pseudo-colored in green, from one of the two independent experiments are shown here.
(C) Incorporation of SARS-CoV-2 S into rVSV particles captured on an ELISA plate was detected using antiserum from a COVID-19 convalescent donor (average ± SD, n = 12 from 3–4 independent experiments). Serum from a COVID-19-negative donor and rVSVs bearing Ebola virus glycoprotein (EBOV GP) were used as negative controls (average ± SD, n = 6 from 2 independent experiments).
(D) Representative images showing Vero cells infected with plaque #2, #3, and #6 viruses at 16 h post-infection (scale bar, 100 μm).
(E) Production of infectious virions at 48 h post-infection from Vero cells infected with the indicated plaque-purified viruses. Titers were measured on Vero cells overexpressing TMPRSS2 (n = 4, from two independent titrations).
We next sequenced six plaque-purified viral isolates derived from the passage 9 (P9) population. All of these viral clones bore the SΔ21 deletion in the S cytoplasmic tail and spread without much syncytia formation (Figure 1D). Interestingly, all of these isolates contained three amino acid changes at S-glycoprotein positions other than 517 or 812—W64R, G261R, and A372T—in addition to the C–terminal SΔ21 deletion (Table S1 and Figure S2). Five of the six isolates also contained mutations H655Y or R685G. Importantly, peak titers of all these viral isolates ranged between 1–3 × 107 infectious units per mL (Figure 1E), suggesting that the mutations they share (or a subset of these mutations) drive rVSV-SARS-CoV-2 S adaptation for efficient spread in tissue culture with little or no syncytium formation.
rVSV-SARS-CoV-2 S Entry Is Cysteine Cathepsin Dependent
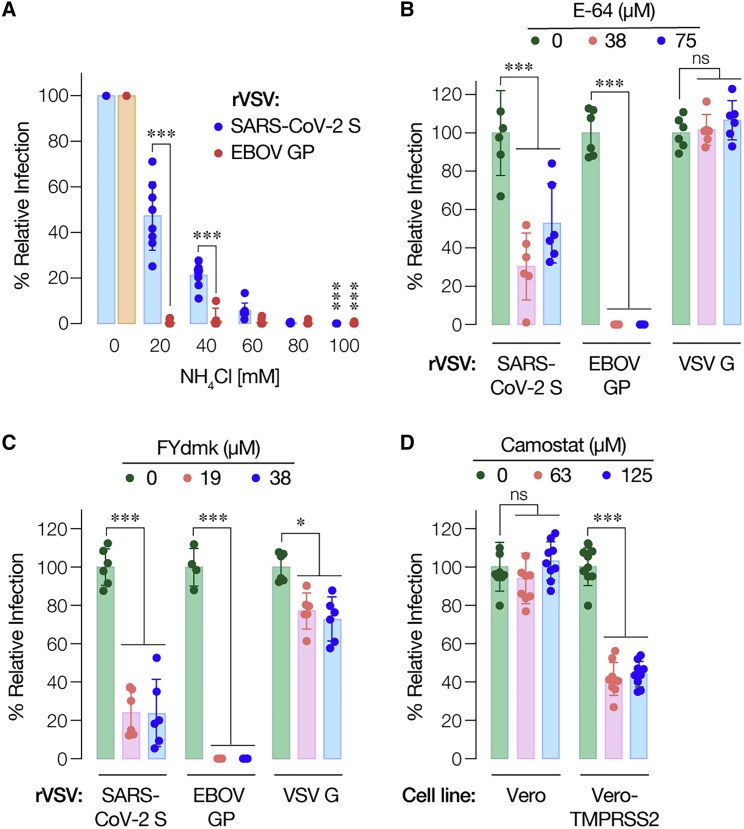
SARS-CoV-2 entry in cells has been shown to be dependent on the proteolytic activity of acid-dependent endosomal cysteine cathepsins, including cathepsin L (Hoffmann et al., 2020a; Wang et al., 2020). Accordingly, we tested the effects of chemical inhibitors of cysteine cathepsins on rVSV-SARS-CoV-2 S infection. Pretreatment of cells with NH4Cl, an inhibitor of endosomal acidification, reduced entry by rVSVs bearing SARS-CoV-2 S or the Ebola virus glycoprotein (EBOV GP) in a dose-dependent manner (Figure 2 A). However, S-mediated entry was comparatively less sensitive to NH4Cl than entry by EBOV GP (Figure 2A). Next, we tested cysteine cathepsin inhibitors E-64 (Figure 2B) and FYdmk (Figure 2C). Pre-treatment of cells with both of these compounds also inhibited S-mediated entry, albeit with reduced sensitivity relative to that observed for EBOV GP-dependent entry (Figures 2B and 2C). Together, these findings confirm that rVSV-SARS-CoV-2 S resembles the authentic agent in its requirements for endosomal acid pH and cysteine cathepsins. They also suggest a reduced dependence on these host factors for entry by SARS-CoV-2 S relative to EBOV GP, a model glycoprotein known to fuse in late endo/lysosomal compartments following extensive endosomal proteolytic processing.
Figure 2.
rVSV-SARS-CoV-2 S Infection Requires the Activity of Cysteine Cathepsin Proteases
(A) Huh7.5.1 cells pre-treated for 1 h at 37°C with the indicated concentrations of NH4Cl were infected with pre-titrated amounts of rVSVs bearing SARS-CoV-2 S or EBOV GP. Infection was scored by eGFP expression at 16–18 h post-infection (average ± SD, n = 8 from 2 independent experiments).
(B) Vero cells pre-treated for 90 min at 37°C with the indicated concentrations of pan-cysteine cathepsin inhibitor E-64 were infected with pre-titrated amounts of rVSVs bearing SARS-CoV-2 S, EBOV GP, or VSV G and scored for infection as above (average ± SD, n = 6 from 3 independent experiments, except n = 4 from 2 independent experiments for EBOV GP).
(C) Vero cells pre-treated for 90 min at 37°C with the indicated concentrations of cathepsin L/B inhibitor FYdmk were infected with pre-titrated amounts of rVSVs bearing SARS-CoV-2 S, EBOV GP, or VSV G. Infection was scored as above (average ± SD, n = 6 from 3 independent experiments).
(D) Vero cells and Vero cells overexpressing TMPRSS2 pre-treated for 120min at 37C with the indicated concentrations of camostat ]were infected with pre-titrated amounts of rVSVs bearing SARS-CoV-2 S and subsequently scored for infection. In panels (B)–(D), all comparisons were made between vehicle- and inhibitor-treated samples. ns,not statistically significant. ∗p < 0.033, ∗∗∗p < 0.001.
TMPRSS2 Can Mediate rVSV-SARS-CoV-2 S Entry
The Type II transmembrane serine protease TMPRSS2 plays a key role in the infection and spread of a number of enveloped viruses in cells of the human airway (Choi et al., 2009; Shen et al., 2017). TMPRSS2 cleavage of the hemagglutinin spike precursors (HA0) of some influenza A and B viruses at a monobasic site generates HA1 and HA2 subunits, thereby priming HA for viral membrane fusion (Böttcher-Friebertshäuser et al., 2014; Limburg et al., 2019; Böttcher-Friebertshäuser et al., 2010; Chaipan et al., 2009). TMPRSS2 can also activate membrane fusion by the spike glycoproteins of human coronaviruses, including those of SARS-CoV and MERS-CoV, by cleaving the spike at the monobasic S2′ site during entry (Kawase et al., 2012; Zhou et al., 2015). Recent work indicates that TMPRSS2 may play a similar role in SARS-CoV-2 entry into human airway and intestinal cells (Bestle et al., 2020; Zang et al., 2020; Hoffmann et al., 2020b). Accordingly, we evaluated the effect of the trypsin-like serine protease inhibitor camostat mesylate (camostat), previously shown to block TMPRSS2 catalytic activity and inhibit viral glycoprotein activation (Zhou et al., 2015; Kawase et al., 2012; Nimishakavi et al., 2015), on rVSV-SARS-CoV-2 S infection. Pretreatment of Vero grivet monkey cells with camostat had little effect, consistent with their low expression levels of TMPRSS2 (Hoffmann et al., 2020b). By contrast, camostat treatment significantly reduced VSV-SARS-CoV-2 S infection in Vero cells transduced to express human TMPRSS2 (Vero-TMPRSS2), as reported previously (Hoffmann et al., 2020a; Figure 2D). These findings suggest that TMPRSS2 can promote cell entry by rVSV-SARS-CoV-2 S.
