Abstract
(Hydroxy)chloroquine ((H)CQ) is being investigated as a treatment for COVID-19, but studies have so far demonstrated either no or a small benefit. However, these studies have been mostly performed in patients admitted to the hospital and hence likely already (severely) affected. Another suggested approach uses prophylactic (H)CQ treatment aimed at preventing either severe acute respiratory syndrome coronavirus 2 infection or the development of disease. A substantial number of clinical trials are planned or underway aimed at assessing the prophylactic benefit of (H)CQ. However, (H)CQ may lead to QT prolongation and potentially induce life-threatening arrhythmias. This may be of particular relevance to patients with preexisting cardiovascular disease and those taking other QT-prolonging drugs. In addition, it is known that a certain percentage of the population carries genetic variant(s) that reduces their repolarization reserve, predisposing them to (H)CQ-induced QT prolongation, and this may be more relevant to female patients who already have a longer QT interval to start with. This review provides an overview of the current evidence on (H)CQ therapy in patients with COVID-19 and discusses different strategies for prophylactic (H)CQ therapy (ie, preinfection, postexposure, and postinfection). In particular, the potential cardiac effects, including QT prolongation and arrhythmias, will be addressed. Based on these insights, recommendations will be presented as to which preventive measures should be taken when giving (H)CQ prophylactically, including electrocardiographic monitoring.
Keywords: Arrhythmia, Chloroquine, COVID-19, ECG, Hydroxychloroquine, Prophylaxis, QT, Recommendations, SARS-CoV-2, Torsades de pointes
A. Introduction
Since December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected >7 million people worldwide as of June 8, 2020 (https://coronavirus.jhu.edu/map.html), with some individuals (particularly those of older age and/or with comorbidities) developing coronavirus disease 2019 (COVID-19), a critical pulmonary illness requiring intensive treatment. In addition, patients with COVID-19 are at an increased risk of cardiovascular complications including myocardial ischemia, heart failure, myocarditis, and arrhythmias.1 Generalized inflammation, cytokine storm, and systemic failure may furthermore compromise cardiac function in patients with COVID-19. Apart from supportive care and mechanical ventilation, specific therapeutic options are limited. Novel antiviral strategies such as remdesivir have shown some promising results,2 but such compounds may not be available for large-scale use in the near future. Similarly, a vaccine is not expected to be successfully developed within the next 6–12 months, necessitating the identification of efficacious prophylactic strategies.
The aminoquinoline (hydroxy)chloroquine ((H)CQ) has been heralded as a therapeutic candidate on the basis of promising in vitro effects, but more recent larger-scale studies in patients with COVID-19 have shown little clinical benefit.3 While various reasons may underlie the observed lack of therapeutic efficacy of (H)CQ, the main question left unanswered is whether (H)CQ used prophylactically and/or initiated in the very early stages of the disease is more efficacious. (H)CQ has potential serious side effects, including cardiac arrhythmia, but these may be of particular relevance to patients clinically affected by COVID-19 but less so to asymptomatic healthy individuals. Indeed, the prophylactic use of CQ is a commonly used antimalarial strategy (making CQ one of the most widely used drugs worldwide),4 and in this setting, significant cardiac toxicity has not been widely reported. Similarly, patients with autoimmune disorders such as systemic lupus erythematosus (SLE) and rheumatoid arthritis use HCQ (a less toxic derivative of CQ) during prolonged periods of time, mostly without serious side effects.5 However, it is known that a certain percentage of the general population carries genetic variant(s) that may predispose them to (H)CQ-induced proarrhythmia. We here explore the potential prophylactic use of (H)CQ in SARS-CoV-2 infection, the expected side effects and toxicity in the population to be targeted, and the recommended safety measures to prevent life-threatening arrhythmias.
B. Antiviral effects of (H)CQ and mechanism of action
Both CQ and HCQ are efficiently absorbed, reach peak serum concentrations within hours, and have a half-life of up to 1 month. Therapeutic doses of (H)CQ typically result in plasma concentrations of 2–5 μM, but accumulation in plasma and tissues occurs after chronic use, which is more pronounced for CQ than for HCQ. Both CQ and HCQ accumulate in lysosomes, destabilizing lysosomal membranes, altering lysosomal and endosomal pH, and interfering with lysosomal activity and autophagosome function.6 Both compounds may furthermore reduce antigen presentation, inhibit cytokine production (interleukin [IL]-1, interferon [IFNα], and tumor necrosis factor [TNF]), and affect Toll-like receptor signaling and cyclic guanosine monophosphate-adenosine monophosphate [GMP-AMP] synthase activity.6 These immunomodulatory and anti-inflammatory effects of (H)CQ have proven beneficial in the chronic treatment of patients with SLE and rheumatoid arthritis. In addition, their lysosomotropic action is thought to account for their antimalarial activity.
