Abstract
Almost all institutions routinely perform cardiac computed tomography (CT) before radiofrequency catheter ablation (RFCA) of atrial fibrillation (AF) to evaluate the cardiac anatomy. The ideal timing of the CT image acquisition is different between for RFCA of AF and for evaluation of coronary artery lesions (CALs). Thus, the aim of this study was to assess whether 64- or 320-line routine cardiac CT scans before RFCA of AF could evaluate both coronary artery lesions and pulmonary veins (LA-PVs) anatomy at the timing of the image acquisition of the LA-PVs in patients with AF who underwent RFCA of AF. The CALs were evaluated in 606 consecutive patients who underwent RFCA of AF assessed by the ideal timing of the CT image acquisition for RFCA of AF, and myocardial ischemia (MI) was also evaluated in patients with severe coronary stenosis (≥ 50%) and unevaluable CALs due to their severe coronary calcification and banding artifact by additional examinations combined with exercise stress testing, 201Tl scintigraphy, and/or fractionated flow reserve measurements. This study revealed that, in patients with AF who underwent RFCA of AF, (1) both 64- and 320-line cardiac CT scans for RFCA of AF could evaluate CALs in 93% of those patients, (2) the prevalence of MI was 9%, (3) significant relationships between the CHADS2 score and prevalence of MI were observed (p = 0.003), and (4) the positive predict values of MI in patients with severe coronary stenosis (≥ 50%) and unevaluable CALs also significantly increased in accordance with the CHADS2 score (p = 0.003). The evaluation of CALs and MI by routine cardiac CT for RFCA of AF combined with the additional examinations may be one of the most feasible modalities for patients with AF.
Keywords: Atrial fibrillation, Catheter ablation, CHADS2 score, Computed tomography, Coronary artery disease, Myocardial ischemia
Introduction
The number of patients with atrial fibrillation (AF) has been increasing, and radiofrequency catheter ablation (RFCA) of AF has proven to be a useful strategy worldwide [1]. The CHADS2 score is well known to be a useful predictor of the risk for not only cerebral infarctions associated with AF [2], but also a new onset of AF [3]. Moreover, the CHADS2 score is constructed by risk factors of coronary artery disease (CAD), and has also been reported as a predictor of cardiovascular/cerebrovascular events in patients with CAD without AF [4, 5]. The close relationship between AF and CAD has been reported [6], and that (1) the prevalence of CAD detected by coronary computed tomography (CT) is significantly higher in patients with AF than in those without [7], and (2) coronary angiography (CAG) can detect coronary narrowing (≥ 50%) in patients with AF who undergo RFCA of AF [8]. Almost all institutions routinely perform cardiac CT before RFCA of AF to evaluate the cardiac anatomy, especially of the left atrium and pulmonary veins (LA-PVs). The ideal timing of the CT image acquisition is different between the LA-PVs and coronary arteries. For example, the imagings which were the ideal timings of the image acquisition of LA-PVs or coronary arteries starts after confirming the pulmonary arteries or ascending aorta have been contrasted by visual observation, respectively. Thus, the aim of this study was to assess whether 64- or 320-line routine cardiac CT scans before RFCA of AF could evaluate both coronary artery lesions and LA-PVs anatomy at the timing of the image acquisition of LA-PVs in patients with AF who underwent RFCA of AF.
Materials and methods
Study population and laboratory analysis
This study was approved by the institutional review committee and ethics review board of our hospitals. From April 2016 to June 2018, 606 consecutive patients (402 males and 204 females with a mean age of 69 ± 0.4 years and body surface area of 1.68 ± 0.01 m2) (Table 1) with AF and without previous histories of CAD who were admitted to our hospitals to undergo RFCA of AF were evaluated. The type of AF was determined according to the 2011 ACC/AHA/ESC guidelines for the management of patients with AF [9]. Patients that could not use contrast because of renal dysfunction (serum creatinine ≥ 1.5 mg/dL) and that underwent hemodialysis were excluded. All patients had their history recorded and underwent a physical examination and laboratory analysis.
Table 1.
