Abstract
Aim
The novel coronavirus infection (COVID-19), now a worldwide public health concern is associated with varied fatality. Patients with chronic underlying conditions like diabetes and hypertension have shown worst outcomes. The understanding of the association might be helpful in early vigilant monitoring and better management of COVID-19 patients at high risk. The aim of the meta-analysis was to assess the association of diabetes and hypertension with severity of disease.
Methods
A literature search was conducted using the databases PubMed and Cochrane until March 31, 2020. Seven studies were included in the meta- analysis, including 2018 COVID-19 patients.
Results
Diabetes was lower in the survivors (OR: 0.56; 95%CI: 0.35–0.90; p = 0.017; I2: 0.0%) and non-severe (OR: 1.66; 95%CI: 1.20–2.30; p = 0.002; I2: 0.0%) patients. No association of diabetes was found with ICU care. Hypertension was positively associated with death (OR: 0.49; 95%CI: 0.34–0.73; p<0.001; I2: 0.0%), ICU care (OR: 0.42; 95%CI: 0.22–0.81; p = 0.009; I2: 0.0%) and severity (OR: 2.69; 95%CI: 1.27–5.73; p = 0.01; I2: 52.4%).
Conclusions
Our findings suggest that diabetes and hypertension have a negative effect on health status of COVID-19 patients. However, large prevalence studies demonstrating the consequences of comorbid diabetes and hypertension are urgently needed to understand the magnitude of these vexatious comorbidities.
Keywords: COVID-19, Coronavirus, Diabetes, Hypertension, SARS-CoV-2
1. Introduction
The novel coronavirus (COVID-19) pandemic is now a worldwide public health concern [1] and is a progressive public health emergency of global significance [2]. The first case was reported to in Wuhan province of China on 31 December 2019 with unexplained lower respiratory infections [3]. The disease has rapid human to human transmission capacity and varied fatality, due to acute respiratory distress syndrome (ARDS), multi-organ failure and other serious complications [4]. These viruses are termed as the Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) as it is very similar to the one that caused the SARS outbreak [3]. They are a large family of single-stranded RNA viruses (+ssRNA) that can be isolated in different animal species [5]. According to recent estimates, 2% of the population is suggested to be healthy carriers of Coronavirus and these viruses are responsible for 5% to 10% of acute respiratory infections [6]. WHO reports above 2,241,359confirmed cases worldwide since the beginning of the epidemic, 152,551deaths covering 213 countries. The epidemiological scenario has been changed drastically, as on beginning of the April. Data obtained from the WHO Health Emergency Dashboard (April 19, 2020, 5:00 pm CEST) showed countries with most confirmed cases are USA (695,353), Spain (191,726), Italy (175,925) Germany (139,897), United Kingdom (114,221), France (110,721) followed by epicenter China (84,201). In India, 15,172 cases confirmed clinically and, in the laboratory, and 507 deaths are reported [3].
Primarily, the COVID-19 patients have fever, myalgia or fatigue, and dry cough at the time of admission [7]. Although most patients are thought to have a favorable prognosis, older patients and those with chronic underlying conditions like diabetes, hypertension, coronary heart diseases etc. show worse outcomes [8], [9]. Patients with severe illness develop dyspnea and hypoxemia within 1 week after onset of the disease, which might quickly progress to ARDS or end-organ failure [8]. Various studies have reported the clinical characteristics of patients with covid-19 who need management in intensive care units. Old age and comorbidities, particularly hypertension and diabetes, are believed to be risk factors for disease severity and death in SARS-Cov-2 infected patients [1], [7], [10]. Another study also reported comorbid hypertension being the most common, followed by diabetes and coronary heart disease [11]. Several studies have demonstrated higher prevalence of diabetes in COVID-19 patients, however, the effect of the prevalence of diabetes on severity of the disease needs further exploration. The understanding of the association might be helpful in early vigilant monitoring and better management of COVID-19 patients at high risk leading to ARDS, organ failure and death. Thus, in this meta-analysis we aim to assess the association of diabetes and hypertension with severity of disease.
2. Materials and methods
This systematic literature review followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines extension for scoping reviews [12].
2.1. Data sources and searches
We searched PubMed, Medline and Cochrane until March 31, 2020 using the keywords “COVID-19 and diabetes”, “2019 novel coronavirus and diabetes”, “2019 novel coronavirus and hypertension” and “Covid-19 and hypertension”. We also searched grey literature using Google Scholar and reference list of eligible articles.