Human ACE2 Is Required for rVSV-SARS-CoV-2 S Entry
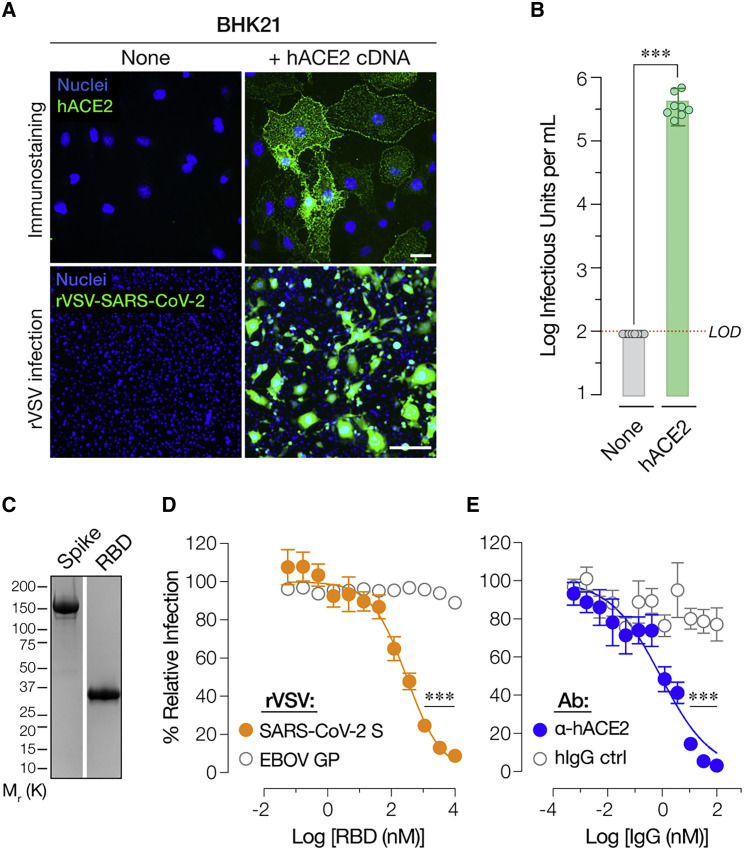
SARS-CoV-2 uses hACE2 as its entry receptor (Letko et al., 2020; Shang et al., 2020b, 2020a). Baby hamster kidney (BHK21) cells do not express detectable levels of ACE2 protein and are resistant to SARS-CoV-2 entry (Chu et al., 2020; Hoffmann et al., 2020a; Wang et al., 2020). Concordantly, we observed no detectable infection by rVSV-SARS-CoV-2 S in BHK-21 cells (Figure 3 A, top left). By contrast, BHK21 cells transduced to express hACE2 (Figure 3A, top right) were highly susceptible to rVSV-SARS-CoV-2 S (Figure 3A, bottom right and Figure 3B).
Figure 3.
rVSV-SARS-CoV-2 S Infection Requires Human ACE2
(A) Naive (None) baby hamster kidney (BHK21) cells or cells transduced with a retrovirus carrying human ACE2 cDNA (+ hACE2 cDNA) were immunostained for hACE2 expression using an anti-ACE2 antibody. Cells were imaged by fluorescence microscopy. The hACE2 signal is pseudo-colored green (top panel, scale bar = 20 μm). These cells were also exposed to serial 5-fold dilutions of rVSV-SARS-CoV-2 S and infection was scored by eGFP expression (bottom panel, scale bar = 50 μm). Representative images from one of 3 independent experiments are shown.
(B) Enumeration of eGFP-positive green cells (Average ± SD, n = 8 from 3 independent experiments). Red dotted line indicates the assay limit of detection (LOD).
(C) Recombinant, Ni-NTA–affinity purified S1-S2 ectodomain (Spike) or the receptor binding domain (RBD) of the SARS-CoV-2 S protein were subjected to SDS-PAGE and Coomassie staining. A representative image from one of two independent purification trials is shown here.
(D) Monolayers of Huh7.5.1 cells were pre-incubated with serial 3-fold dilutions of the purified RBD for 1 h at 37°C and then infected with pre-titrated amounts of rVSVs bearing SARS-CoV-2 S or EBOV GP. At 16–18 h post-infection, cells were fixed, nuclei counter-stained with Hoechst-33342, and infection (eGFP expression) was scored by fluorescence microscopy. It is represented as % relative infection (no RBD = 100%, Average ± SEM, n = 8 from 3–4 [rVSV-SARS-CoV-2 S] or n = 4 from 2 [rVSV-EBOV GP] independent experiments).
(E) Monolayers of Huh7.5.1 cells pre-incubated for 1 h at 37°C with 3-fold serial dilutions of anti-human ACE2 antibody or negative control (hIgG) were infected with pre-titrated amounts of rVSV-SARS-CoV-2 S. Infection was scored as above and is represented as % relative infection (no antibody = 100%, Average ± SD, n = 8 from 3–4 independent experiments). ∗∗∗p < 0.001.
To directly establish an entry-relevant interaction between rVSV-SARS-CoV-2 S and hACE2, we expressed and purified the spike RBD (Figure 3C) and pre-incubated it with target cells. RBD pre-treatment inhibited rVSV-SARS-CoV-2 S entry in a specific and dose-dependent manner (Figure 3D). Moreover, pre-incubation of cells with an hACE2-specific mAb, but not an isotype-matched control mAb, potently abolished rVSV-SARS-CoV-2 S entry (Figure 3E). These findings provide evidence that rVSV-SARS-CoV-2 S entry and infection, like that of the authentic agent, requires spike RBD-hACE2 engagement.
rVSV-SARS-CoV-2 S Infects Cells of Human Airway Origin in an ACE2-Dependent Manner
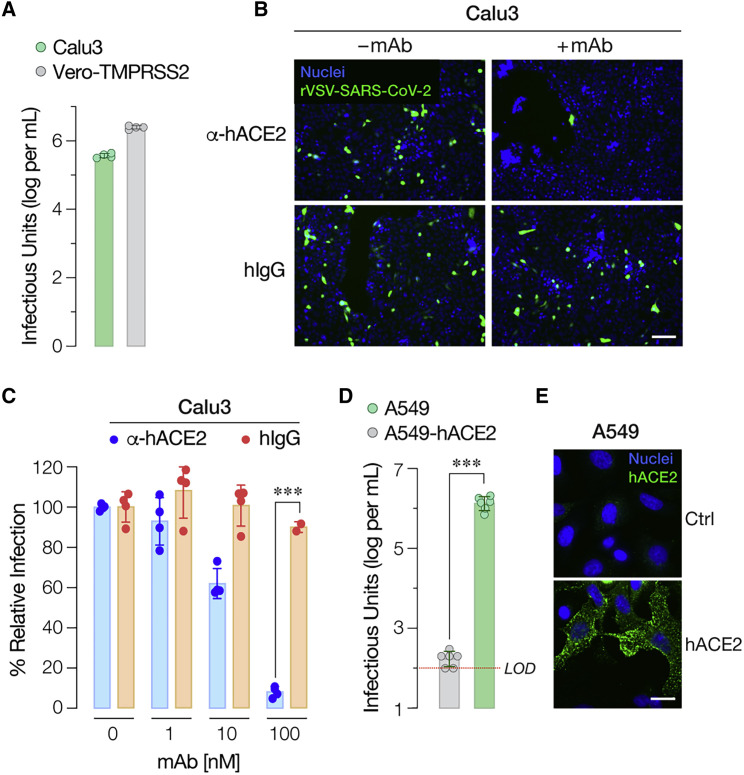
SARS-CoV-2 can infect multiple cell types in the human airway, including ciliated epithelial cells of the bronchial and bronchiolar mucosae and type I and II pneumocytes of the lung (Rockx et al., 2020; Hui et al., 2020). We assessed rVSV-SARS-CoV-2 entry and infection in epithelial cell lines that serve as models of human respiratory function. Specifically, we found that the human lung adenocarcinoma cell line Calu3 was highly susceptible to infection (Figure 4 A) in a manner that was sensitive to treatment with a hACE2-specific mAb (Figures 4B and 4C). By contrast, the human lung adenocarcinoma cell line A549 was refractory to infection (Figure 4D), as previously documented with authentic SARS-CoV-2 and a single-cycle VSV vector bearing SARS-CoV-2 S (Chu et al., 2020; Harcourt et al., 2020; Hoffmann et al., 2020a; Hui et al., 2020). Because poorly differentiated A549 cells express little ACE2 (Jia et al., 2005; Mossel et al., 2005; Figure 4E), we transduced these cells to express hACE2 and then exposed them to virus. The A549-hACE2 cells were more susceptible than their parental cells by a factor of ≈104 (Figure 4E). Thus, rVSV-SARS-CoV-2 S can enter and infect cells of human airway origin in an ACE2-dependent fashion.
Figure 4.
rVSV-SARS-CoV-2 S Infection in Human Airway Epithelial Cells is ACE2-Dependent
(A) Infectivity of rVSV-SARS-CoV-2 S was measured in human airway epithelial Calu3 cells and Vero-TMPRSS2 cells by applying serial dilutions of the virus. Infections were scored as described in Figure 3B (Average ± SD, n = 4 from two independent titrations).
(B and C) Monolayers of Calu3 cells pre-incubated for 1 h at 37°C with indicated amounts of anti-human ACE2 antibody or negative control (hIgG) were infected with pre-titrated amounts of rVSV-SARS-CoV-2 S. (B) Representative images from one of the two independent experiments are shown (scale bar, 100 μm). (C) Infection was scored as above and is represented as % relative infection (no antibody = 100%, Average ± SD, n = 4 from two independent experiments, except for n = 2 for hIgG at 100 nM).
(D) Infectivity of rVSV-SARS-CoV-2 S in human respiratory epithelial A549 cells transduced with a retrovirus carrying human ACE2 cDNA or empty vector was evaluated by exposing cells to serial dilutions of rVSV-SARS-CoV-2 S. Infections were scored as described in Figure 3B (Average ± SD, n = 6 from 3 independent titrations). Red dotted line indicates the assay limit of detection (LOD). Means were compared by unpaired t test.