Patient characteristics
| All | 64-line | 320-line | p value | |
|---|---|---|---|---|
| n | 606 | 410 (68%) | 196 (32%) | – |
| Male | 408 (67%) | 268 (65%) | 140 (71%) | 0.137 |
| Age (years) | 69 ± 9.0 | 69 ± 9.2 | 68 ± 8.4 | 0.075 |
| Body mass index (kg/m2) | 23.1 ± 3.5 | 23.3 ± 3.5 | 22.8 ± 3.6 | 0.146 |
| Body surface area (m2) | 1.68 ± 0.19 | 1.67 ± 0.19 | 1.69 ± 0.17 | 0.082 |
| CHADS2 score | 2.05 ± 1.29 | 2.04 ± 1.30 | 2.07 ± 1.29 | 0.791 |
| 0 | 56 (9%) | 38 (9%) | 18 (9%) | 0.981 |
| 1 | 163 (27%) | 110 (27%) | 53 (27%) | 0.941 |
| 2 | 200 (33%) | 140 (34%) | 60 (31%) | 0.332 |
| 3 | 108 (18%) | 70 (17%) | 38 (19%) | 0.432 |
| 4 | 47 (8%) | 32 (8%) | 15 (8%) | 0.955 |
| ≥ 5 | 32 (5%) | 20 (5%) | 12 (6%) | 0.430 |
| Congestive heart failure | 293 (48%) | 188 (46%) | 105 (54%) | 0.243 |
| Hypertension | 424 (70%) | 287 (70%) | 137 (70%) | 0.899 |
| Age (≥ 75 years old) | 164 (27%) | 122 (33%) | 42 (21%) | 0.053 |
| Diabetes mellitus | 158 (26%) | 107 (26%) | 51 (26%) | 0.941 |
| History of CVA/TIA | 103 (17%) | 66 (16%) | 37 (19%) | 0.255 |
| Dyslipidemia | 200 (33%) | 135 (33%) | 65 (33%) | 0.951 |
| Ex or current smoking | 170 (28%) | 115 (28%) | 55 (28%) | 0.977 |
| Type of atrial fibrillation | ||||
| Paroxysmal | 369 (61%) | 240 (59%) | 129 (66%) | 0.086 |
| Persistent | 209 (33%) | 146 (36%) | 62 (32%) | 0.336 |
| Long-lasting | 29 (5%) | 24 (6%) | 5 (3%) | 0.075 |
| Laboratory analysis | ||||
| Serum creatinine (mg/dl) | 0.89 ± 0.24 | 0.89 ± 0.24 | 0.90 ± 0.23 | 0.409 |
| Left-ventricular ejection fraction (%) | 63 ± 9.9 | 63 ± 9.1 | 64 ± 11 | 0.268 |
| Diameter of left atrium (mm) | 39 ± 6.7 | 39 ± 6.6 | 39 ± 6.7 | 0.309 |
| Parameters during CT imaging | ||||
| Heart rate (bpm) | 66 ± 15 | 66 ± 15 | 66 ± 16 | 0.829 |
| Sinus rhythm | 398 (66%) | 261 (64%) | 137 (70%) | 0.131 |
| Medications during CT imaging | ||||
| Beta-blocker (%) | 421 (69%) | 293 (71%) | 128 (65%) | 0.124 |
| Minor tranquilizer (%) | 172 (28%) | 119 (29%) | 52 (27%) | 0.524 |
CVA cerebrovascular apoplexy, TIA transient ischemic attack, CT computed tomography
Computed tomography
All patients gave written informed consent before imaging. The imaging technique has been described previously [10]. In brief, after sublingual administration of 0.3 mg of nitroglycerin, following a test bolus, 50–60 ml of nonionic contrast medium (Omnipaque 300, Amersham Health, Oslo, Norway) was injected at 20–22 mgI/kg/s via an antecubital vein. The imaging which was the ideal timing of the image acquisition of LA-PVs started after confirming the pulmonary arteries had been contrasted by visual observation. Scanning was performed in a single breath hold in the cranio-caudal direction at the level of the aortic arch using a simultaneous acquisition of eight sections (each 0.5 mm), with a prospective electrocardiogram (ECG)-triggering set at 75% of the RR interval, beam collimation of 10 mm, and table speed 16.75 mm/0.5 s or fixed table (0.5 or 0.275 s tube rotation time, 120 kV, 500 mA, or 750 mA) by 64-line or 320-line CT scans. Contiguous 0.5 mm axial CT (Aquilion, 64-line, 320-line, TOSHIBA, Tokyo, Japan) slices were reconstructed from the CT data using a soft-tissue algorithm and the resulting DICOM data were recorded onto a CD-ROM. When the patients’ heart rate was more than 70 bpm, the oral administration of 20 mg of the beta-blocker metoprolol tartrate and/or 0.5 mg of the minor tranquilizer etizolam were used.