Like SARS-CoV, SARS-CoV-2 infects cells by binding to the angiotensin-converting enzyme 2 (ACE-2) receptor through its spike domain whereas the Middle East respiratory syndrome coronavirus uses human CD26 or dipeptidyl peptidase-4 (DPP4) receptors for cell entry. SARS-CoV-2 entry into the cell depends on pH-dependent internalization and fusion with intracellular organelles such as endosomes and lysosomes. In addition to elevating the pH of acidic endosomes and disrupting the intracellular transport of the virus, (H)CQ may affect the glycosylation of ACE-2, potentially reducing SARS-CoV-2 binding to ACE-2 and preventing entry of the virus into the cell. Wang et al7 tested the in vitro antiviral efficiency of a number of drugs in African green monkey kidney Vero E6 cells (ATCC-1586) inoculated with SARS-CoV-2 and found that CQ potently blocked virus infection. Importantly, CQ was shown to decrease virus yield by 80% when cells pretreated with the drug for 1 hour before viral infection and still by 50%–60% when it was added to the cells 2 hours postinfection, indicating that the drug functioned at both entry and postentry stages of infection.7 Similarly, previous studies had demonstrated antiviral activity of CQ in Vero E6 cells inoculated with SARS-CoV when administered both at the time of inoculation or postinfection.8 , 9 Anti–SARS-CoV-2 activity was subsequently also demonstrated for HCQ in Vero E6 cells in 2 studies.10 , 11 However, both an apparent lower potency of HCQ than of CQ10 and a higher antiviral activity for HCQ than for CQ was demonstrated.11 Similar to CQ, administration of HCQ was found to inhibit both entry and postentry stages of SARS-CoV-2 infection.10
In addition to its direct effects on SARS-CoV-2 replication, (H)CQ may modulate the innate and adaptive immune response. As previously discussed,12 HCQ may on one hand dampen the overactive immune response during the inflammatory phase of the infection and modulate IFNγ production and on the other hand weaken the innate immune response to the virus and impair adaptive immune responses. Importantly, these various effects of (H)CQ may differ depending on dosing and disease stage and severity; however, such effects on immune modulation in patients with COVID-19 are as yet unknown.
C. Limited therapeutic efficacy of (H)CQ in patients with symptomatic COVID-19
Various studies have looked at the therapeutic potential of (H)CQ in patients hospitalized for symptomatic COVID-19. Initial studies reported (H)CQ to be effective in reducing viral replication13 , 14 or in reducing time to clinical recovery.15 However, these studies had small sample sizes (<100) and various methodological flaws (nonrandomized, nonblinded) and were underpowered for primary end points such as mortality.16 Meanwhile, more recent larger studies have not been able to confirm the beneficial effects of (H)CQ.17, 18, 19, 20 These studies found that in patients hospitalized for COVID-19, HCQ therapy was not associated with a reduction in intensive care unit admission or death,17 a reduction in intubation or death,18 or a reduction in death alone.19 In fact, Magagnoli et al20 reported an increased risk of mortality for HCQ, although this may have been biased because of baseline differences between the control and the intervention group. In addition, various studies have looked at the potential benefit of adding a macrolide, such as azithromycin or clarithromycin, to (H)CQ. However, again no benefit was observed in any of the studies.19 , 20
A variety of reasons could underlie the fact that the above-mentioned studies were unsuccessful in finding a positive effect of (H)CQ therapy. All studies investigated the effects of (H)CQ in patients hospitalized for COVID-19, meaning that treatment was initiated at a relatively advanced stage of the disease. As described above, in vitro studies demonstrated that part of the antiviral effects of (H)CQ is mediated by inhibition of receptor binding and membrane fusion of SARS-CoV-2, that is, during early stages of virus infection. In contrast, patients hospitalized for COVID-19 are likely already advanced beyond the early disease stage, and hence it is possible that treatment with (H)CQ simply initiated too late in these studies. In addition, the deleterious immune modulatory effects of (H)CQ (on the innate and adaptive immune responses) may in fact have mitigated some of its beneficial effects. Finally, it must not be overseen that discordances between in vitro and in vivo observations of (H)CQ may also occur in SARS-CoV-2, as has been the case for other viruses.21