(E) A549 cells transduced as in panel 3D were immunostained for hACE2 expression as described in Figure 3A. using an anti-ACE2 antibody. Cells were imaged by fluorescence microscopy. The hACE2 signal is pseudo-colored green and representative images are shown (scale bar = 20 μm). ∗∗∗p < 0.001.
S-Protein-Targeting Antibodies in COVID-19 Convalescent Sera Specifically Account for rVSV-SARS-CoV-2 S Neutralization
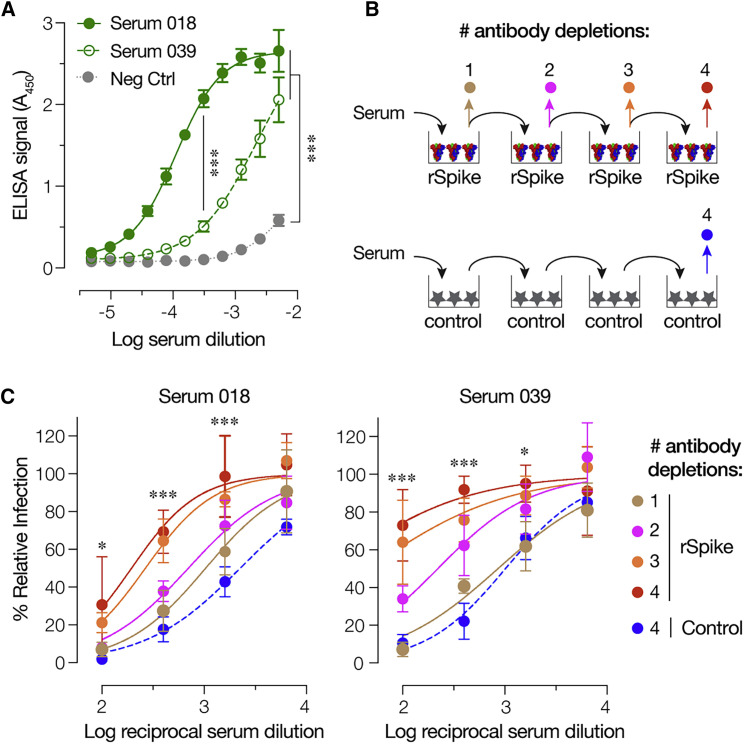
Prior to examining the performance of rVSV-SARS-CoV-2 S in neutralization assays with human antisera, we sought to establish a specific role for interaction between anti-spike antibodies in these sera and the VSV-borne S protein. Accordingly, we first evaluated the reactivity of two sera with rVSV-neutralizing activity (Figure S3) against viral particles by ELISA. Both sera specifically recognized rVSV-SARS-CoV-2 S particles (Figure 5 A) and were also shown to be reactive against a purified, trimeric preparation of the spike glycoprotein (Wrapp et al., 2020; R.H.B., unpublished data). Further, serial pre-incubation of each serum with purified S immobilized on a high-binding plate depleted its capacity to inhibit rVSV-SARS-CoV-2 S infection to a degree that was commensurate with its content of S-specific antibodies (Figures 5B and 5C). By contrast, parallel pre-incubations with blocked plates had little or no effect (Figure 5C). These results indicate that S-glycoprotein-targeting antibodies in COVID-19 convalescent sera specifically mediate rVSV-SARS-CoV-2 S neutralization.
Figure 5.
rVSV-SARS-CoV-2 S Neutralization Is Mediated by S-Glycoprotein-Targeting Antibodies in Human Antisera
(A) ELISA plates coated with rVSV-SARS-CoV-2 S were incubated with serial 2-fold dilutions of serum 18, serum 39, or negative control serum. Bound S-specific antibodies were detected with an anti-human HRP-conjugated secondary antibody (average ± SD, n = 4 from 2 independent experiments).
(B) Schematic of the antibody depletion study.
(C) Pre-titrated amounts of serum 18 and serum 39 were sequentially incubated with SARS-CoV-2 S-coated high-binding plates to deplete S-specific antibodies. Capacity of the depleted sera (and control sera incubated with only the blocking agent) to neutralize rVSV-SARS-CoV-2 S was then estimated by incubating pre-titrated amounts of rVSV at the indicated dilutions of sera at 37°C for 1 h prior to infecting monolayers of Huh7.5.1 cells. Cells were scored for infection as above (average ± SD, n = 4 from 2 independent experiments). In panels (A), (C), and (D), p values for pairwise comparisons of the untreated sample and inhibitor-treated sample means are shown, unless otherwise indicated. In panels (C) and (D), the depletion #4 and depletion control were compared. ∗p < 0.033,∗∗∗p < 0.001.
The Susceptibilities of rVSV-SARS-CoV-2 S and Authentic SARS-CoV-2 to Antibody-Mediated Neutralization Are Highly Correlated
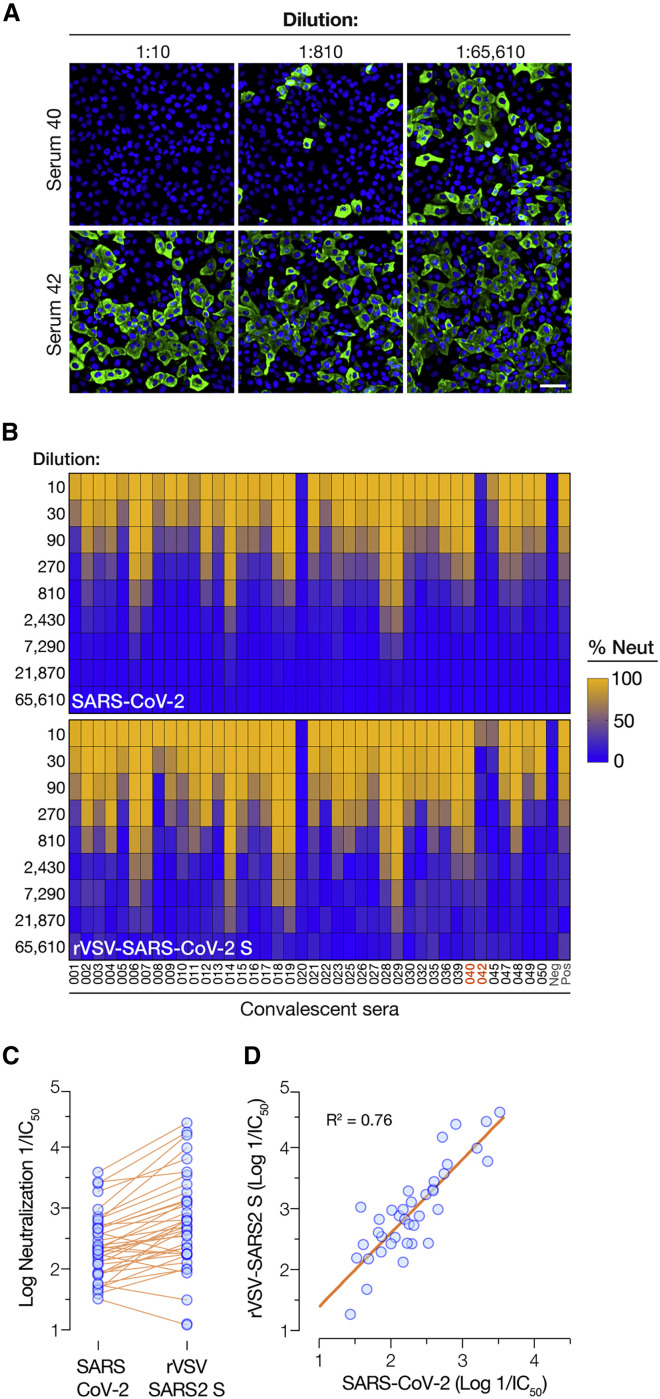
We compared the capacities of human antisera derived from 40 COVID-19 convalescent donors to block infection by rVSV-SARS-CoV-2 S and authentic SARS-CoV-2 in a microneutralization format. Briefly, pre-titrated amounts of viral particles were incubated with serial dilutions of each antiserum, and target cells were then exposed to the virus-antiserum mixtures. Viral infection was determined by enumerating eGFP-positive cells (rVSV) as above (Figure 1) or cells immunoreactive with a SARS-CoV-2 nucleocapsid protein-specific antibody (authentic virus) (Figure 6 A). Heatmaps of viral infectivity revealed similar antiserum donor- and dose-dependent neutralization patterns for rVSV-SARS-CoV-2 S and authentic SARS-CoV-2 (Figure 6B). Comparison of the serum dilutions at half-maximal neutralization derived from logistic curve fits (neutralization IC50) revealed a 3- to10-fold shift toward enhanced neutralization with rVSV-SARS-CoV-2 S (Figure 6C). The origin of this difference is unclear but does not appear to arise from viral-passage-dependent changes in the rVSV-encoded spike gene sequence (Figure S4). Rather, it may reflect assay-specific differences in the rVSV and authentic virus microneutralization formats employed herein. Nevertheless, the relative potencies of the antisera against rVSV-SARS-CoV-2 S and authentic SARS-CoV-2 were well correlated (R2 = 0.76) (Figure 6D). In sum, these findings demonstrate the suitability of rVSV-SARS-CoV-2 S for rapid, high-throughout, reporter-based assays of spike-glycoprotein-dependent entry and its inhibition.