Evaluation of coronary artery lesions by cardiac CT and the detection of myocardial ischemia
The coronary lesions were evaluated in all patients who underwent cardiac CT scans at the timing of the image acquisition of LA-PVs for RFCA of AF. Mild-to-moderate and severe stenoses were defined as ≤ 50% and > 50% stenosis, respectively. Unevaluable coronary artery lesions were defined as severe coronary calcifications and/or banding artifacts [11] that made the evaluation of the coronary artery lesions impossible. The patients with severe coronary stenosis (> 50%) and/or unevaluable coronary artery lesions were evaluated for myocardial ischemia before or after the RFCA of AF. To evaluate the myocardial ischemia, they underwent examinations combined with exercise stress testing, 201Tl scintigraphy, and/or fractionated flow reserve (FFR) measurements [12].
Statistical analysis
The numerical results are expressed in the text as the mean ± standard deviation. Paired data were compared by a Fisher’s exact test and Student’s t test. The trend in the proportions and correlation between the prevalence of severe coronary stenosis (> 50%), unevaluable coronary artery lesions, or myocardial ischemia and the CHADS2 score was determined by a Cochran–Armitage analysis. All analyses were performed with SAS version 9.2 software (SAS Institute, Cary, NC, USA). A p of < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics and laboratory analysis (Table 1)
Cardiac CT scans for RFCA of AF were performed in 606 patients with AF and without a previous history of CVD at our hospitals. Because three patients did not agree to the use of contrast for the cardiac CT because of their renal dysfunction, they were excluded from the statistical analysis. No patients suffered from any cardiac CT-related complications except for a contrast-induced eruption. In all patients, the mean CHADS2 score was 2.05 ± 1.29 points. The numbers of patients were 56 (9%), 163 (27%), 200 (33%), 108 (18%), 47 (8%), and 32 (5%) for those with a CHADS2 score of 0, 1, 2, 3, 4, and ≥ 5 point(s), respectively. The prevalence of congestive heart failure, hypertension, age over 75 year old, diabetes mellitus, history of cerebrovascular apoplexy/transient ischemic attack, dyslipidemia, ex- or current smoking, paroxysmal AF, persistent AF, and long-lasting AF was 239 (48%), 424 (70%), 164 (27%), 158 (26%), 103 (17%), 200 (33%), 170 (28%), 369 (61%), 209 (33%), and 29 (5%), respectively. The values of the serum creatinine, left-ventricular ejection fraction (LVEF), and diameter of the left atrium (LA) (LAD) by echocardiography were 0.89 ± 0.24 mg/dL, 63 ± 9.9%, and 39 ± 6.7 mm, respectively. The mean heart rare and prevalence of sinus rhythm during the CT imaging were 66 ± 15 bpm and 398 (66%), respectively. The prevalence of the use of beta-blockers and/or minor tranquilizers during the CT imaging was 421 (69%) and 172 (28%), respectively.
Evaluation of the coronary artery lesions and myocardial ischemia (Tables 2, 3)
Table 2.