D. Increased vulnerability to (H)CQ-induced corrected QT prolongation in patients with COVID-19?
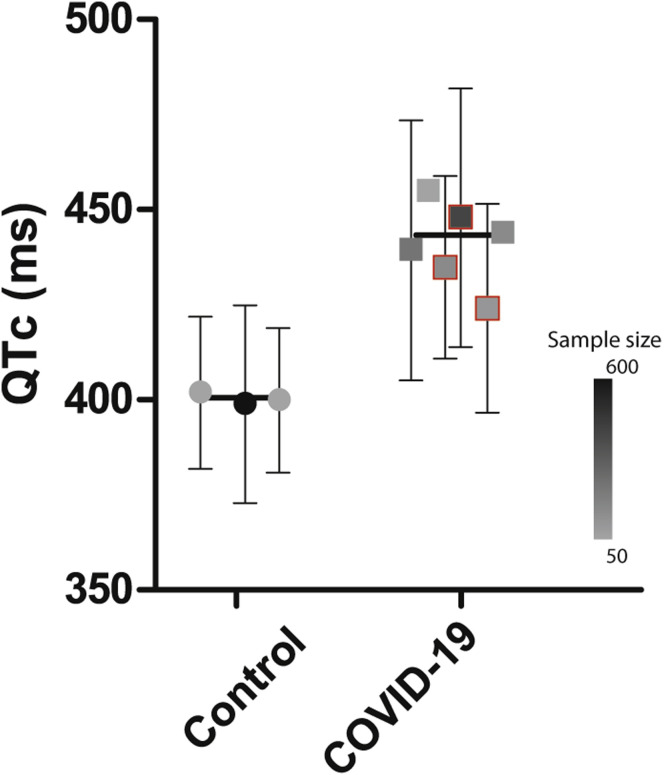
Another interesting possibility could be that patients with COVID-19 are more vulnerable for drug-induced corrected QT (QTc) prolongation and accompanying potentially lethal side effects, thereby (partly) masking a potential benefit of (H)CQ treatment. (H)CQ is a moderate inhibitor of the human ether-à-go-go related gene encoding the potassium channel responsible for the delayed rectifier current.22 The drug furthermore inhibits the inward-rectifier potassium channel Kir2.1. (H)CQ-induced block of cardiac potassium currents impairs ventricular repolarization and consequently leads to action potential and QT prolongation, thereby increasing the risk of malignant arrhythmias (torsades de pointes [TdP]). In general, the risk of drug-induced QT prolongation and TdP is increased by the concomitant use of other QT-prolonging drugs, electrolyte disturbances, and cardiac disease. Crucially, from the available electrocardiographic (ECG) data in studies published so far, it appears that patients with COVID-19 already have a prolonged QTc interval, even without (H)CQ use: the baseline mean QTc interval in patients with COVID-19, as presented in previous studies,23, 24, 25, 26 is much longer (in the 425–455 ms range) than that reported in healthy volunteers (±400 ms) (Figure 1 ).27 , 28
Figure 1.
Baseline corrected QT (QTc) interval in the population with coronavirus disease 2019 (COVID-19) compared to the control population. Manually measured QTc values were used unless unavailable or unspecified; the latter are indicated by a red outline.24, 25, 26Error bars indicate standard deviations of each separate study (not available for 2 studies23,29). The filled symbols (circles and squares) represent the sample size of the studies (see inset color scale). The horizontal black line indicates the overall mean value of all the studies for each group.