Figure 6.
Correlation of Convalescent Serum-Mediated Neutralization of rVSV-SARS-CoV-2 S and Authentic SARS-CoV-2
(A) Pre-titrated amounts of SARS-CoV-2 were incubated with serial 3-fold dilutions of antisera from COVID-19 convalescent donors or negative control at 37°C for 1 h. Virus-serum mixtures were then applied to monolayers of Vero-E6 cells. At 24 h post-infection, cells were fixed, permeabilized and immunostained with a SARS-CoV nucleocapsid-specific antibody. Nuclei were counterstained, and infected cells were scored for the presence of nucleocapsid antigen. Representative images from one of the 2 independent experiments are shown (scale bar, 200 μm).
(B) Pre-titrated amounts of rVSV-SARS-CoV-2 S were incubated with serial 3-fold dilutions of antisera from COVID-19 convalescent patients or negative control at 37°C for 1 h. Virus:serum mixtures were then applied to monolayers of Vero cells. At 7 h post-infection, cells were fixed, nuclei were counterstained, and infected cells were scored by GFP expression. Heatmaps showing % neutralization of authentic SARS-CoV-2 or rVSV-SARS-CoV-2 S by the panel of 40 antisera are shown (Averages of n = 4 from 2 independent experiments).
(C and D) Comparison of the neutralizing activities of the antisera (log reciprocal IC50 values) against authentic SARS-CoV-2 and rVSV-SARS-CoV-2 S (“rVSV SARS2 S”). (D) Linear regression analyses of neutralization IC50 values from panel C.
Discussion
There is an urgent need for vaccines and therapeutics to prevent and treat COVID-19. The rapid development of SARS-CoV-2 countermeasures is contingent on the availability of robust, scalable, and readily deployable surrogate viral systems to screen antiviral humoral responses and define correlates of immune protection. Such tools would also facilitate the efficient down-selection of candidate antivirals and studies of their mechanisms of action. Here, we describe a highly infectious recombinant vesicular stomatitis virus bearing the SARS-CoV-2 spike glycoprotein S that closely resembles the authentic agent in its entry-related properties. We show that rVSV-SARS-CoV-2 S affords the high-throughput, reporter-based screening of small-molecule and antibody-based inhibitors targeting the viral spike glycoprotein with performance characteristics comparable to those of SARS-CoV-2.
rVSV-SARS-CoV-2 S initially replicated poorly in cell culture following its rescue from plasmids, but we noted accelerated viral growth at passage 5 (Figure 1). This coincided with the emergence of viral variants bearing S glycoproteins with 21- or 24-amino-acid truncations of their cytoplasmic tails, as also observed by Case and co-workers (Case et al., 2020). The cytoplasmic tails of the S glycoproteins of SARS-CoV and SARS-CoV-2 are highly similar and carry signals for their retention in the endoplasmic reticulum (ER), including a conserved Kx Hxx motif located near the C–terminus (McBride et al., 2007, Ujike et al., 2016). 18- to 19-amino-acid deletions in the cytoplasmic tails of SARS-CoV S (Fukushi et al., 2006; Fukushi et al., 2005) and SARS-CoV-2 S (Ou et al., 2020) have been shown to increase the infectivity of single-cycle VSV-S pseudotypes. As previously observed for ER/Golgi-localizing hantavirus glycoproteins (Slough et al., 2019), these deletions likely redistribute S glycoproteins to the cell surface, thereby relieving the spatial mismatch in budding between VSV and SARS-CoV2 (plasma membrane versus ER, respectively) and enhancing S incorporation into VSV particles.
Accelerated growth by rVSV-SARS-CoV-2 S around passage 5 was accompanied by a marked increase in the occurrence of syncytia (see Figure 1B) due to S-mediated cell-cell fusion (Figure S1). This may reflect a functional property of the cytoplasmic-tail-deleted S variants, including perturbations in their subcellular localization, as discussed above. Strikingly, passage 9 stocks and highly infectious viral plaque isolates from these stocks displayed a pattern of spreading infection more typical for rVSVs, with few large syncytia in evidence (Figure 1B). In this regard, it is tempting to speculate that one or more additional S glycoprotein mutations detected in the passage 5–9 viral populations and in the six plaque isolates (Table S1) arose as compensatory changes to suppress the syncytiogenic propensity of the rVSV-encoded SΔ21 glycoprotein spikes. Indeed, several mutations in the S1 NTD and RBD may serve to modulate spike glycoprotein fusogenicity, as also may mutations near or at the S1–S2 cleavage site (H655Y and R685G, respectively) and/or at the S2′ cleavage site (P812R) (Table S1 and Figure S2). Further, at least one mutation (H655Y), present in five of six rVSV-SARS-CoV-2 S plaque isolates, has arisen during natural SARS-CoV-2 evolution in humans (Yang et al., 2020), during transmission studies in a hamster model (Chan et al., 2020), and possibly during SARS-CoV-2 passage in tissue culture (this report). Our current efforts are aimed at understanding what role(s) these mutations in the S glycoprotein ectodomain play in the maintenance of high levels of rVSV infectivity without the formation of large numbers of syncytia. These findings also highlight a feature of rVSV-SARS-CoV-2 S not shared by any of the viral entry surrogates described to date: its utility for forward genetics. This can be used to dissect structure-function relationships in the SARS-CoV-2 spike glycoprotein and to elucidate the mechanisms of action of spike- or entry-targeted antivirals.
We demonstrate that rVSV-SARS-CoV-2 S can be used to rapidly and faithfully assess the neutralizing activities of large panels of COVID-19 convalescent sera (this report, Figure 6) and spike-directed mAbs (Wec et al., 2020). We have exploited the fidelity and high throughput of our rVSV-based 384-well plate microneutralization assay to rapidly pre-screen >300 COVID-19 convalescents and identify potential convalescent plasma donors first for the expanded access program and now for an ongoing randomized controlled trial of convalescent plasma therapy (Casadevall and Pirofski, 2020; NYU Langone Health/Albert Einstein College of Medicine, 2020; K.C. and L.-a.P., unpublished data). The utility and reliability of this approach is further enhanced by its synergy with the new SARS-CoV-2 microneutralization assay also described herein (Figures 6A and 6B), which provides a rapid and non-subjective alternative to classical PRNT assays. When used in combination, these assays should afford the rapid mechanistic interrogation of cellular factors and antivirals that act at any step of the viral multiplication cycle—entry hits should affect both the rVSV and the authentic virus, whereas post-entry hits should affect only the latter.
As the COVID-19 pandemic continues apace and the development of plasma-, hyperimmune globulin-, mAb-, and small-molecule-based countermeasures accelerates, the need for highly scalable viral assays continues to mount. The availability of highly infectious rVSV surrogates that can be scaled up with relative ease for antiviral screening and readily deployed in reporter-based microneutralization assays will facilitate these efforts.
