Abstract
COVID-19 has created havoc in the world by causing thousands of demises in a short period of time. Up till now, several attempts have been made for potential therapeutics against SARS-COV2. In this retrospective, single-center study, we extracted data from 122 COVID-19, RT-PCR confirmed patients. who were treated with a new treatment strategy of lianhuaqingwen with Arbidol Hydrochloride. The patients were either asymptomatic or had mild symptoms for COVID-19 disease. Of 122 patients 21 (17.21%) patients developed severe conditions of COVID-19, while total 111 (90.9%) experienced mild symptoms such as fever in 93 (76.22%) patients, cough in 23 (20.17%) and muscle pain were observed in total 8 (7%) patients. Furthermore our newly applied drugs combination (Lianhuaqingwen and Arbidol Hydrochloride) showed therapeutic effects in 5–7 days in patients with mild symptoms with 98% recovery rate. These results indicate that COVID-19 patients with mild symptoms can be treated with Lianhuaqingwen and Arbidol Hydrochloride. However, extensive clinical investigations are required to confirm the effectiveness of these drugs.
Keywords: COVID-19, Therapeutics, Mild symptoms, TCM, Recoveries
1. Introduction
COVID-19 caused by SARS-CoV has been declared as a global pandemic by WHO on March 11, 2020. The disease has paralyzed the world and is currently causing thousands of mortalities and morbidities worldwide (Khan et al., 2020, Hamza et al., 2020). Besides transmission and biological features, previous studies have largely focused on clinical characteristics and treatment responses. At the outset, a total of 41 cases of COVID-19 reported by Huang et al. (2020) manifested fever, dry cough, myalgia, fatigue, and pneumonia, which in severe cases caused organ dysfunction(Huang et al., 2020). Similarly, Wang et al. (2020) studied the clinical features from 138 patients and found that these patients revealed, fever (98%), fatigue (70%), and dry nonproductive cough (60%) (Wang et al., 2020). In addition, Guan et al. (2020) reported that fever was developed in only 43% of COVID-19 patients on admission, however, the number increased to 88.7% during hospitalization (Guan et al., 2020). On the other hand, studies have also focused on the recovery from COVID-19 in response to treatment with antiviral drugs such as, remdesivir and chloroquine were found effective against COVID-19 disease (Li et al., 2020, Studemeister et al., 2020, Gao et al., 2020). Recently, a Traditional Chinese Medicine (TCM) called Lianhuaqingwen (LH) has received enormous attention in China, particularly in treating COVID-19 patients with mild symptoms. The effect of LH can be enhanced through its combinational therapy with arbidol Hydrochloride (Yang et al., 2020).
LH is a Chinese patent medicine composed of Herbs. The major ingredients of LH consisted of Lonicera japonica, Forsythia suspensa, Isatis indigotica, Ephedra sinica, Pogostemon cablin, Rheum palmatum, Glycyrrhiza uralensis, Houttuynia cordata, Dryopteris crassirhizoma, Rhodiola crenulata, Prunus sibirica, gypsum and 1-mentho (Hu et al., 2020). LH exerted broad-spectrum activities on a number of influenza viruses by inhibiting viral propagation and regulating immune responces and exhibited similar therapeutic efficacy while used with Oseltamivir in reducing the course of H1N1 virus infection (Duan et al., 2011). LH showed its anti-coronavirus activity by inhibiting virus replication and decreasing the cytokine release from host recently reported by (Runfeng et al., 2020) However, limited details are available regarding the recovery of patients with mild symptoms or asymptomatic patients. in response to the combination of Traditional Chinese Medicines (TCM), antiviral drugs, and/or antibiotics. In this study, we briefly demonstrate the characteristics of 122 hospitalized patients with COVID-19 and compare them with previously reported information. We further report the effectiveness of LH in combination with the antiviral drug for COVID-19 in asymptomatic patients or patients with mild symptoms.
2. Methods
2.1. Study design
This retrospective, cohort, single-center study was conducted in Wuchang Fencang hospital, a makeshift hospital in Wuhan, Hubei China. Patients were admitted to the Fenceng hospital after the onset of the specified symptoms and based on selected criteria such as if an individual RT-PCR test were found positive or respiratory rate > 30 and blood oxygen saturation > 93%. All cases in this study were confirmed according to WHO recommended laboratory diagnosis guidelines. The clinical outcomes (i-e admission, length of stay, discharged, and mortality) were monitored from Feb-4, 2020 to March 17, 2020. The data including demographic data exposure history, signs and symptoms, medical history, laboratory findings, and chest computed tomographic (CT) scan were reviewed by a team of trained and experienced physicians. The date of disease onset defined from the day when the first symptoms were noticed (symptomatic patients) or the SARS-CoV-2 was detected in samples (in case of asymptomatic patients). Treatment strategies were recommended by expert medical team, mainly composed of physicians (infection department, respiratory department, endocrinology department, oncology department, traditional Chinese medicine department, and psychology department, etc.), radiologists, and laboratory doctors and the responses were observed until the termination of treatment. Informed consent was obtained from patients and permission was obtained from the National Health Commission of China regarding the publication of this data.