2.2. Inclusion and exclusion
The studies describing the prevalence of diabetes and hypertension according to disease severity were included. We excluded duplicate publications, reviews, editorials, case reports, letters, meta-analysis, protocols, studies in language other than English and studies not reporting the required data. First author (RP) searched data and screened article for eligibility. Senior author (NA) double checked all the included articles and any dispute was resolved by consensus.
2.3. Quality assessment
Two reviewers (RP and NS) assessed the quality of data in the included studies using the National Institute of Health (NIH) quality assessment tools [13]. We preferred the NIH tool because it is comprehensive and widely accepted for an exhaustive assessment of data quality. We rated the overall quality of included studies as good, fair and poor, and incorporated them in the meta-analysis results.
2.4. Data extraction
Data were inputted into a standardized data extraction table (Excel) and independently checked by a second reviewer (NS) for accuracy. The following variables were extracted: name of the first author, year of publication, study design, age, gender, current smoking, number of patients in severe and non-severe condition with comorbidities hypertension and diabetes. The included studies designated severe group as having respiratory distress, RR ≥ 30 beats/minute in a resting state, a mean oxygen saturation of ≤ 93%, and an arterial blood oxygen partial pressure (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg [14].
2.5. Data synthesis
We performed an exploratory meta-analysis to understand the magnitude and direction of effect estimate. Odds ratios (ORs) were calculated and presented with respective 95% confidence intervals (CIs). Mantel-Haenszel random-effects meta-analysis using DerSimonian and Laird method was used to pool ORs [15]. Heterogeneity between studies was assessed using the χ2-based Cochran's Q statistic (p < 0.1 considered as the presence of heterogeneity) and I-squared (I2) statistics (>50% representing moderate heterogeneity) [15]. Forest plot was produced and studies were grouped according to their design. Publication bias was not assessed as a total number of studies were less than ten [15].
3. Results
3.1. Search results
The systematic search yielded a total of 105 publications. Out of 105 studies 40 studies were found using the keyword” COVID-19 and diabetes”, 16 studies with keyword “2019 novel coronavirus and diabetes”, 33 studies with the term “Covid-19 and hypertension”, and 14 studies with keyword “2019 novel coronavirus and hypertension”. Two studies were found from other sources. After removing duplicates, 53 articles were found to be potential publications for screening. After the application of predefined inclusion and exclusion criteria, a total of seven studies were included for the meta-analysis (see Fig. 1 ).
Fig. 1.
Flow diagram of the number of studies screened and included in the meta-analysis.
3.2. Study characteristics
All the included studies were conducted in China. The prevalence of diabetes and hypertension were reported in survivors and non-survivors in two studies, ICU care and non-ICU care patients in two studies, while the prevalence of diabetes and hypertension in severe and non-severe patients was reported in three studies. Among the seven included studies, three were cohort studies [11], [14], [16], while four were case-series [1], [7], [10], [17]. The studies enrolled a total of 2018 patients, including 1175 males and 840 females. The demographic characteristics of the subjects included in these studies are provided in Table 1 .