Limitations of Study
rVSV-SARS-CoV-2 S has only been tested in cell culture. Thus, we cannot conclusively rule out that it may not be well tolerated in vivo. Given this theoretical safety concern, lab workers should exercise due caution in handling an agent that is potentially infectious in humans. Until the safety of rVSV-SARS-CoV-2 S has been evaluated and established in appropriate animal model(s), we will only distribute it to researchers for in vitro work to be performed at biosafety level 2 or higher, as approved by their institutional biosafety committee.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat hACE2-specific antibody | R&D systems | Cat# AF933 RRID: AB_355722 |
| Rabbit Coronavirus Nucleocapsid Antibody | SinoBiological | Cat# 40143-R001 RRID: AB_2827974 |
| Goat AlexaFluor 488-conjugated anti-Rabbit IgG | ThermoFisher | Cat# A32731 RRID: AB_2633280 |
| Human gamma globulin | Jackson ImmunoResearch | Cat# 009-000-002 RRID: AB_2337042 |
| Donkey AlexaFluor 594-conjugated anti-goat IgG | Invitrogen | Cat# A32758 RRID: AB_2762828 |
| Goat anti-human IgG-HRP | Invitrogen | Cat# 31410 RRID: AB_228269 |
| Bacterial and Virus Strains | ||
| SARS-CoV-2 (Washington State isolate) | USAMRIID | MT020880.1 |
| rVSV-EBOV/Mayinga GP (EBOV/H.sap-tc/COD/76/Yambuku-Mayinga) | (Wong et al., 2010) | N/A |
| rVSV- G | (Whelan et al., 1995) | N/A |
| rVSV-SARS-CoV-2 S (Wuhan-Hu-1 isolate) | This study | A passage 9 viral stock will be distributed for in vitro non-commercial use under a UBMTA. |
| Biological Samples | ||
| Antisera from COVID-19 convalescent donors obtained with IRB approval and informed consent | This study | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Cysteine cathepsin inhibitor E-64 | Wako Chemicals | Cat# 331-40963 |
| Camostat mesylate | Tocris | Cat# 3193 |
| Cathepsin L/B inhibitor Z-Phe-tyr-dmk | Calbiochem | Cat# 219427 |
| Ammonium chloride | Acros organics | Cat# 123340010 |
| Ultra-TMB colorimetric substrate | Thermo Fisher | Cat# 34029 |
| Critical Commercial Assays | ||
| RNeasy Mini Kit (250) | QIAGEN | Cat# 7410 |
| SuperScript™ IV Reverse Transcriptase | Invitrogen | Cat# 18090050 |
| Deposited Data | ||
| Does not apply | - | - |
| Experimental Models: Cell Lines | ||
| Human hepatoma Huh7.5.1 | Laboratory of Dr. Jan Carrette (Originally from Dr. Frank Chisari) | N/A |
| 293FT | ThermoFisher | Cat# R70007 RRID: CVCL_6911 |
| Vero | ATCC | CCL-81 RRID: CVCL_0059 |
| Vero-TMPRSS2 | This study | N/A |
| Vero C1008 cells (VERO 76, clone E6, Vero E6) | ATCC | CRL-1586 RRID: CVCL_0574 |
| ExpiCHO-S cells | ThermoFisher | Cat# A29127 |
| BHK21 [C13] | Laboratory of Dr. Margaret Kielian /ATCC | CCL10 RRID: CVCL_1915 |
| BHK21-hACE2 | This study | N/A |
| FreeStyle 293F | ThermoFisher | Cat# R79007 |
| Calu3 | Laboratory of Dr. Simon Anthony/ ATCC | HTB-55 RRID: CVCL_0609 |
| A549 | Laboratory of Dr. Balaji Manicassamny/ ATCC |
CCL-185 RRID: CVCL_0023 |
| Experimental Models: Organisms/Strains | ||
| Does not apply | - | - |
| Oligonucleotides | ||
| MluI-nSARS-CoV-2 S-F CAGAGATCGATCTGTTTCCTTGACACGC GTGCCACCATGTTCGTGTTCCTG |
IDT | N/A |
| NotI-nSARS-CoV-2 S-R GTTCAAACATGAAG AATCTGTGTGCAGGGCGGCCGCTCAGGT GTAGTGCAGCTTCACG |
IDT | N/A |
| S-549F (rVSV SARS-CoV-2 S -Sanger sequencing) GGCAACTTCAAGAACCTGA GA | IDT | N/A |
| S-1159F (rVSV SARS-CoV-2 S -Sanger sequencing) TGAATGACCTGTGCTTCACC | IDT | N/A |
| S-1758F (rVSV SARS-CoV-2 S -Sanger sequencing) ATTACACCCTGCTCCTTCGG | IDT | N/A |
| S-2357F (rVSV SARS-CoV-2 S -Sanger sequencing) GCAGATCTACAAGACCCCACC | IDT | N/A |
| S-2946F (rVSV SARS-CoV-2 S -Sanger sequencing) AGACTGGACAAGGTGGAAGC | IDT | N/A |
| VSV-2860F (rVSV SARS-CoV-2 S -Sanger sequencing) AGGCCTTAATGTTTGGCCTG | IDT | N/A |
| VSV-4773R (rVSV SARS-CoV-2 S -Sanger sequencing) AAATCATTGAACTCGTCGGTCTC | IDT | N/A |
| Recombinant DNA | ||
| Plasmid: VSV antigenome plasmid | (Whelan et al., 1995) | N/A |
| Plasmids: plasmids expressing T7 polymerase and VSV N, P, M, G and L | (Witko et al., 2006) | N/A |
| Plasmid: pBabe-puro-hACE2 | This study | N/A |
| Plasmid: pBabe-puro-TMPRSS2 | This study | N/A |
| Plasmid: pBabe-puro | (Morgenstern and Land, 1990) | Addgene plasmid # 1764 |
| Plasmid: VSV-G | (Stewart et al., 2003) | Addgene plasmid # 8454 |
| Plasmid: pUMVC | (Stewart et al., 2003) | Addgene plasmid # 8449 |
| Plasmid: pCAGGS SARS-CoV-2 Spike | Laboratory of Dr. Jason McLellan | N/A |
| Plasmid: pCAGGS SARS-CoV-2 RBD | Laboratory of Dr. Florian Krammer (BEI resources) | Cat# NR-52366 |
| Synthetic, codon-optimized (humanized) SARS-2-CoV-2 S GenBank MN908947.3 | TwistBioscience | N/A |
| Plasmid: hACE2 | Laboratory of Dr. Hyeryun Choe | Addgene plasmid #1786 |
| Plasmid: TMPRSS2 | Laboratory of Dr. Roger Reeves | Addgene plasmid #53887 |
| Software and Algorithms | ||
| GraphPad Prism (version 8.3.0(538)) | GraphPad Software | https://www.graphpad.com/ |
| Adobe Photoshop (version 21.1.3) | Adobe | https://www.adobe.com/ |
| Adobe Illustrator (version 24.1.3) | Adobe | https://www.adobe.com/ |
| Pymol 2.4 | Schrödinger, LLC | https://pymol.org/2/ |
| UCSF Chimera | (Pettersen et al., 2004) | https://www.cgl.ucsf.edu/chimera/ |
| SnapGene | SnapGene | https://www.snapgene.com/ |
| Other | ||
| Zeiss Axio Observer.Z1 inverted fluorescence microscope | Carl Zeiss AG | N/A |
| Cytation 5 Cell Imaging Multi-Mode Reader | BioTek | N/A |
| Operetta High Content Imaging System | Perkin Elmer | N/A |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Kartik Chandran (kartik.chandran@einsteinmed.org).
Materials Availability
Plasmids and a passage 9 stock of rVSV-SARS-CoV-2 S generated in this study, together with documenting information, will be made available upon request and following execution of a UBMTA between Albert Einstein College of Medicine and the recipient institution. rVSV-SARS-CoV-2 S will be made available for in vitro research only (please see the ‘Limitations of the Study’ section).
Data and Code Availability
Primary data are available on request. This study did not generate any new software code.
Experimental Model and Subject Details
Cells
Human hepatoma Huh7.5.1 (received from Dr. Jan Carette; originally from Dr. Frank Chisari) and 293FT (ThermoFisher) cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM high glucose, GIBCO) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Atlanta Biologicals), 1% Penicillin/Streptomycin (P/S, GIBCO) and 1% Gluta-MAX (GIBCO). The African vervet monkey kidney Vero cells and baby hamster kidney BHK21 cells were maintained in DMEM (high glucose) supplemented with 2% heat-inactivated FBS, 1% P/S and 1% Gluta-MAX. Vero-E6 cells were grown in Minimum Essential Medium (MEM) supplemented in 10% FBS and Gentamicin (all from Sigma). A549 cells were maintained in DMEM (high glucose) supplemented with 10% heat-inactivated FBS, 1% P/S and 1% Gluta-MAX. These cell lines were passaged every 2-3 days using 0.05% Trypsin/EDTA solution (GIBCO). Calu3 cells were cultured in Eagle’s Minimum Essential Medium (EMEM, ATCC) supplemented with 10% heat-inactivated FBS, 1% P/S, and 1% Gluta-MAX and passaged weekly using 0.05% Trypsin/EDTA solution.
Generation of Cells Overexpressing hACE2 or TMPRSS2
Human ACE2 and TMPRSS2 coding sequences were PCR-amplified from the hACE2 plasmid Addgene #1786 (a generous gift from Hyeryun Choe) and TMPRSS2 plasmid Addgene #53887 (a generous gift from Roger Reeves), respectively and cloned into a retroviral pBabe-puro vector. Retroviruses were produced by transfecting 293FT cells with the hACE2 and TMPRSS2 expressing pBabe-puro plasmids along with those expressing the Moloney murine leukemia virus (MMLV) gag-pol and VSV G proteins. Retroviral supernatants passed through a 0.45-μm filter were used to transduce BHK21, Vero cells or A549 cells. Transfected cells were selected with puromycin (2 μg/mL).
Convalescent Serum Samples
Serum samples were collected from healthy adult volunteers residing in Westchester County, NY who had recovered from COVID-19 in April 2020. Patients had reported a positive nasopharyngeal swab by PCR for SARS-CoV-2 during illness and had been asymptomatic for at least 14 days prior to sample collection. After obtaining informed consent, serum was obtained by venipuncture (BD Vacutainer, serum), centrifuged, aliquoted and stored at −80°C prior to use. The sera were heat-inactivated at 56°C for 30 min and stored at 4°C prior to analysis. Protocol approval was obtained by the Institutional Review Board (IRB) of the Albert Einstein College of Medicine.
Generation of rVSV-SARS-CoV-2
A plasmid encoding the VSV antigenome was modified to replace its native glycoprotein, G, with the full-length wild-type S glycoprotein gene of the Wuhan-Hu-1 isolate of SARS-CoV-2 (GenBank MN908947.3). The VSV antigenome also encodes for an eGFP reporter gene as a separate transcriptional unit. Plasmid-based rescue of the rVSV was carried out as described previously (Kleinfelter et al., 2015; Whelan et al., 1995; Wong et al., 2010). Briefly, 293FT cells were transfected with the VSV antigenome plasmid along with plasmids expressing T7 polymerase and VSV N, P, M, G and L proteins by using polyethylenimine. Supernatants from the transfected cells were transferred to Huh7.5.1 cells every day (day 2-7 post-transfection) till the appearance of eGFP-positive cells. The poorly spreading virus was initially propagated by cell subculture. RNA was isolated from viral supernatants at different passages and Sanger sequencing was used to verify S gene sequences. A passage #9 viral stock was plaque-purified on Vero cells. Supernatants were aliquoted and stored at −80°C. The generation of rVSV-SARS-CoV-2 S and its use in tissue culture at biosafety level 2 was approved by the Environmental Health and Safety Department and the Institutional Biosafety Committee at Albert Einstein College of Medicine.