2.2. Definitions
The severity of COVID-19 was defined by satisfying at least one of these factors; the ratio of the partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) 300 mmHg (1 mmHg ¼ 0.133 kPa) breathing rate > 30/min and pulse oximeter oxygen saturation at rest < 93%, Critical illness if satisfying at least one of the following items, the respiratory failure occurred and individuals received mechanical ventilation, failure of other organs, shock, and received care in the intensive care unit.
2.3. Laboratory confirmation
RT PCR was performed for detection of SARS-CoV-2 in nasal swab samples from the COVID-19 patients or suspected individuals, according to the established protocol by the Chinese Center for Disease Control (Huang et al., 2020). Two genes, nucleocapsid protein (N) and Open Reading Frame1ab (ORF1ab) were amplified and targeted by RT-PCR. Target 1 (ORF1ab): Forward primer CCCTGTGGGTTTTACACTTAA, Reverse primer as ACGATTGTGCATCAGCTGA; Probe 5′-VIC-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3′. For Target 2 (N): Forward primer GGGGAACTTCTCCTGCTAGAAT; and Reverse primer CAGACATTTTGCTCTCAAGCTG; with Probe as 5′-FAM- TTGCTGCTGCTTGACAGATT-TAMRA-3′. The extracted RNA was assayed for real-time RT-PCR using a SARS-COV2 nucleic acid detection kit as per the manufacturer’s protocol (Shanghai bio germ Medical Technology Co Ltd).
2.4. Statistical analysis
The statistical analyses were performed using software R, where different parameters were described in percentages, frequency rates, and continuous variables described via Interquartile range (IQR) mean and median. IQR Interquartile Range.
3. Results
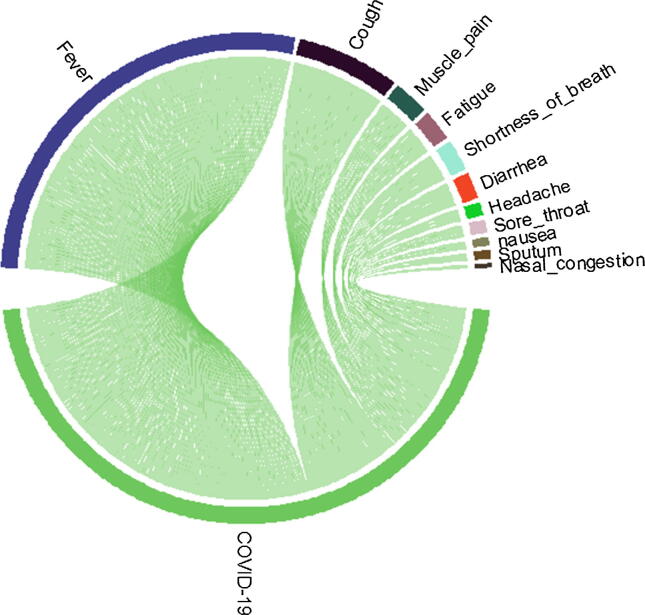
The median age of patients in this study was 49 years comprised of 70 (57.3%) females and 52 (42.27%) males. Of these patients total of 8 (6.5%) were found asymptomatic (Table 1). At the onset of the disease, fever [93 (76.22%) patients], cough [23 (20.17%) patients] and muscle pain [8 (7%) patients] were the most common symptoms, and less common symptoms were diarrhea, nausea, headache, and vomiting Figure 1. The median duration of stay in the hospital was 18 days, and the average incubation period was 7 days. The significant alternation in laboratory findings during hospitalization was a high level of C reactive protein, lymphopenia, increases in WBC, and neutrophil count (Table 2). Out of the total 122 patient 111 recovered, female accounted (n = 60, 54%) and (n = 42, 37%) were male, with median hospitalization of 17.99 days. However, 21 patients were shifted to another hospital including 11 (9%) female and 10 (8.1%) male, where the majority (n = 17, 81%) were later recovered.