Several mechanisms may underlie the high baseline QTc interval in patients with COVID-19. First, patients with COVID-19 are frequently burdened with various comorbidities, such as diabetes, obesity, and cardiovascular diseases (coronary artery disease, heart failure, or cardiac arrhythmias). These comorbidities can cause QTc prolongation as such,30 but also through the medication subscribed for these comorbidities.31 Second, specific components of the COVID-19 pathophysiological process may play a role. Patients with COVID-19 display an excessive immune response, leading to a cytokine storm with high plasma levels of IL-1, IL-6, and TNFα, which can all prolong the action potential and consequently the QTc interval.32 In addition, electrolyte disturbances (ie, hypokalemia or magnesemia) and hypoxia, often seen in patients with COVID-19, can prolong the QTc interval.33 Moreover, patients with COVID-19 may use other antiviral QT-prolonging drugs, such as azithromycin. Overall, these factors may reduce repolarization reserve, leading to a prolonged baseline QTc interval in patients with COVID-19 as well as a more pronounced QTc prolongation after (H)CQ therapy. Indeed, recent studies have reported average increases in a QTc interval of ∼28–35 ms after (H)CQ treatment in patients hospitalized for COVID-19,23, 24, 25 and these results are in line with other published data summarized in a recent review.34 Crucially, up to 20% of patients had QTc prolongation in the range of ≥500 ms, which is generally considered the cutoff value for discontinuing treatment. Although TdP arrhythmias appeared to occur infrequently, this has been reported35 and it is clear that (severely) ill patients hospitalized for COVID-19 may be more susceptible to (H)CQ-induced cardiotoxicity. However, the question remains whether preventive treatment initiated early in the disease course is of clinical benefit.
E. Prophylactic use of (H)CQ in COVID-19: Potential strategies and initial findings
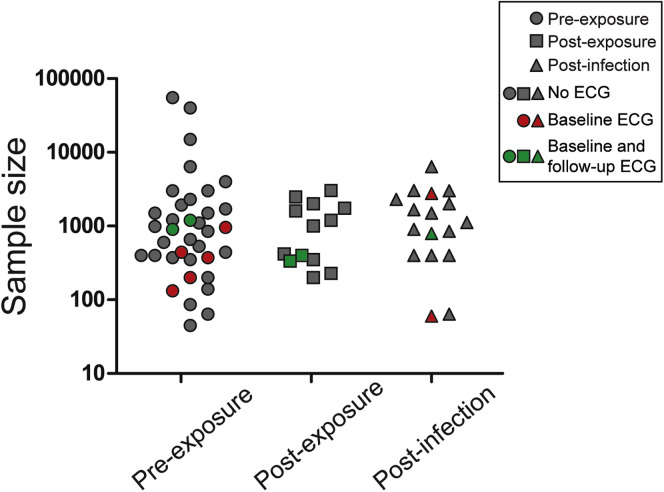
According to the in vitro findings, early use of (H)CQ may prevent virus infection and/or reduce disease severity, similar to its previous use as antimalarial therapy. As such, early treatment with (H)CQ in COVID-19 as a prophylactic therapy is conceivable. Various strategies can be considered, depending on whether therapy is given preinfection or postinfection, for example, targeted vs mass prophylaxis. Preinfection relates either to individuals who are “preexposed,” such as health care workers or high-risk patient groups, or to individuals “postexposed” to confirmed cases. Postinfection prophylaxis is aimed at recently infected, nonhospitalized, SARS-CoV-2–positive individuals who are still asymptomatic or only mildly affected. Currently, 60 studies listed on ClinicalTrials.gov are using such a prophylactic approach, enlisting a cumulative estimated sample size of 175,000 individuals (Figure 2 ). Of these, 36 (sub)studies initiate therapy in preexposed individuals, 13 in confirmed postexposed individuals, and 17 in postinfected individuals. Depending on the proposed strategy, different dosing strategies and duration of (H)CQ use will be used, varying from days to months. So far, results from only 2 studies asserting the effect of HCQ on post–exposure-confirmed SARS-CoV-2 cases have been published. One small nonrandomized, noncontrolled study administered HCQ in 211 SARS-CoV-2–negative individuals, who were all exposed to 1 index patient, and found that none of these cases had a positive polymerase chain reaction test after 14 days of quarantine.36 However, this study is severely limited by the lack of a control group and the fact that almost none of the exposed individuals fell in the high-risk exposure group. More recently, the first randomized controlled trial testing (H)CQ as prophylaxis has been published.37 In this study, 821 postexposed individuals, who were recruited via social media, were treated with HCQ or placebo within 4 days of exposure. The incidence of developing COVID-19, which was defined through clinical evaluation in the vast majority of cases (∼80%) because of a limited access to diagnostic tests, was relatively low and not different between the groups (ie, 12%–14%). However, the study was limited by the fact that polymerase chain reaction diagnosis was not performed in most participants, and follow-up data collection was through self-reporting. Overall, the study design may not have been optimal to identify a beneficial, preventive effect of HCQ in mildly affected patients. In addition, whether (H)CQ is effective in preexposed individuals remains unknown, and it will be interesting to see the results of other ongoing randomized controlled trials on this topic.
Figure 2.