SARS-CoV-2 Stock Preparation
All work with authentic SARS-CoV-2 was completed in BSL-3 laboratories at USAMRIID in accordance with federal and institutional biosafety standards and regulations. Vero-76 cells were inoculated with SARS-CoV-2 (GenBank MT020880.1) at a MOI = 0.01 and incubated at 37°C with 5% CO2 and 80% humidity. At 50 h post-infection, cells were frozen at −80°C for 1 h, allowed to thaw at room temperature, and supernatants were collected and clarified by centrifugation at ∼2,500 × g for 10 min. Clarified supernatant was aliquoted and stored at −80°C. Sequencing data from this virus stock indicated a single mutation in the spike glycoprotein (H655Y) relative to Washington state isolate MT020880.1.
SARS-CoV2 Spike Glycoprotein RBD Expression and Purification
The pCAGGS SARS-CoV2 RBD plasmid (a generous gift from Florian Krammer) was used for the expression of recombinant RBD as previously described (Amanat et al., 2020; Stadlbauer et al., 2020). FreeStyle 293F cells (ThermoFisher Scientific) were transfected with the plasmid DNA diluted in PBS (0.67 μg total plasmid DNA per ml of culture) using polyethylenimine (Polysciences, Inc.) at a DNA-to-PEI ratio of 1:3. At 6 days post-transfection, cultures were harvested by centrifugation at 4,000 x g for 20 min, and supernatant was incubated with Ni-NTA resin (GoldBio) for 2 h at 4°C. Resin was collected in columns by gravity flow, washed with a wash buffer (50 mM Tris HCl pH 8.0, 250 mM NaCl, 20 mM Imidazole) and eluted with an elution buffer (50 mM Tris HCl pH 8.0, 250 mM NaCl, 250 mM Imidazole). Eluant was concentrated in Amicon centrifugal units (EMD Millipore) and buffer was exchanged into the storage buffer (50 mM Tris HCl pH 8.0, 250 mM NaCl). Protein was analyzed by SDS-PAGE, aliquoted, and stored at −80°C.
SARS-CoV2 Spike Glycoprotein Expression and Purification
The pCAGGS SARS-CoV2 plasmid encoding stabilized S glycoprotein gene (a generous gift from Jason McLellan) was used for the expression of recombinant S protein as described previously (Wrapp et al., 2020) with several modifications. ExpiCHO-S cells (ThermoFisher) were transiently transfected with plasmid DNA diluted in OptiPRO Serum-Free Medium (0.8 μg total DNA per ml of culture) using ExpiFectamine (ThermoFisher) at a DNA-to-ExpiFectamine ratio of 1:4. At 8 days post-transfection, cultures were harvested by centrifugation at 4,000 x g for 20 min. Clarified supernatant was dialyzed in 50 mM Tris HCl pH 8.0, 250 mM NaCl at a clarified supernatant to dialysis buffer ratio of 1:25 prior affinity chromatography. Dialyzed supernatant was incubated with Ni-NTA resin (GoldBio) for 2 h at 4°C. Resin was collected in columns by gravity flow, washed with wash buffer (50 mM Tris HCl pH 8.0, 250 mM NaCl, 20 mM Imidazole) and eluted with elution buffer (50 mM Tris HCl pH 8.0, 250 mM NaCl, 250 mM Imidazole). Eluate was concentrated in Amicon centrifugal units (EMD Millipore) and exchanged into a storage buffer (50 mM Tris HCl pH 8.0, 250 mM NaCl). Protein was analyzed by SDS-PAGE, aliquoted, and stored at −80°C.
Method Details
Detection of S Protein in rVSV-SARS-CoV-2
High-protein binding 96-well ELISA plates (Corning) were coated with 25 μl of concentrated rVSV-SARS-CoV-2 S or rVSV-EBOV (2.73 μg/mL) overnight at 4°C, and blocked with 3% nonfat dry milk in PBS (PBS-milk) for 1 h at 25°C. Plates were extensively washed and incubated with serum 18, serum 39 or negative serum diluted to 1:100 first then with serial 2-fold dilutions in PBS milk 1% -Tween 0.1% for 1 h at 25°C. Plates were washed three times and incubated with Goat anti-human IgG-HRP (#31410 Invitrogen) diluted 1:3000 (PBS milk 1% -Tween 0.1%) for 1 h at 25°C and detected using 1-Step Ultra TMB-ELISA Substrate Solution (Thermo Fisher Scientific, Waltham, MA). Plates were read using a Cytation 5 imager (BioTek) at 450 nm.
NH4Cl Inhibition Experiments
Huh7.5.1 cell monolayers were incubated for 1 h with 20–100 mM NH4Cl in DMEM. Next, pre-titrated rVSVs expressing EBOV GP or SARS-CoV-2 S were used to infect cells. Infection was scored 16-18 h later as described above.
Cathepsin Inhibitor Experiments
Monolayers of Vero cells pre-treated for 1.5 h at 37°C with E-64 (37.5 or 75 μM), Z-Phe-tyr-dmk (FYdmk, 18.75 or 37.5 μM), or 1.5% DMSO (vehicle control) were infected with pre-titrated amounts of rVSVs carrying SARS-CoV 2 S, EBOV GP or VSV G. At 1 h post-infection, 20 mM NH4Cl was added. Infected cells were fixed 16–18 h later and scored for infection as described above.
TMPRSS2 Inhibitor Experiments
Monolayers of Vero or Vero-TMPRSS2 cells pre-treated for 2 h at 37°C with camostat mesylate (Tocris) or 1% DMSO (vehicle control) were infected with pre-titrated amounts of rVSV-SARS-CoV 2 S. At 1 h post-infection, 20 mM NH4Cl was added to terminate viral entry. Infected cells were fixed 7 h later and scored for infection as described above.
Detection of hACE2 in BHK21 and A549 Transfected Cells
To stain for surface-expressed hACE2, BHK21, A549, BHK21-hACE2 and A549-hACE2 cells or the control cells were seeded onto fibronectin-coated glass coverslips were incubated with 0.4 μg/mL of hACE2-specific goat antibody (#AF933, R&D systems) at 4°C in media containing 25 mM HEPES. Next, cells were washed with cold PBS, fixed with 4% paraformaldehyde, and blocked with buffer (2% (w/v) bovine serum albumin, 5% (v/v) glycerol, 0.2% (v/v) Tween20 in Ca2+/Mg2+-free PBS). Secondary donkey AlexaFluor 594-conjugated anti-goat IgG (#A32758 Invitrogen) was used to detect the hACE2 signal. Coverslips were mounted in Prolong with DAPI (Invitrogen) and imaged on an Axio Observer inverted microscope (Zeiss).
rVSV-SARS-CoV-2 S Microneutralization Assay
Serum samples were serially diluted and incubated with virus for 1 h at room temperature. Serum-virus mixtures were then added in duplicate to 384-well plates (Corning) containing Huh7.5.1 cells or 96-well plates (Corning) containing Vero cells. Plates were incubated for 7 h at 37°C and 5% CO2. Cells were fixed with 4% paraformaldehyde (Sigma), washed with PBS, and stored in PBS containing Hoechst-33342 (Invitrogen) at a dilution of 1:2,000. Viral infectivity was measured by automated enumeration of GFP-positive cells from captured images using a Cytation5 automated fluorescence microscope (BioTek) and analyzed using the Gen5 data analysis software (BioTek). The half-maximal inhibitory concentration (IC50) of the mAbs was calculated using a nonlinear regression analysis with GraphPad Prism software.
Anti-hACE2 Antibody Blocking Assay
Huh7.5.1 cells were seeded into a 384-well plate and Calu3 cells in a 96-well plate pre-coated with 1% gelatin/PBS, respectively. Nex day, goat anti-human ACE2 antibody (#AF933, R&D Systems) was serially diluted and applied to the cells. After 1 h incubation at 37°C and 5% CO2, cells were infected with rVSV-SARS-CoV-2 S. At 16-18 h (Huh-7.5.1) or 8 h (Calu3) post-infection, cells were fixed and scored for infection as described above. Human gamma globulin (009-000-002) purchased from Jackson ImmunoResearch was used as negative control.
RBD Competition Assay
Monolayers of Huh7.5.1 cells in a 384-well plate were incubated with serial dilutions of recombinant RBD domain for 1 h at 37°C and 5% CO2. Cells were then infected with pre-titrated amounts of rVSV-SARS-CoV-2 or rVSV-EBOV GP and scored for infection 16–18 h later.
S-Mediated Antibody Depletion Assay
High-protein binding 96-well ELISA plates (Corning) coated with PBS alone or with 2 μg/mL of SARS-CoV-2 S protein in PBS overnight at 4°C were blocked for 1 h with 3% nonfat dry milk (Biorad) in PBS. Serum samples diluted in DMEM (1:50 dilution) were serially incubated 4 times for 1 h each at 37°C on S protein-coated or control wells. The depleted sera were tested for their neutralization capacity as described above.