Table 1.
Baseline characteristics of COVID-19 Patients.
| Gender (total = 122) | Number | % |
|---|---|---|
| Female | 70 | 57.37 |
| Male | 52 | 42.6 |
| Mean | 49.2 | – |
| Median | 49 | – |
| Range | 11–72 | – |
| Symptoms | ||
| Fever (including low fever) | 93 | 76.22 |
| Cough | 23 | 18.85 |
| Muscle pain | 8 | 6.56 |
| Fatigue | 7 | 5.74 |
| Shortness of breath | 7 | 5.74 |
| Diarrhea | 6 | 4.92 |
| headache | 3 | 2.46 |
| Sore throat | 3 | 2.46 |
| nausea | 2 | 1.64 |
| Sputum | 2 | 1.64 |
| Nasal congestion | 1 | 0.82 |
Fig. 1.
Ratio of Sign and Symptoms of in 122, COVID-19 Patients.
Table 2.
Laboratory Findings of COVID-19 Patients.
| Blood | Median (IQR) |
|---|---|
| WBC | 1.725 (2.18–11.14) |
| Neutrophil count | 1.44 (1.13–8.53) |
| Lymphocytes count | 0.64 (0.57–3.45) |
| ALY# 10^9/L | 0.01 (0–0.05) |
| RBC (/L) | 0.625 (1.9–6.06) |
| NRBC# 10^9/L | 1.955 (0.13–79.71) |
| HGB (g/L) | 18 (48–166) |
| Platelets 10^9/L | 165 (125–188) |
| FR-CRP (mg/L) | 7.59 (0.08–173.81) |
| hs-CRP (mg/L) | 2.3125 (0.08->10) |
| CRP (mg/L) | 38.645 (<10–10.43) |
Both patients with mild symptoms and asymptomatic, received TCM LH Capsule in combination with Arbidol Hydrochloride tablets, with dosage and duration detail in (Table 3). This newly applied combination showed effects in 5–7 days for patients with mild symptoms and was found effective with 98% recovery rate. As a result, no mechanical support was required to any of the recovered patients, during the overall course treatment. Interestingly the recovery response of the aged people and patients with other coexisting conditions such as asthma and hypertension were also noticeable. A total 47 (85%) out of 55 with the age of 60 or above were fully recovered. Similarly, in the age group of 40–60 total 61 (81.3%) patients were recovered. In our observations, this strategy with zero adverse effects (Table 4) demonstrated significant recovery responses from all age groups. Moreover, the severity and complications of the COVID-19 were found in male patients as compared to female patients. However asymptomatic cases were found in females more which can be potential source of infection.
Table 3.
Treatment Strategy for mild or asymptomatic COVID-19 Patients.
|
S. No |
Medicine Chinese/Western |
Disease stage |
Out comes/ Complication
|
| 1. | i. Arbidol Hydrochloride (2 tables/each time, three times one day) for 5 days ii. TCM* (Lianghua Qingwen) (4 capsules/each time, three times one day) for 10 to 14 days. |
Mild Symptoms/ Asymptomatic confirmed All 122 (100%) Patients received this combination |
|
| 2. | Moxifloxacin‘Hydrochloride (1 table/each time, one times one day) for 7–14 days along with combination S.No.1 |
Symptomatic Patients with Fever, cough, muscle pain etc.Total 39 Patients received this additional Antibiotics |
Table 4.
Age and Gender wise recovery responses groups.