Evaluation of coronary artery lesions
| All | 64-line | 320-line | p value | |
|---|---|---|---|---|
| Number of patients | 606 | 410 (68%) | 196 (32%) | – |
| Coronary arterial stenosis | ||||
| Evaluable coronary artery lesions | 562 (93%) | 381 (93%) | 181 (92%) | 0.809 |
| Mild-to-moderate (≤ 50%) | 418 (69%) | 283 (69%) | 135 (69%) | 0.801 |
| Severe (> 50%) | 144 (24%) | 98 (24%) | 46 (23%) | 0.818 |
| Unevaluable coronary artery lesions | ||||
| Severe coronary calcification | 41 (7%) | 26 (6%) | 15 (8%) | 0.548 |
| Banding artifacts | 3 (0.5%) | 3 (0.7%) | 0 (0%) | 0.231 |
Table 3.
Evaluation and detection of myocardial ischemia
| Evaluation of myocardial ischemia | |
| Number of patients | 188 (31%) |
| Exercise stress testing | 180 (30%) |
| 201Tl scintigraphy | 82 (14%) |
| Fractionated flow reserve | 62 (10%) |
| Detection of myocardial ischemia | |
| Number of patients | 54 (9%) |
The amount of the contrast medium and radiation exposure time when evaluating the LA-PV anatomy plus the coronary arteries were the almost same when compared with evaluating the LA-PV anatomy only. They were about 60 ml or 50 ml, and 20 s or 8 s by 64-line or 320-line CT scans, respectively. However, the extra time about 15 min was needed to reconstruction and evaluation of LA-PV anatomy plus the coronary arteries compared to LA-PV anatomy only. The coronary artery lesions in 562 (93%) out of the 606 patients could be evaluated, but not in the remaining 44 (7%) patients, defined as unevaluable coronary artery lesions, because of severe coronary calcifications (n = 41; 7%) or banding artifact (n = 3; 0.5%). The prevalence of mild-to-moderate (≤ 50%), severe stenosis (> 50%), and unevaluable coronary artery lesions, was 418 (69%), 144 (24%), and 44 (7%), respectively. Myocardial ischemia was evaluated in 188 (31%) patients, including 144 (24%) patients with a > 50% coronary artery stenosis and 44 (7%) with unevaluable coronary artery lesions by cardiac CT, exercise stress testing (30%), 201Tl scintigraphy (14%), and/or fractionated flow reserve (FFR) measurements [12] (10%). Finally, myocardial ischemia was detected in 54 (9%) patients. There were no patients, including both those 144 patients with severe (> 50%) coronary artery stenosis and the remaining 462 patients, who had major adverse cardiovascular events including acute coronary syndrome and heart failure before, during, and after RFCA of AF. After RFCA of AF, 51 patients out of 54 patients with myocardial ischemia underwent re-vascularization of coronary arteries with percutaneous coronary interventions. Remaining 3 patients with myocardial ischemia were treated by optimal medical therapies.
Correlation between the CHADS2 score and myocardial ischemia (Fig. 1)
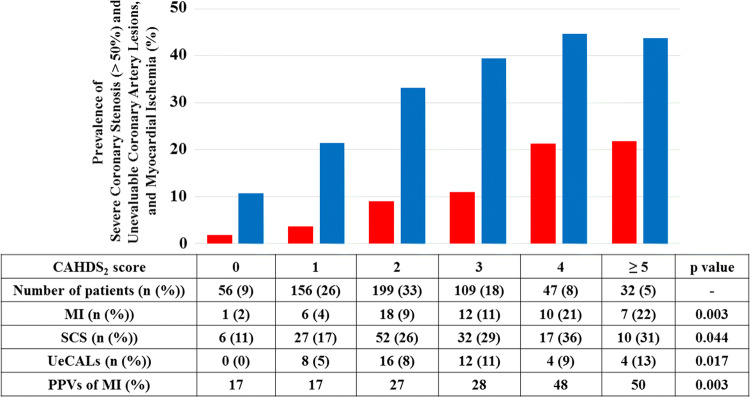
Fig. 1.