SARS-CoV-2 Neutralization Assay
Serially diluted serum samples were mixed with pre-diluted SARS-CoV-2 in infection media (EMEM/2% FBS/Gentamicin) and incubated for 1 h at 37°C/5% CO2/80% humidity. Virus/serum inoculum was added to Vero-E6 cells, seeded in 96 well plates, at a MOI of 0.4 and incubated for 1 h at 37°C/5% CO2/80% humidity. Virus/serum inoculum was removed and cells were washed with PBS prior to addition of culture media (MEM/10% FBS/Gentamicin). Following 24 h incubation at 37°C/5% CO2/80% humidity, media was removed and cells were washed with PBS. PBS was removed and cells were submerged in 10% formalin for 24 h. Formalin was removed and cells were washed with PBS prior to permeabilization with 0.2% Triton-X for 10 min at room temperature. Cells were blocked for 2 h, then immunostained with SARS-1 nucleocapsid protein-specific antibody (Sino Biologic; Cat# 40143-R001 ) and AlexaFluor 488 labeled secondary antibody. Cells were imaged using an Operetta (Perkin Elmer) high content imaging instrument and infected cells were determined using Harmony Software (Perkin Elmer).
Syncytia Inhibition Assay
Vero cells were infected with pre-titrated amounts of rVSV-SARS-CoV-2 S for 2 h at 37°C and 5% CO2. Following the removal of virus inocula, cells were washed with PBS to remove any residual virus and indicated dilutions of convalescent sera were applied to the infected cells. Cells were fixed, their nuclei were counterstained, and syncytia formation was imaged by eGFP expression at 16 h post-infection.
Quantification and Statistical Analysis
The n number associated with each dataset in the figures indicates the number of biologically independent samples. The number of independent experiments and the measures of central tendency and dispersion used in each case are indicated in the figure legends. Dose-response neutralization curves were fit to a logistic equation by nonlinear regression analysis. Unless otherwise indicated in the figure legends, statistical comparisons were carried out by two-way ANOVA with a post hoc correction for family-wise error rate (Dunnett test for comparison of an untreated sample mean to treated sample means, Tukey test for all possible comparisons of sample means). Testing level (alpha) was 0.05 for all statistical tests. All analyses were carried out in GraphPad Prism 8.
Acknowledgments
We thank I. Gutierrez, E. Valencia, L. Polanco, and S. Diaz for laboratory management and technical support. We thank J. McLellan for his generous gifts of wild-type and recombinant SARS-CoV-2 spike constructs. We also thank F. Krammer, H. Choe, and R. Reeves for their generous gifts of SARS-CoV-2 RBD, hACE2, and TMPRSS2 constructs, respectively. We are grateful to J. Carette for his generous gift of Huh-7.5.1 cells and to S. Anthony, E. Choi, B. Gomperts, B. Maniccasamy, K. Stapleford, and C. Sen for their generous gifts of airway epithelial cell lines. This work was supported in part by National Institutes of Health (NIH) grants U19AI142777 and R01AI132633 (to K.C.), R01AI143453 and R01AI123654 (to L.P.), R01AI125462 (to J.R.L.) and R21AI141367 (to J.P.D). M.E.D. is a Latin American Fellow in the Biomedical Sciences, supported by the Pew Charitable Trusts. R.H.B. and R.J.M. were partially supported by NIH training grant 2T32GM007288-45 (Medical Scientist Training Program) at Albert Einstein College of Medicine. Opinions, conclusions, interpretations, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army. The mention of trade names or commercial products does not constitute endorsement or recommendation for use by the Department of the Army or the Department of Defense.
Author Contributions
Conceptualization, M.E.D., D.H., K.C., R.K.J.; Methodology, M.E.D., D.H., J.M.D., A.S.H., K.C., R.K.J.; Investigation, M.E.D., D.H., R.H.B., G.L., O.V., S.A.A., A.S.H., R.K.J.; Resources, M.E.D., D.H., R.H.B., A.S.W., O.V., J.M.F., E.L., C.F., R.J.M., G.G., L.-a.P., J.P.D., J.M.D., J.R.L., K.C., R.K.J.; Data Curation, M.E.D., D.H., R.H.B., A.S.W., E.L., C.F., A.M., D.K., J.Q., K.C., R.K.J.; Writing—Original Draft, M.E.D., D.H., A.S.H., K.C., R.K.J.; Writing—Review & Editing, all authors; Visualization, M.E.D., D.H., R.H.B., G.L., K.C., R.K.J.; Supervision, L.-a.P., J.P.D., J.M.D., J.R.L., A.S.H., K.C., R.K.J.; Project administration, K.C., R.K.J.; Funding Acquisition, J.B., L.-a. P., J.P.D., J.M.D., J.R.L., K.C.
Declaration of Interests
K.C. is a member of the scientific advisory board of Integrum Scientific, LLC.
Published: September 9, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.chom.2020.06.020.
Supplemental Information
References
- Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020 doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestle D., Heindl M.R., Limburg H., Van Lam van T., Pilgram O., Moulton H., Stein D.A., Hardes K., Eickmann M., Dolnik O. TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-CoV-2 in human airway epithelial cells and provide promising drug targets. bioRxiv. 2020 doi: 10.1101/2020.04.15.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch B.J., van der Zee R., de Haan C.A.M., Rottier P.J.M. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher-Friebertshäuser E., Freuer C., Sielaff F., Schmidt S., Eickmann M., Uhlendorff J., Steinmetzer T., Klenk H.-D., Garten W. Cleavage of influenza virus hemagglutinin by airway proteases TMPRSS2 and HAT differs in subcellular localization and susceptibility to protease inhibitors. J. Virol. 2010;84:5605–5614. doi: 10.1128/JVI.00140-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher-Friebertshäuser E., Garten W., Matrosovich M., Klenk H.D. The hemagglutinin: a determinant of pathogenicity. Curr. Top. Microbiol. Immunol. 2014;385:3–34. doi: 10.1007/82_2014_384. [DOI] [PubMed] [Google Scholar]
- Caì Y., Yú S., Jangra R.K., Postnikova E.N., Wada J., Tesh R.B., Whelan S.P.J., Lauck M., Wiley M.R., Finch C.L. Human, nonhuman primate, and bat cells are broadly susceptible to tibrovirus particle cell entry. Front. Microbiol. 2019;10:856. doi: 10.3389/fmicb.2019.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L., Zhu Q., Zhang X., Zheng Y., Geng C. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 2020;182:73–84.e16. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case J.B., Rothlauf P.W., Chen R.E., Liu Z., Zhao H., Kim A.S., Bloyet L.-M., Zeng Q., Tahan S. Neutralizing antibody and soluble ACE2 inhibition of a replication-competent VSV2 SARS-CoV-2 and a clinical isolate of SARS-CoV-2. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaipan C., Kobasa D., Bertram S., Glowacka I., Steffen I., Tsegaye T.S., Takeda M., Bugge T.H., Kim S., Park Y. Proteolytic activation of the 1918 influenza virus hemagglutinin. J. Virol. 2009;83:3200–3211. doi: 10.1128/JVI.02205-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.-W., Zhang A.J., Yuan S., Poon V.K.-M., Chan C.C.-S., Lee A.C.-Y., Chan W.-M., Fan Z., Tsoi H.-W., Wen L. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin. Infect. Dis. 2020:ciaa325. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.-Y., Bertram S., Glowacka I., Park Y.W., Pöhlmann S. Type II transmembrane serine proteases in cancer and viral infections. Trends Mol. Med. 2009;15:303–312. doi: 10.1016/j.molmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Chu H., Chan J.F.-W., Wang Y., Yuen T.T.-T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020:ciaa410. doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi S., Mizutani T., Saijo M., Matsuyama S., Miyajima N., Taguchi F., Itamura S., Kurane I., Morikawa S. Vesicular stomatitis virus pseudotyped with severe acute respiratory syndrome coronavirus spike protein. J. Gen. Virol. 2005;86:2269–2274. doi: 10.1099/vir.0.80955-0. [DOI] [PubMed] [Google Scholar]
- Fukushi S., Mizutani T., Saijo M., Kurane I., Taguchi F., Tashiro M., Morikawa S. Evaluation of a novel vesicular stomatitis virus pseudotype-based assay for detection of neutralizing antibody responses to SARS-CoV. J. Med. Virol. 2006;78:1509–1512. doi: 10.1002/jmv.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt J., Tamin A., Lu X., Kamili S., Sakthivel S.K., Murray J., Queen K., Tao Y., Paden C.R., Zhang J. Vol. 26. 2020. pp. 1266–1273. (Severe Acute Respiratory Syndrome Coronavirus 2 From Patient With Coronavirus Disease, United States). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Pöhlmann S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell. 2020;78:779–784.e5. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K.P.Y., Cheung M.-C., Perera R.A.P.M., Ng K.-C., Bui C.H.T., Ho J.C.W., Ng M.M.T., Kuok D.I.T., Shih K.C., Tsao S.-W. Tropism, replication competence, and innate immune responses of the coronavirus SARS-CoV-2 in human respiratory tract and conjunctiva: an analysis in ex-vivo and in-vitro cultures. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30193-4. S2213-2600(20)30193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jae L.T., Raaben M., Riemersma M., van Beusekom E., Blomen V.A., Velds A., Kerkhoven R.M., Carette J.E., Topaloglu H., Meinecke P. Deciphering the glycosylome of dystroglycanopathies using haploid screens for lassa virus entry. Science. 2013;340:479–483. doi: 10.1126/science.1233675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangra R.K., Herbert A.S., Li R., Jae L.T., Kleinfelter L.M., Slough M.M., Barker S.L., Guardado-Calvo P., Román-Sosa G., Dieterle M.E. Protocadherin-1 is essential for cell entry by New World hantaviruses. Nature. 