Overview of (sub)trials listed on ClinicalTrials.gov assessing the prophylactic use of (hydroxy)chloroquine in severe acute respiratory syndrome coronavirus 2. Trials are listed by estimated sample size and prophylaxis strategy. Trials not conducting any electrocardiography (ECG) are depicted in gray, trials conducting baseline ECG in red, and trials conducting baseline and follow-up ECG in green.
F. (H)CQ prophylaxis for COVID-19: Cardiac considerations and recommendations
When considering potential large-scale (H)CQ prophylaxis, the potential off-target effects should be taken into account. Cardiac rhythm disorders such as sinus bradycardia or conduction disorders (atrioventricular block and bundle branch blocks) have been reported in (H)CQ users.38 As discussed above, (H)CQ may furthermore lead to potentially dangerous QTc prolongation in patients with COVID-19, although this may be less problematic in healthy individuals given (H)CQ prophylactically. In healthy volunteers, 600 mg of CQ increased the QTc interval on average by 16 ms.27 The widespread use of CQ for antimalarial prophylaxis has not been associated with an increased risk of ventricular arrhythmias such as TdP or significant QTc prolongation.4 , 39 Here, potential confounding effects of high fever and/or its defervescence on cardiac repolarization may also be of relevance but are as yet incompletely understood.40 In 85 patients with SLE or other connective tissue diseases treated with HCQ for >1 year, the average QTc interval was 410 ms (range 349–464 ms) and no other significant effects on ECG parameters were observed.5 In another study of 409 patients with SLE treated chronically with (H)CQ, a prolonged QTc interval (according to the Minnesota criteria) was reported in only 3 patients (0.7%).41 Moreover, in 76,822 adverse drug reaction cases from the World Health Organization pharmacovigilance database (VigiBase), only 53 cases of QT prolongation (0.07%) and 83 cases of ventricular tachycardia including TdP (0.11%) were recently reported,42 whereas no safety signal for TdP/QT interval was observed for (H)CQ in an analysis of the US Food and Drug Administration Adverse Event Reporting System.43 It must be noted, however, that the latter 2 databases likely underestimate the true cardiac toxicity as a result of potential underreporting of adverse events. In general, the specificity and sensitivity of the reported incidence of cardiac adverse events varies significantly depending on the type of study involved (spontaneous reporting, observational studies, randomized controlled trials, or hospital metadata) as well as the sample size.44 These are important considerations when considering the apparent conflicting results reported in relation to COVID-19.
Despite the seemingly low prevalence of serious side effects associated with (H)CQ use, particularly in healthy individuals and when used for a short period of time, certain individuals may be at an increased risk of potentially life-threatening side effects. CQ and HCQ are both metabolized by cytochrome P450 (CYP3A4), and hence plasma levels may be increased during the concomitant use of CYP3A4-inhibiting drugs. (H)CQ metabolism is also compromised in individuals with inherited glucose-6-phosphate dehydrogenase deficiency.45 A certain percentage of the population furthermore carries genetic variant(s) that reduces their repolarization reserve, predisposing them to drug-induced QT prolongation. Such genetic factors that predispose to QTc prolongation in response to certain drugs include the KCNE2 variant D85N, which is present in ±2% of the general population and importantly enriched in cohorts with drug-induced adverse events.46 Additionally, ∼10% of African American individuals carry the SCN5A allele 1103Y, which is also associated with a strong predisposition for excessive QTc prolongation.47 Even considering the lowest estimation of 2% of the population carrying QT-prolonging alleles, this would mean that of the 175,000 individuals being enrolled in the various (H)CQ prophylactic studies, 1750 of them are at an increased risk of (H)CQ-induced excessive QTc prolongation and potentially life-threatening arrhythmias (assuming 1:1 randomization).48 The functional impact of such genetic variants may be more relevant to female patients who already have a longer QT interval to start with.