| Number of Patients with age group Total 122 |
Recovered Patients. 111/122 (90.9%) |
Serious 21/122 17.2% |
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||
| 4 < 30 y | 4 (100%) | 01 (25%) | 03 (75%) | 0 | NA | NA |
| 22 (30–40) | 18 (81.8%) | 07 (38%) | 11 (61%) | 04 (18.1%) | 01 (25%) | 03 (75%) |
| 41 (40–50) | 32 (78%) | 11 (34.3) | 21 (65.6%) | 09 (21.9%) | 04 (44.4%) | 05 (55.5%) |
| 34 (50–60) | 29 (85%) | 13 (44.8%) | 16 (55.1%) | 05 (14.7%) | 02 (40%) | 03 (60%) |
| 20 (60–70) | 17 (85%) | 09 (52.9%) | 08 (47%) | 03 (15%) | 0266.6%) | 01 (33.3%) |
| 1 > 70 y | 1 (100%) | N/A | 01 | 0 | N/A | N/A |
4. Discussion
Findings of the current study have shown that the treatment of COVID-19 patients with LH capsule in combination with Arbidol Hydrochloride resulted in significant recovery. Out of 122 patients, 111 were fully recovered and discharged from the hospital. However, with higher infection proportion in females, our finding confers recently published results (Wang et al., 2020), while disagreeing with the early report by Chen et al. (Chen et al., 2020), which showed that males are most likely affected by COVID-19. Moreover, we found that asymptomatic cases were more common in females, as reported earlier (Noelle Breslin et al., 2020). Similarly another study showed that the majority of COVID-19 infected females developed mild symptoms and recovered quickly as compared male patients (Liu et al., 2020). No convincing evidence is available to support the phenomenon that most of the females develop mild symptoms or remain asymptomatic. However, it is well konwn that women are less susceptible to viral infections as compared to men due differences in innate immunity factors related to sex chromosomes and steroidal hormones (Conti, 2020), which could be one of the reasons that most of the COVID-19 infected females do not develop severe symptoms. The occurrences and risk of COVID-19 infection are similar in different ages there are no significant differences found, unlike reported previously (Huang et al., 2020).
Furthermore, the combination of LH and Aribidol Hydrochrolride we applied showed significant effects against COVID-19 in 5 to 7 days (Table No.4), where the symptoms reduced very rapidly as compared to many other tested drugs including Arbidol Hydrochloride, when used alone or in combination with other drugs (Li et al., 2020, Ashraf et al., 2020). It may be due to the mechanism of action of LH (Dong et al., 2014) that can effectively reduce systematic and airway inflammation by regulation of immune systems through inhibiting the release of the corresponding inflammatory factors. Moreover, recovery responses of patients aged over 60 years were also notable with 85% recovery, indicating that Arbidol’s role by inhibiting the fusion of the viral envelope to the target host cell membrane (Liu et al., 2020) and LH anti-inflammation role are supportive to elderly recovery.
By applying this new treatment strategy, the mild symptoms were relieved without the application of mechanical or any other therapeutics support. However, patients with bacterial infection additionally received Moxifloxacin Hydrochloride. Despite the interesting results presented in this study, the combination of TCM with western medicines should further be investigated prior to clinical recommendation for COVID-19 patients on large scale. This will help determining the most promising therapeutic combination against COVID-19 disease.
5. Conclusion
The ratio of patients remained asymptomatic and patients with mild symptoms was found higher in females as compaored to males, suggesting that males are more suscepitible to develope symptoms. Furthermore, the combination of LH with Aribidol hydrochloride can be used as effective therapeutic option against COVID-19, specifically in the case of patients with mild symptoms. However further studies and clinical investigations are recommended to confirm its efficacy.
6. Ethics approval and consent to participate
Approval was obtained from the National Health Commission of China, Hospital Institutional eview board approval and informed patient’s consent was obtained.
Funding
This project received the funding from the Postdoctoral research grants from the Second Affiliated Hospital of Zhengzhou University, Zhengzhou China and the Chinese Postdoctoral Science Foundation (for S.K.).
Contributors
J.Hu, T. Wang, G. Han conducted the main study and collected the data, S.Khan analyzed the data, A.Ali and S.Khan conceived the idea and wrote initial draft, R.Siddique, and Shabana did the literature search and wrote the initial draft, and S.Khan, G. Nabi, G. Han, W.Zaman M.Dong review, revised the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge grant support from the National Natural Science Foundation of China (grant numbers 81870942, 81471174 and 81520108011) and CAS-TWAS Fellowship Programme by Chinese Academy of Sciences.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Suliman Khan, Email: suliman.khan18@mails.ucas.ac.cn.
Guang Han, Email: hg7913@hotmail.com.
References
- Ashraf, M.A., Shokouhi, N., Shirali, E., Davari-tanha, F., Memar, O., Kamalipour, A., Azarnoush, A., Mabadi, A., Ossareh, A., Sanginabadi, M., Mokhtari Azad, T., Aghaghazvini, L., Ghaderkhani, S., Poordast, T., Pourdast, A., Nazemi, P., 2020. COVID-19 in Iran, a comprehensive investigation from exposure to treatment outcomes. MedRxiv, 2020.04.20.20072421. https://doi.org/10.1101/2020.04.20.20072421.