2018;563:559–563. doi: 10.1038/s41586-018-0702-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H.P., Look D.C., Shi L., Hickey M., Pewe L., Netland J., Farzan M., Wohlford-Lenane C., Perlman S., McCray P.B., Jr. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J. Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia S.U., Rose J.K., Lamirande E., Vogel L., Subbarao K., Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340:174–182. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia S.U., Simon I.D., Rose J.K. SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector. Virology. 2008;376:165–172. doi: 10.1016/j.virol.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J. Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfelter L.M., Jangra R.K., Jae L.T., Herbert A.S., Mittler E., Stiles K.M., Wirchnianski A.S., Kielian M., Brummelkamp T.R., Dye J.M., Chandran K. Haploid genetic screen reveals a profound and direct dependence on cholesterol for hantavirus membrane fusion. MBio. 2015;6:e00801. doi: 10.1128/mBio.00801-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C., Qian K., Li T., Zhang S., Fu W., Ding M., Hu S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020;11:2070. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limburg H., Harbig A., Bestle D., Stein D.A., Moulton H.M., Jaeger J., Janga H., Hardes K., Koepke J., Schulte L. TMPRSS2 is the major activating protease of influenza A virus in primary human airway cells and influenza B virus in human type II pneumocytes. J. Virol. 2019;93:e00649-19. doi: 10.1128/JVI.00649-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Wang J., Shao Y., Wang X., Zhang H., Shuai L., Ge J., Wen Z., Bu Z. A recombinant VSV-vectored MERS-CoV vaccine induces neutralizing antibody and T cell responses in rhesus monkeys after single dose immunization. Antiviral Res. 2018;150:30–38. doi: 10.1016/j.antiviral.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier K.E., Jangra R.K., Shieh K.R., Cureton D.K., Xiao H., Snapp E.L., Whelan S.P., Chandran K., Levy M. A new transferrin receptor aptamer inhibits new world hemorrhagic fever mammarenavirus entry. Mol. Ther. Nucleic Acids. 2016;5:e321. doi: 10.1038/mtna.2016.32. [DOI] [PubMed] [Google Scholar]
- McBride C.E., Li J., Machamer C.E. The Cytoplasmic Tail of the Severe Acute Respiratory Syndrome Coronavirus Spike Protein Contains a Novel Endoplasmic Reticulum Retrieval Signal That Binds COPI and Promotes Interaction with Membrane Protein. Journal of Virology. 2007;81:2418–2428. doi: 10.1128/JVI.02146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J.P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossel E.C., Huang C., Narayanan K., Makino S., Tesh R.B., Peters C.J. Exogenous ACE2 expression allows refractory cell lines to support severe acute respiratory syndrome coronavirus replication. J. Virol. 2005;79:3846–3850. doi: 10.1128/JVI.79.6.3846-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., Zhang L., Nie L., Qin H., Wang M. Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2. Emerg. Microbes Infect. 2020;9:680–686. doi: 10.1080/22221751.2020.1743767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimishakavi S., Raymond W.W., Gruenert D.C., Caughey G.H. Divergent Inhibitor Susceptibility among Airway Lumen-Accessible Tryptic Proteases. PLoS One. 2015;10:e0141169. doi: 10.1371/journal.pone.0141169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYU Langone Health/Albert Einstein College of Medicine Convalescent Plasma to Limit COVID-19 Complications in Hospitalized Patients. ClinicalTrials.gov Identifier: NCT04364737. 2020. https://clinicaltrials.gov/ct2/show/NCT04364737?term=Montefiore&cond=COVID-19&draw=2&rank=2 Retrieved on May, 19th 2020.
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pinto D., Park Y.-J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- Pu T., Ding C., Li Y., Liu X., Li H., Duan J., Zhang H., Bi Y., Cun W. Evaluate severe acute respiratory syndrome coronavirus 2 infectivity by pseudoviral particles. J. Med. Virol. 2020 doi: 10.1002/jmv.25865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaben M., Jae L.T., Herbert A.S., Kuehne A.I., Stubbs S.H., Chou Y.-Y., Blomen V.A., Kirchhausen T., Dye J.M., Brummelkamp T.R., Whelan S.P. NRP2 and CD63 are host factors for lujo virus cell entry. Cell Host Microbe. 2017;22:688–696.e5. doi: 10.1016/j.chom.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M.M., Oude Munnink B.B., de Meulder D., van Amerongen G., van den Brand J., Okba N.M.A. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.T., Limbo O., Smith C., Song G., Woehl J. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020:eabc7520. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L.W., Mao H.J., Wu Y.L., Tanaka Y., Zhang W. TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1–10. doi: 10.1016/j.biochi.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slough M.M., Chandran K., Jangra R.K. Two point mutations in old world hantavirus glycoproteins afford the generation of highly infectious recombinant vesicular stomatitis virus vectors. MBio. 2019;10:e02372-18. doi: 10.1128/mBio.02372-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer D., Amanat F., Chromikova V., Jiang K., Strohmeier S., Arunkumar G.A., Tan J., Bhavsar D., Capuano C., Kirkpatrick E. SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr. Protoc. Microbiol. 2020;57:e100. doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S.A., Dykxhoorn D.M., Palliser D., Mizuno H., Yu E.Y., An D.S., Sabatini D.M., Chen I.S.Y., Hahn W.C., Sharp P.A. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.W., Chia W.N., Chen M.I.-C., Hu Z., Young B.E., Tan Y.-J., Yi Y., Lye D.C., Anderson D.E., Wang L.-F. A SARS-CoV-2 surrogate virus neutralization test (sVNT) based on antibody-mediated blockage of ACE2-spike (RBD) protein-protein interaction. 2020. 10.1002/jmv.25865 [DOI] [PubMed]
- Ujike M., Huang C., Shirato K., Makino S., Taguchi F. The contribution of the cytoplasmic retrieval signal of severe acute respiratory syndrome coronavirus to intracellular accumulation of S proteins and incorporation of S protein into virus-like particles. Journal General Virology. 2016;97:1853–1864. doi: 10.1099/jgv.0.000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.-Y. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell. 2020;181:894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wec A.Z., Wrapp D., Herbert A.S., Maurer D.P., Haslwanter D., Sakharkar M., Jangra R.K., Dieterle M.E., Lilov A., Huang D. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science. 2020:eabc7424. doi: 10.1126/science.abc7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan S.P., Ball L.A., Barr J.N., Wertz G.T. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc. Natl. Acad. Sci. USA. 1995;92:8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witko S.E., Kotash C.S., Nowak R.M., Johnson J.E., Boutilier L.A.C., Melville K.J., Heron S.G., Clarke D.K., Abramovitz A.S., Hendry R.M. An efficient helper-virus-free method for rescue of recombinant paramyxoviruses and rhadoviruses from a cell line suitable for vaccine development. J. Virol. Methods. 2006;135:91–101. doi: 10.1016/j.jviromet.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Wong A.C., Sandesara R.G., Mulherkar N., Whelan S.P., Chandran K. A forward genetic strategy reveals destabilizing mutations in the Ebolavirus glycoprotein that alter its protease dependence during cell entry. J. Virol. 2010;84:163–175. doi: 10.1128/JVI.01832-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Situation report-147, Coronavirus disease (COVID-19) 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200615-covid-19-sitrep-147.pdf?sfvrsn=2497a605_2 Retrieved on June 15th, 2020.
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang F., Shen C., Peng W., Li D., Zhao C., Li Z., Li S., Bi Y., Yang Y. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020;368:1274–1278. doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H.-L., Wu Y.-T., Cao J.-L., Yang R., Ma J., Qiao X.-Y., Yao X.-Y., Zhang B.-H., Zhang Y.-L., Hou W.-H. Robust neutralization assay based on SARS-CoV-2 S-bearing vesicular stomatitis virus (VSV) pseudovirus and ACE2-overexpressed BHK21 cells. bioRxiv. 2020 doi: 10.1101/2020.04.08.026948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Dong N., Chan W.-C., Chen S. MedRxiv; 2020. Identification of super-transmitters of SARS-CoV-2. [DOI] [Google Scholar]
- Zang R., Gomez Castro M.F., McCune B.T., Zeng Q., Rothlauf P.W., Sonnek N.M., Liu Z., Brulois K.F., Wang X., Greenberg H.B. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020;5:eabc3582. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Vedantham P., Lu K., Agudelo J., Carrion R., Jr., Nunneley J.W., Barnard D., Pöhlmann S., McKerrow J.H., Renslo A.R., Simmons G. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zost S.J., Gilchuk P., Chen R.E., Case J.B., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N., Chen E.C. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. bioRxiv. 2020 doi: 10.1101/2020.05.12.091462. 2020.05.12.091462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Primary data are available on request. This study did not generate any new software code.