Prevalence of patients with severe coronary stenosis (SCS) (> 50%) and unevaluable coronary artery lesions (UeCALs) (blue bar), and myocardial ischemia (MI) (red bar) according to the CHADS2 score. In accordance with the CHADS2 score, the prevalence of those parameters (blue bar; p = 0.033 and red bar; p = 0.003) significantly increases. Moreover, in accordance with the CHADS2 score, the positive predict values (PPVs) of MI in patients with SCS and UeCALs significantly increase (p = 0.003). The numbers of patients were 56, 156, 199, 109, 47, and 32 for CHADS2 scores of 0, 1, 2, 3, 4, and ≥ 5 point(s), respectively
In accordance with the CHADS2 score, the prevalence of patients with severe coronary stenosis (> 50%) (p = 0.044) and unevaluable coronary artery lesions (blue bar) (p = 0.017), and myocardial ischemia (red bar) (p = 0.003) has been significantly increasing. Interestingly, in accordance with the CHADS2 score, the positive predict values of myocardial ischemia in patients with severe coronary stenosis (> 50%) and unevaluable coronary artery lesions also have been significantly increasing (p = 0.003).
Comparison between the 64- and 320-line CT (Tables 1, 2)
The prevalence of the use of a 64- and 320-line CT scans was 410 (68%) and 196 (32%), respectively. There were no statistical differences in the patient characteristics (Table 1), prevalence of evaluable or unevaluable coronary artery lesions, and prevalence of mild, moderate, and severe coronary artery lesions (Table 2), between the two groups. However, there were only 3 (0.5%) patients with rapid AF (the mean heart rate: 91 ± 3 bpm) during the CT imaging whose coronary artery lesions could not be evaluated by the 64-line scans because of banding artifact [11] due to rapid AF, even though they received beta-blockers and minor tranquilizers (Table 1, 2).
Discussion
This study revealed that, in patients with AF who underwent RFCA of AF, (1) both 64- and 320-line routine cardiac CT scans for RFCA of AF at the timing of the image acquisition of LA-PVs could evaluate coronary artery lesions in 93% of those patients, (2) the prevalence of myocardial ischemia was 9%, (3) significant relationships between the CHADS2 score and prevalence of severe coronary stenosis (> 50%) and unevaluable coronary artery lesions, and myocardial ischemia were observed, and (4) the positive predict values of myocardial ischemia in patients with severe coronary stenosis (> 50%) and unevaluable coronary artery lesions also significantly increased in accordance with the CHADS2 score (p = 0.003).
Evaluation of the coronary artery lesions by cardiac CT scans for RFCA of AF
Because the scanning performed during a single breath hold with the prospective ECG-triggering set at 75% of the RR interval during the cardiac CT imaging, the scanning becomes very difficult during an AF rhythm that has an irregular RR interval and/or tachycardia of more than 100 bpm that has a shorter RR interval [13]. Those conditions sometimes cause banding artifact, which makes it difficult to evaluate coronary artery lesions. In addition, the unique problems of the 64-line cardiac CT include the approximately 20 s single breath hold. Because the approximately 20 s breath hold may be difficult for patients with lung disease and/or older patients such as those more than 80 years old, the 320-line cardiac CT scan, which has a shorter single breath hold time than the 64-line CT scan, may be more suitable for those older patients.
Detection of myocardial ischemia by cardiac CT scans for RFCA of AF
Although it has previously been reported that the relatively high prevalence of CAD in patients with AF who undergo RFCA of AF and its relation to CHADS2 score have already been reported using CAG [8], CAG may be more invasive than the cardiac CT. This study revealed that the cardiac CT for RFCA of AF at the timing of the image acquisition of LA-PVs could evaluate coronary artery lesions in 93% of the patients who underwent RFCA of AF. Finally, myocardial ischemia was detected in 9% of those patients by cardiac CT for RFCA of AF at the timing of the image acquisition of LA-PVs combined with the additional examinations including with exercise stress testing, 201Tl scintigraphy, and/or fractionated flow reserve (FFR) measurements [12]. Moreover, a significant relationship between the CHADS2 score and myocardial ischemia (red bar in Fig. 1) (p = 0.003) was observed in this study. Furthermore, the positive predict value of myocardial ischemia in patients with severe coronary stenosis (> 50%) and unevaluable coronary artery lesions also significantly increased in accordance with the CHADS2 score (Fig. 1) (p = 0.003). These findings may support the previous findings that a high CHADS2 score has been proven to be a predictor of cardiovascular/cerebrovascular events in patients with documented CAD [4, 5]. Moreover, it has been reported that approximately 75% of culprit coronary artery lesions in patients who suffer from cardiovascular events such as acute coronary syndrome result from plaque ruptures developing a clot formation with mild-to-moderate coronary stenosis [14, 15]. Because 24% of the patients with AF who underwent RFCA of AF had severe coronary stenosis (> 50%) in this study, the future cardiovascular event risk may be decreased by performing primary prevention using diet, exercise, optimal medical therapies including statins, and re-vascularization in those patients.