Table 1.
Demographic characteristics.
| Author, year | Study design | Groups | No. of patients | Age | Sex |
Current smoker | |
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| Chen et al, 2020 | Case series | Total | 274 | 62.0 (44.0–70.0) | 171 (62) | 103 (38) | 12 (4) |
| Deaths | 113 | 68.0 (62.0–77.0) | 83 (73) | 30 (27) | 7 (6) | ||
| Recovered patients | 161 | 51.0 (37.0–66.0) | 88 (55) | 73 (45) | 5 (3) | ||
| P value | NA | NA | NA | ||||
| Zhou et al, 2020 | Cohort | Total | 191 | 56·0 (46·0–67·0) | 119 (62) | 72 (38) | 11 (6) |
| Deaths | 54 | 69·0 (63·0–76·0) | 38 (70) | 16 (30) | 5 (9) | ||
| Recovered patients | 137 | 52·0 (45·0–58·0) | 81 (59) | 56 (41) | 6 (4) | ||
| Huang et al, 2020 | Case-series | Total | 41 | 49·0 (41·0–58·0) | 30 (73) | 11 (27) | 3 (7) |
| ICU care | 13 | 49·0 (41·0–61·0) | 11 (85) | 2 (15) | 0 | ||
| Non-ICU care | 28 | 49·0 (41·0–57·5) | 19 (68) | 9 (32) | 3 (11) | ||
| P value | 0·60 | 0·24 | 0·31 | ||||
| Wang et al, 2020 | Case-series | Total | 138 | 56 (42–68) | 75 (54.3) | 63 (45.7) | NA |
| ICU care | 36 | 66 (57–78) | 22 (61.1) | 14 (38.9) | NA | ||
| Non-ICU care | 102 | 51 (37–62) | 53 (52.0) | 49 (48.0) | NA | ||
| Jin-jin et, 2020 | Cohort | Total | 140 | 57 (25–87) | 71 (50.7) | 69 (49.3) | 2 (1.4) |
| Non-severe | 82 | 51.5 (26–78) | 38 (46.3) | 44 (53.7) | 0 (0) | ||
| Severe | 58 | 64 (25–87) | 33 (56.9) | 25 (43.1) | 2 (3.4) | ||
| P value | < 0.001 | 0.219 | 0.17 | ||||
| Wan et al, 2020 | Case-series | Total | 135 | 47 (36–55) | 72 (53.3) | 63 (46.7) | 9 (6.7) |
| Non-severe | 95 | 44 (33–49) | 52 (54.7) | 43 (45.3) | 8 (8.4) | ||
| Severe | 40 | 56 (52–73) | 21 (52.5) | 19 (47.5) | 1 (2.5) | ||
| Guan et al, 2020 | Cohort | Total | 1099 | 47.0 (35.0–58.0) | 637 (58.1)* | 459 (41.9)* | 137 (12.6)^ |
| Non-severe | 926 | 45.0 (34.0–57.0) | 537 (58.2)** | 386 (41.8)** | 108 (11.8)^^ | ||
| Severe | 173 | 52.0 (40.0–65.0) | 100 (57.8) | 73 (42.2) | 29 (172)^^^ | ||
Data is presented as Median (IQR) or number (%).
No., number; ICU, intensive care unit; NA, not available; IQR, inter quartile range.
*Out of 1096, **Out of 923, ^Out of 1085, ^^Out of 913, ^^^Out of 172.
3.3. Quality assessment
We assessed the quality of data in the included studies using the NIH quality assessment tools. The quality assessment indicated that most included studies were of acceptable quality. All the papers clearly stated the research question or objective, the study population was clearly specified and defined and all the subjects were selected from the same or similar populations. The detailed result of the quality assessment is provided in Supplementary File.
3.4. Association between diabetes and disease severity
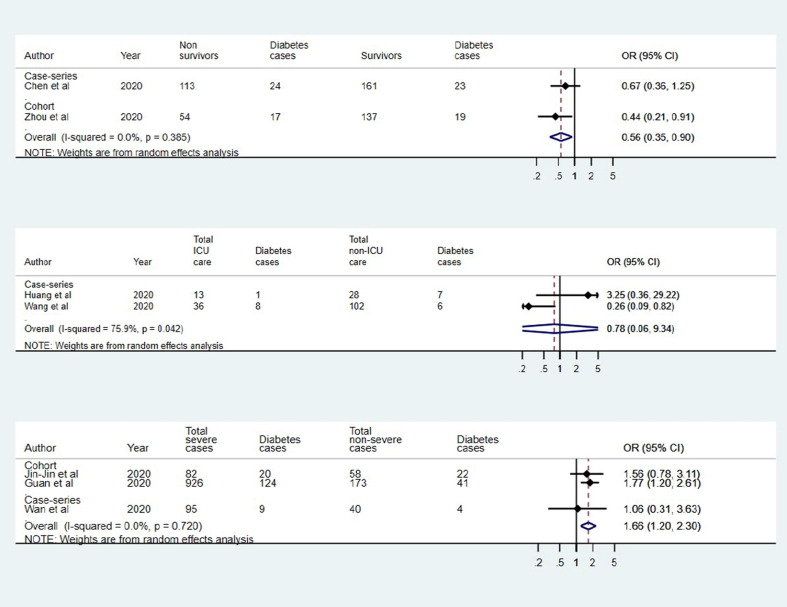
For death outcome, two studies (one case-series and one cohort) qualified for inclusion in quantitative analysis. The pooled estimate (OR: 0.56; 95% CI: 0.35–0.90; p = 0.017; I2: 0.0%) suggested that diabetes was significantly lower in the survivors (Fig. 2 a). The analysis of two case series suggested that diabetes was not statistically associated ICU care (OR: 0.78; 95% CI: 0.06–9.34; p = 0.842; I2: 75.9%) with substantial heterogeneity in individual study estimates (Fig. 2b). Further, the pooled estimate of two cohort studies and one case-series suggested significant association between diabetes and severity (OR: 1.66; 95% CI: 1.20–2.30; p = 0.002; I2: 0.0%) (Fig. 2c).
Fig. 2.
Association of diabetes and covid-19 in (a) survivors and non-survivors, (b) ICU and non-ICU care, and (c) severe and non-severe.