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P, Y. A., 2020. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J. Biol. Regul. Homeost Agents. https://www.ncbi.nlm.nih.gov/pubmed/32253888 [DOI] [PubMed]
- Dong L., Xia J.W., Gong Y., Chen Z., Yang H.H., Zhang J., He J., Chen X.D. Effect of lianhuaqingwen capsules on airway inflammation in patients with acute exacerbation of chronic obstructive pulmonary disease. Evid.-Based Complement. Alternat. Med. 2014;2014 doi: 10.1155/2014/637969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z.-P., Jia Z.-H., Zhang J., Liu S., Chen Y., Liang L.-C., Zhang C.-Q., Zhang Z., Sun Y., Zhang S.-Q., Wang Y.-Y., Wu Y.-L. Natural herbal medicine Lianhuaqingwen capsule anti-influenza A (H1N1) trial: a randomized, double blind, positive controlled clinical trial. Chin. Med. J. 2011;124(18):2925–2933. http://europepmc.org/abstract/MED/22040504 [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(1):72–73. doi: 10.5582/BST.2020.01047. [DOI] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Zhong N. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;1–13 doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza M., Ali A., Khan S., Ahmed S., Attique Z., Rehman S.U., Khan A., Ali H., Rizwan M., Khan A.M., Siddique F., Mehmood A., Hamza M., Ali A., Khan S., Ahmed S., Attique Z. nCOV-19 peptides mass fingerprinting identification, binding, and blocking of inhibitors flavonoids and anthraquinone of Moringa oleifera and hydroxychloroquine. J. Biomol. Struct. Dynam. 2020:1–11. doi: 10.1080/07391102.2020.1778534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, K., Guan, W.-J., Bi, Y., Zhang, W., Li, L., Zhang, B., Liu, Q., Song, Y., Li, X., Duan, Z., Zheng, Q., Yang, Z., Liang, J., Han, M., Ruan, L., Wu, C., Zhang, Y., Jia, Z.-H., Zhong, N.-S., 2020. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine 153242. 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Nabi G., Han G., Siddique R., Lian S., Shi H., Bashir N., Ali A., Shereen M.A. Novel coronavirus : how things are in Wuhan. Clin. Microbiol. Infect. 2020;26(4):399–400. doi: 10.1016/j.cmi.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q., Fang, X., Tian, L., Chen, X., Chung, U., Wang, K., Li, D., Dai, X., Zhu, Q., Xu, F., Shen, L., Wang, B., Yao, L., & Peng, P., 2020. The effect of Arbidol Hydrochloride on reducing mortality of Covid-19 patients: a retrospective study of real world date from three hospitals in Wuhan. MedRxiv, 2020.04.11.20056523. https://doi.org/10.1101/2020.04.11.20056523.
- Noelle Breslin, MD; Caitlin Baptiste, MD; Cynthia Gyamfi-Bannerman, MD, MPH; Russell Miller, MD; Rebecca Martinez, M., Kyra Bernstein, MD; Laurence Ring, MD; Ruth Landau, MD; Stephanie Purisch, MD; Alexander M. Friedman, MD, M., Karin Fuchs, MD; Desmond Sutton, XX; Maria Andrikopoulou, MD; Devon Rupley, MD; Jean-Ju Sheen, MD; Janice Aubey, M., Noelia Zork, MD; Leslie Moroz, MD; Mirella Mourad, MD; Ronald Wapner, MD; Lynn L. Simpson, MD; Mary E. D’Alton, M., & Dena Goffman, M. (2020). Case Series. Am. J. Obstet. Gynecol. MFM, april 9. https://doi.org/10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed]
- Runfeng, L., Yunlong, H., Jicheng, H., Weiqi, P., Qinhai, M., Yongxia, S., Chufang, L., Jin, Z., Zhenhua, J., Haiming, J., Kui, Z., Shuxiang, H., Jun, D., Xiaobo, L., Xiaotao, H., Lin, W., Nanshan, Z., Zifeng, Y., 2020. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res. 156, 104761. 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed]
- Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., Monforte A.D.A., Ismail S., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate Use of Remdesivir for Patients with Severe. 2020;Covid-19:1–10. doi: 10.1056/NEJMoa2007016. [DOI] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA – J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Islam, S., Wang, J., Li, Y., & Chen, X., 2020. Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. 16. https://doi.org/10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed]
- Li, Y., Xie, Z., Lin, W., Cai, W., Wen, C., Guan, Y., Mo, X., Wang, J., Wang, Y., Peng, P., Chen, X., Hong, W., Xiao, G., Liu, J., Zhang, L., Hu, F., Li, F., Zhang, F., Deng, X., Li, L., 2020. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial. Med. https://doi.org/10.1016/j.medj.2020.04.001 [DOI] [PMC free article] [PubMed]