Positive predict values of myocardial ischemia
The positive predict values of myocardial ischemia in patients with severe coronary stenosis (> 50%) and unevaluable coronary artery lesions significantly increased in accordance with the CHADS2 score (Fig. 1) (p = 0.003). However, those values, especially in patients with less than 1 point with the CHADS2 score, were relatively low (17%) compared with more than 4 points with that score (about 50%). Moreover, it costs medical expenses for the additional examinations to evaluate myocardial ischemia. Thus, it is unknown whether it meets a law of nature to perform those examinations to evaluate myocardial ischemia in patients with severe coronary stenosis (> 50%) and unevaluable coronary artery lesions, even though their CHADS2 score is low.
Limitations of the study
Although our study was a multi-center trial, it was limited by the retrospective design and its relatively small number of patients. Patients with AF who did not undergo RFCA of AF, especially with long-lasting AF, were not included in this study. Thus, whether our results can safely be extrapolated to a larger number of patients including patients with AF who do not undergo RFCA of AF should be determined in further prospective studies.
Conclusions
This study revealed that both 64- and 320-line routine cardiac CT scans for RFCA of AF at the timing of the image acquisition of LA-PVs could be steadily detected coronary artery lesions. Moreover, the additional examinations combined with exercise stress testing, 201Tl scintigraphy, and/or fractionated flow reserve (FFR) measurements [12] could precisely detect myocardial ischemia in 9% of patients with AF who underwent RFCA of AF. Thus, physicians should be aware of this condition when examining patients with AF, especially in the presence of a high CHADS2 score, and that may be one of the most important risk factors for the progression of cardiovascular events in patients with AF. Finally, the evaluation of coronary artery lesions and myocardial ischemia by routine cardiac CT for RFCA of AF at the timing of the image acquisition of LA-PVs combined with the additional examinations including with exercise stress testing, 201Tl scintigraphy, and/or fractionated flow reserve (FFR) measurements [12] may be one of the most feasible modalities for patients with AF.
Acknowledgements
We thank Mr. John Martin for his linguistic assistance with this paper.
Abbreviations
- AF
Atrial fibrillation
- BMI
Body mass index
- CAG
Coronary angiography
- CAD
Coronary artery disease
- CT
Computed tomography
- FFR
Fractionated flow reserve
- RFCA
Radiofrequency catheter ablation
Funding
None.
Compliance with ethical standards
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Takahiro Mito, Yoshibumi Antoku, Akihiro Masumoto, and Masatsugu Nozoe have contributed equally to this paper.