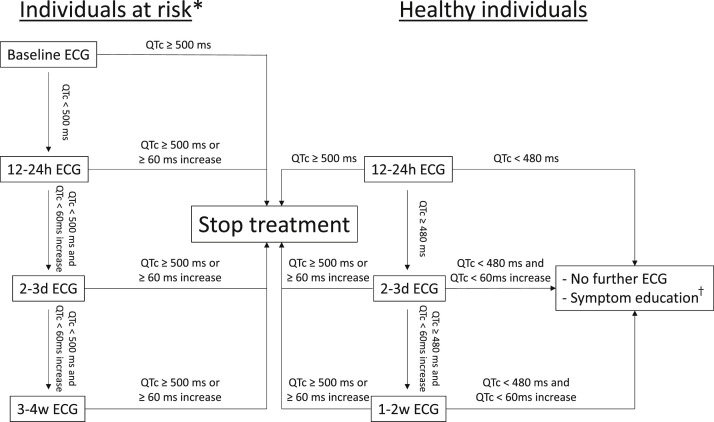
Despite these considerations, only 12 of the 60 studies of (H)CQ prophylaxis listed on ClinicalTrials.gov explicitly state that baseline ECGs will be collected and only 5 also mention follow-up ECG(s). The vast majority does not list QTc prolongation as an exclusion criterion or only exclude individuals with known QTc prolongation; those with a positive family history for long QT syndrome, TdP, or sudden death; or those using other QTc-prolonging drugs. Six studies mention specific QTc cutoffs in their exclusion criteria, but do not specify whether this relates to previously available ECGs or whether an ECG will be performed in each included individual. On the basis of the considerations discussed above, we recommend that the prophylactic use of (H)CQ for COVID-19 should include some form of ECG monitoring. In accordance with our previous recommendations,49 an ECG before the initiation of treatment is advised in patients with a known history of congenital or acquired long QT syndrome, structural heart disease, bradycardia, or use of other QT-prolonging drugs, and in these patients (H)CQ should not be initiated when the baseline ECG shows QTc interval ≥ 500 ms. If the QTc interval is <500 ms, an ECG shortly after the initiation of treatment is advised in addition to a repeated ECG after a few days. Long-term use would require a follow-up ECG after a few weeks, since (H)CQ plasma accumulation may occur. An ECG showing QTc interval ≥ 500 ms or an increase of 60 ms is an indication to discontinue therapy. In all other individuals receiving (H)CQ prophylaxis, we do not consider a pretreatment ECG necessary but do recommend at least 1 ECG 12–24 hours after the first dose in order to identify those at an increased risk of (H)CQ-induced excessive QT prolongation and TdP. If the QTc interval exceeds 500 ms, (H)CQ should be discontinued; if the QTc interval is ≥480 ms, a second follow-up ECG is advised within 1–2 days. Again, when the QTc interval exceeds 500 ms or is increased by 60 ms, therapy should be discontinued. No follow-up ECG is necessary if the QTc interval is <480 ms. However, these individuals should be educated about possible arrhythmic symptoms (eg, dizziness, syncope, and palpitations) and reassessment of their arrhythmogenic risk is prompted when such symptoms are experienced. During treatment, all patients should be informed about other potential QT-prolonging risk factors, such as other QT-prolonging drugs and hypokalemia secondary to, for example, chronic diarrhea. Naturally, in the case of COVID-19–related illness, appropriate measures should be taken when necessary, including careful electrolyte monitoring. A schematic overview of these recommendations is presented in Figure 3 .
Figure 3.
Schematic diagram of the recommendations for electrocardiographic (ECG) monitoring during (hydroxy)chloroquine prophylaxis. ∗Individuals at risk are defined as those with a known history of congenital or acquired long QT syndrome, structural heart disease, bradycardia, use of other QT-prolonging drugs, and conditions with an increased risk of electrolyte disorders (such as chronic diarrhea or chronic kidney disease). †Symptom education for patients, ensuring awareness of potential arrhythmic symptoms (eg, dizziness, syncope, and palpitations). QTc = corrected QT.
Conclusion
While (H)CQ treatment has so far not shown therapeutic benefit in patients with COVID-19, (H)CQ prophylaxis may prove beneficial and a large number of clinical studies are planned and underway to address this issue. Given the known QT-prolonging effects of (H)CQ, its prophylactic use may potentially induce life-threatening arrhythmias, particularly in patients with preexisting cardiovascular disease and those taking other QT-prolonging drugs. In addition, a certain percentage of the population carries genetic variant(s) that reduces their repolarization reserve, and this may be more relevant to female patients. Based on these insights, ECG monitoring after (H)CQ is recommended.
Footnotes
This work was funded by an Innovational Research Incentives Scheme Vidi grant from the Netherlands Organisation for Health Research and Development (ZonMw 91714371 to Dr Remme), a PhD scholarship from the AMC graduate school (to Dr Offerhaus), the Netherlands CardioVascular Research Initiative CVON (Dutch Heart Foundation, Dutch Federation of University Medical Centres, ZonMw, and the Royal Netherlands Academy of Sciences; PREDICT2 CVON2018-30 to Drs Wilde and Remme) and by the Fondation Leducq (to Drs Remme and Wilde).