3.5. Association between hypertension and disease severity
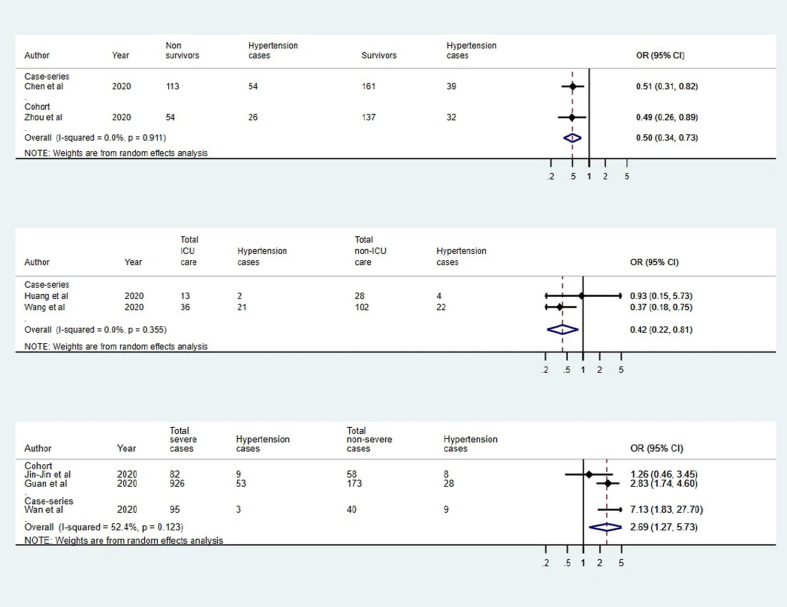
For death outcome, two studies (one case-series and one cohort) qualified for inclusion in quantitative analysis. The pooled estimate (OR: 0.49; 95% CI: 0.34–0.73; p <0.001; I2: 0.0%) suggested that hypertension was significantly lower in the survivors (Fig. 3 a). Similarly, the analysis of two case series suggested that hypertension was statistically associated with ICU care (OR: 0.42; 95% CI: 0.22–0.81; p = 0.009; I2: 0.0%) (Fig. 3b). Further, the pooled estimate of two cohort studies and one case-series suggested significant association between hypertension and severity (OR: 2.69; 95% CI: 1.27–5.73; p = 0.01; I2: 52.4%) (Fig. 3c).
Fig. 3.
Association of hypertension and covid-19 in (a) survivors and non-survivors, (b) ICU and non-ICU care, and (c) severe and non-severe.
4. Discussion
Recent evidence on SARS-CoV-2 suggest that the presence of comorbidities increase mortality risk in COVID-19 patients. Diabetes is one the most important components to predict adverse outcomes [18]. Thus, the present meta-analysis was conducted to assess the association of diabetes with disease severity in COVID-19 patients. The present meta-analysis was based on data from 7 studies on COVID-19 patients. The present meta-analysis demonstrated that the prevalence of diabetes is lower in survivors than in non-survivors of COVID-19 patients. However, there was no difference in diabetes prevalence in patients requiring or not requiring ICU care. Additionally, a positive association between diabetes and disease severity was found. Several studies have demonstrated higher prevalence of diabetes in COVID-19 patients, however, the effect of the prevalence of diabetes on severity of the disease needs further exploration. A recent meta-analysis on the comorbidities suggested diabetes as one of the most prevalent comorbidities (8 ± 6, 95% CI 6–11%), in COVID-19 patients. However, no significant difference was found in diabetes between severe and non-severe group [19]. Another similar meta-analysis demonstrated the pooled prevalence of diabetes to be 7.87% (95%CI 6.57–9.28%) [20]. A meta-analysis reported the proportions of diabetes in patients with COVID-19 to be 9.7%. The incidences of diabetes were about two folds higher in ICU/severe cases than in their non-ICU/severe counterparts [18]. A retrospective study showed that 85.54% of severe patients had diabetes or cardiovascular diseases, which was significantly higher than that of the mild group [21]. A case-series reported 58% COVID-19 patients to have comorbid diabetes mellitus [22].