References
- 1.Piccini JP, Fauchier L. Rhythm control in atrial fibrillation. Lancet. 2016;388:829–840. doi: 10.1016/S0140-6736(16)31277-6. [DOI] [PubMed] [Google Scholar]
- 2.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 3.Zuo ML, Liu S, Chan KH, Lau KK, Chong BH, Lam KF, Chan YH, Lau YF, Lip GY, Lau CP, Tse HF, Siu CW. The CHADS2 and CHA 2DS 2-VASc scores predict new occurrence of atrial fibrillation and ischemic stroke. J Interv Card Electrophysiol. 2013;37:47–54. doi: 10.1007/s10840-012-9776-0. [DOI] [PubMed] [Google Scholar]
- 4.Tabata N, Yamamoto E, Hokimoto S, Yamashita T, Sueta D, Takashio S, Arima Y, Izumiya Y, Kojima S, Kaikita K, Matsui K, Fujimoto K, Sakamoto K, Shimomura H, Tsunoda R, Hirose T, Nakamura N, Sakaino N, Nakamura S, Yamamoto N, Matsumura T, Kajiwara I, Koide S, Sakamoto T, Nakao K, Oshima S, Tsujita K, Kumamoto Intervention Conference Study Investigators Prognostic value of the CHADS2 score for adverse cardiovascular events in coronary artery disease patients without atrial fibrillation—a multi-center observational cohort study. J Am Heart Assoc. 2017;6:e006355. doi: 10.1161/JAHA.117.006355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Wang J, Lv L, Xu C, Liu H. Usefulness of the CHADS2 and R2CHADS2 scores for prognostic stratification in patients with coronary artery disease. Clin Interv Aging. 2018;13:565–571. doi: 10.2147/CIA.S156208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawada S, Watanabe A, Morimoto Y, Nakagawa K, Nishii N, Nakamura K, Morita H, Ito H. Radiofrequency catheter ablation prior to percutaneous coronary intervention in patients with atrial fibrillation coexisting with stable coronary artery disease: a single-center pilot study. Heart Vessels. 2019;34:632–640. doi: 10.1007/s00380-018-1280-8. [DOI] [PubMed] [Google Scholar]
- 7.Nucifora G, Schuijf JD, Tops LF, van Werkhoven JM, Kajander S, Jukema JW, Schreur JH, Heijenbrok MW, Trines SA, Gaemperli O, Turta O, Kaufmann PA, Knuuti J, Schalij MJ, Bax JJ. Prevalence of coronary artery disease assessed by multislice computed tomography coronary angiography in patients with paroxysmal or persistent atrial fibrillation. Circ Cardiovasc Imaging. 2009;2:100–106. doi: 10.1161/CIRCIMAGING.108.795328. [DOI] [PubMed] [Google Scholar]
- 8.Tomomatsu T, Morishima I, Okumura K, Tsuboi H, Morita Y, Takagi K, Yoshida R, Nagai H, Ikai Y, Shibata N, Tsuzuki K, Sone T, Murohara T. Comparison of frequency and characteristics of patients with atrial fibrillation having ablation with versus without coronary narrowing (≥ 50%) by angiography. Am J Cardiol. 2017;119:1770–1775. doi: 10.1016/j.amjcard.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 9.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC, Jr, Priori SG, Estes NA, 3rd, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Ohman EM, Stevenson WG, Tarkington LG, Yancy CW, American College of Cardiology Foundation/American Heart Association Task F 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 10.Kistler PM, Rajappan K, Jahngir M, Earley MJ, Harris S, Abrams D, Gupta D, Liew R, Ellis S, Sporton SC, Schilling RJ. The impact of CT image integration into an electroanatomic mapping system on clinical outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:1093–1101. doi: 10.1111/j.1540-8167.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- 11.Mori S, Endo M, Asakura H. Improvement in banding artefacts in four-dimensional computed tomography for radiotherapy planning. Phys Med Biol. 2006;51:5231–5244. doi: 10.1088/0031-9155/51/20/010. [DOI] [PubMed] [Google Scholar]
- 12.De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius-Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd K, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nuesch E, Juni P, Investigators FT. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. doi: 10.1056/NEJMoa1408758. [DOI] [PubMed] [Google Scholar]
- 13.Jarvinen V, Uusitalo V, Tolvanen T, Saraste A, Kuusisto J, Sinisalo J, Knuuti J. The accuracy of left ventricular and left atrial volumetry using 64-slice computed tomography. In vitro validation study with human cardiac cadaveric casts. J Comput Assist Tomogr. 2018;42:754–759. doi: 10.1097/RCT.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 14.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2) N Engl J Med. 1992;326:310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- 15.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1) N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]