References
- 1.Gupta A.K., Jneid H., Addison D. Current perspectives on coronavirus 2019 (COVID-19) and cardiovascular disease: a white paper by the JAHA editors. J Am Heart Assoc. 2020;2019:e017013. doi: 10.1161/JAHA.120.017013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19—preliminary report [published online ahead of print May 22, 2020]. N Engl J Med. https://doi.org/10.1056/NEJMoa2007764 [DOI] [PubMed]
- 3.Tang W., Cao Z., Han M. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White N.J. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7:549–558. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 5.Costedoat-Chalumeau N., Hulot J.-S., Amoura Z. Heart conduction disorders related to antimalarials toxicity: an analysis of electrocardiograms in 85 patients treated with hydroxychloroquine for connective tissue diseases. Rheumatology. 2007;46:808–810. doi: 10.1093/rheumatology/kel402. [DOI] [PubMed] [Google Scholar]
- 6.Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 7.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keyaerts E., Vijgen L., Maes P., Neyts J., Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent M.J., Bergeron E., Benjannet S. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J., Cao R., Xu M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao X., Ye F., Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyerowitz E.A., Vannier A.G.L., Friesen M.G.N. Rethinking the role of hydroxychloroquine in the treatment of COVID-19. FASEB J. 2020;34:6027–6037. doi: 10.1096/fj.202000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gautret P., Lagier J.-C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. Article 105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z., Hu J., Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial [published online ahead of print April 10, 2020]. medRxiv. https://doi.org/10.1101/2020.03.22.20040758
- 16.Alexander P.E., Debono V.B., Mammen M.J. COVID-19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J Clin Epidemiol. 2020;123:120–126. doi: 10.1016/j.jclinepi.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahevas M., Tran V.-T., Roumier M. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial [published online ahead of print April 14, 2020]. medRxiv. https://doi.org/10.1101/2020.04.10.20060699
- 18.Geleris J., Sun Y., Platt J. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. May 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg E.S., Dufort E.M., Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magagnoli J., Narendran S., Pereira F. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19 [published online ahead of print April 23, 2020]. medRxiv. https://doi.org/10.1101/2020.04.16.20065920 [DOI] [PMC free article] [PubMed]
- 21.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antiviral Res. 2020;177:104762. doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polak S., Wiśniowska B., Brandys J. Collation, assessment and analysis of literature in vitro data on hERG receptor blocking potency for subsequent modeling of drugs’ cardiotoxic properties. J Appl Toxicol. 2009;29:183–206. doi: 10.1002/jat.1395. [DOI] [PubMed] [Google Scholar]
- 23.van den Broek M.P.H., Möhlmann J.E., Abeln B.G.S., Liebregts M., van Dijk V.F., van de Garde E.M.W. Chloroquine-induced QTc prolongation in COVID-19 patients. Neth Heart J. 2020;28:406–409. doi: 10.1007/s12471-020-01429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chorin E., Wadhwani L., Magnani S. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;182:264–277. doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinkeler F.S., Berger F.A., Muntinga H.J., Jansen M.M.P.M. The risk for QTc interval prolongation in COVID-19 patients treated with chloroquine. Neth Heart J. 2020;28:418–423. doi: 10.1007/s12471-020-01462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borba M.G.S., Val F.F.A., Sampaio V.S. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 27.Mzayek F., Deng H., Mather F.J. Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin Trials. 2007;2:e6. doi: 10.1371/journal.pctr.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vink A.S., Neumann B., Lieve K.V.V. Determination and interpretation of the QT interval. Circulation. 2018;138:2345–2358. doi: 10.1161/CIRCULATIONAHA.118.033943. [DOI] [PubMed] [Google Scholar]
- 29.Mercuro N.J., Yen C.F., Shim D.J. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) [published online ahead of print May 1, 2020]. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1834 [DOI] [PMC free article] [PubMed]