Although the pathophysiology involved in this comorbidity remains unexplained, several hypotheses have been suggested. Blood glucose level is suggested to play an essential role in the pathogenesis of infectious diseases. The immune system of diabetes patients might be altered by the abnormal blood glucose level, resulting in dysregulation and reduced responses of immune components. Consequently, these patients are susceptible to SARS-CoV-2 and various other types of bacteria [21]. Another hypothesis states that, human pathogenic coronaviruses, SARS-CoV and SARSCoV-2 bind to their target cells through angiotensin-converting enzyme 2 (ACE2), which is expressed by epithelial cells of the lung, intestine, kidney, and blood vessels. The expression of ACE2 is significantly increased in diabetes patients, being treated with ACE inhibitors and angiotensin II type-I receptor blockers. Use of thiazolidinediones and ibuprofen can also be increased ACE2. Consequently, the increased expression of ACE2 would facilitate infection with COVID-19 [23].
The present meta-analysis showed that hypertension is lower in survivors than in non-survivors of COVID-19 patients. Additionally, a positive association of hypertension with disease severity and ICU care was found. A recent meta-analysis demonstrated hypertension to be the most prevalent comorbidity (17 ± 7, 95% CI 14–22%) in COVID-19 patients. Additionally, higher risk with hypertension was found in the severe group compared to those in Non-severe group, (OR 2.36, 95% CI: 1.46–3.83) [19]. According to another meta-analysis, the pooled prevalence of hypertension in people infected with SARS-CoV-2 were estimated as 16.37% (95%CI: 10.15–23.65%) [20]. A meta-analysis reported the proportions of hypertension in patients with COVID-19 to be 17.1%. The incidence of hypertension were about two folds higher in ICU/severe cases than in their non-ICU/severe counterparts [18]. Hypertension is treated with ACE inhibitors and ARBs, which results in an upregulation of ACE2 and SARSCoV-2 binds to their target cells through ACE2. Consequently, the increased expression of ACE2 in hypertension patients receiving ACE inhibitors would facilitate infection with COVID-19 [23].
Diseases such as hypertension, diabetes and cardiovascular diseases, and their susceptibility conditions, may be linked to the pathogenesis of COVID-19. Chronic diseases share several standard features with infectious disorders, such as the proinflammatory state, and the attenuation of the innate immune response. Patients having any comorbidity had poorer clinical outcomes than those without any comorbidity. A higher number of comorbidities correlated with poorer clinical outcomes. A thorough assessment of comorbidities may help establish risk stratification of patients with Covid-19 upon hospital admission [16], [19]. Major gaps in the knowledge of the origin, epidemiology, duration of human transmission, and clinical spectrum of disease need fulfilment by future studies [7]. There are several limitations that needs to be mentioned. The number of studies included in the meta-analysis is small. Substantial heterogeneity was observed among the studies. Additionally, case-series were included in the present meta-analysis. Although we did an extensive search, we may have inadvertently missed relevant studies. Exclusion of studies in languages other than English may have resulted in missing of relevant studies.
To conclude, our findings showed that comorbid diabetes and hypertension have a negative effect on health status of COVID-19 patients. However, large prevalence studies demonstrating the consequences of comorbid diabetes and hypertension are urgently needed to understand the magnitude of these vexatious comorbidities. Extensive studies are required to fill the major gaps in understanding of the disease to establish risk stratification of the patients.
Contributors
All authors contributed significantly to this manuscript.
Declaration of Competing Interests
None declared.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2020.108295.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with, novel coronavirus-infected pneumonia in Wuhan China. JAMA. 2019;2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khot W.Y., Nadkar M.Y. The 2019 novel coronavirus outbreak – a global threat. J Assoc Physicians India. 2020 Mar;68(3):67–71. [PubMed] [Google Scholar]
- 3.https://www.who.int. accessed on 6 April, 2020.
- 4.Lian J., Jin X., Hao S., et al. Analysis of Epidemiological and Clinical features in older patients with Corona Virus Disease, (COVID-19) out of Wuhan. Clin Infect Dis. 2019;2020 doi: 10.1093/cid/ciaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cascella M, Rajnik M, Cuomo A, et al. Features, Evaluation and Treatment Coronavirus (COVID-19) [Updated 2020 Mar 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/. [PubMed]
- 7.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher and Heymann Q&A: The novel coronavirus outbreak causing COVID-19. BMC Med. 2020;18:57. doi: 10.1186/s12916-020-01533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2019;2020 doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Fei, Ting Yu., Ronghui Du., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tricco A.C., Lillie E., Zarin W., et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 13.https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 14.Jin-Jin Z., Dong X., Cao Y.Y., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT GS (March 2011) Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
- 16.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2019;2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan S., Xiang Y., Fang W., et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020 doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B., Yang J., Zhao F., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020 doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J., Zheng Y., Gou X., et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Diseases. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emami A., Javanmardi F., Pirbonyeh N., et al. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J., Li W., Shi X., et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med. 2020 doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 22.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically Ill patients in the seattle region - case series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.