- 30.Park B., Lee Y.-J. Metabolic syndrome and its components as risk factors for prolonged corrected QT interval in apparently healthy Korean men and women. J Clin Lipidol. 2018;12:1298–1304. doi: 10.1016/j.jacl.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Dapro B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Suppl. 2001;3:K70–K80. [Google Scholar]
- 32.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, arrhythmic risk, and inflammation: mind the gap! Circulation. 2020;142:7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 33.Belardinelli L., Giles W.R., Rajamani S., Karagueuzian H.S., Shryock J.C. Cardiac late Na+ current: proarrhythmic effects, roles in long QT syndromes, and pathological relationship to CaMKII and oxidative stress. Heart Rhythm. 2015;12:440–448. doi: 10.1016/j.hrthm.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Jankelson L., Karam G., Becker M.L., Chinitz L.A., Tsai M. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: a systematic review. Heart Rhythm. 2020;17:1472–1479. doi: 10.1016/j.hrthm.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szekely Y., Lichter Y., Shrkihe B.A., Bruck H., Oster H.S., Viskin S. Chloroquine-induced torsades de pointes in a patient with coronavirus disease 2019. Heart Rhythm. 2020;17:1452–1455. doi: 10.1016/j.hrthm.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S.H., Son H., Peck K.R. Can post-exposure prophylaxis for COVID-19 be considered as an outbreak response strategy in long-term care hospitals? Int J Antimicrob Agents. 2020;55:105988. doi: 10.1016/j.ijantimicag.2020.105988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulware D.R., Pullen M.F., Bangdiwala A.S. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19 [published online ahead of print June 3, 2020]. N Engl J Med. https://doi.org/10.1056/NEJMoa2016638 [DOI] [PMC free article] [PubMed]
- 38.Chatre C., Roubille F., Vernhet H., Jorgensen C., Pers Y.-M. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41:919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 39.Haeusler I.L., Chan X.H.S., Guérin P.J., White N.J. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med. 2018;16:200. doi: 10.1186/s12916-018-1188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan X.H.S., Win Y.N., Haeusler I.L. Factors affecting the electrocardiographic QT interval in malaria: a systematic review and meta-analysis of individual patient data. PLoS Med. 2020;17:e1003040. doi: 10.1371/journal.pmed.1003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGhie T.K., Harvey P., Su J., Anderson N., Tomlinson G., Touma Z. Electrocardiogram abnormalities related to anti-malarials in systemic lupus erythematosus. Clin Exp Rheumatol. 2018;36:545–551. [PubMed] [Google Scholar]
- 42.Nguyen L.S., Dolladille C., Drici M.-D. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin: an analysis of the World Health Organization pharmacovigilance database. Circulation. 2020;142:303–305. doi: 10.1161/CIRCULATIONAHA.120.048238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarayani A., Cicali B., Henriksen C.H., Brown J.D. Safety signals for QT prolongation or torsades de pointes associated with azithromycin with or without chloroquine or hydroxychloroquine [published online ahead of print April 19, 2020]. Res Soc Adm Pharm. https://doi.org/10.1016/j.sapharm.2020.04.016 [DOI] [PMC free article] [PubMed]
- 44.Drici M. Estimates of population-based incidence of malignant arrhythmias associated with medication use—a narrative review. Fundam Clin Pharmacol. 2020;34:416–417. doi: 10.1111/fcp.12582. [DOI] [PubMed] [Google Scholar]
- 45.Capoluongo E.D., Amato F., Castaldo G. The friendly use of chloroquine in the COVID-19 disease: a warning for the G6PD-deficient males and for the unaware carriers of pathogenic alterations of the G6PD gene. Clin Chem Lab Med. 2020;58:1162–1164. doi: 10.1515/cclm-2020-0442. [DOI] [PubMed] [Google Scholar]
- 46.Kääb S., Crawford D.C., Sinner M.F. A large candidate gene survey identifies the KCNE1 D85N polymorphism as a possible modulator of drug-induced torsades de pointes. Circ Cardiovasc Genet. 2012;5:91–99. doi: 10.1161/CIRCGENETICS.111.960930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giudicessi J.R., Roden D.M., Wilde A.A.M., Ackerman M.J. Genetic susceptibility for COVID-19-associated sudden cardiac death in African Americans. Heart Rhythm. 2020;17:1487–1492. doi: 10.1016/j.hrthm.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gollob M.H. COVID-19, clinical trials and QT-prolonging prophylactic therapy in healthy subjects: first, do no harm. J Am Coll Cardiol. 2020;75:3184–3186. doi: 10.1016/j.jacc.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C.-I., Postema P.G., Arbelo E. SARS-CoV-2, COVID-19 and inherited arrhythmia syndromes. Heart Rhythm. 2002;17:1456–1462. doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]