Highlights
-
•
Ruxolitinib, a JAK inhibitor, improves polycythemia vera and myelofibrosis symptoms.
-
•
Tuberculosis (disseminated) reactivation reported after ruxolitinib treatment.
-
•
Screening for/treating latent tuberculosis suggested before administering ruxolitinib.
-
•
This is especially pertinent in areas where the risk of tuberculosis is high.
Keywords: Mycobacterium tuberculosis, Prophylaxis, Ruxolitinib
Abstract
Ruxolitinib, a Janus kinase inhibitor, considerably improves symptoms of patients with polycythemia vera and primary or secondary myelofibrosis. However, its association with the development of infectious complications is a concern. Herein, we report the case of an 80-year-old man with primary myelofibrosis who developed disseminated tuberculosis during treatment with ruxolitinib at 15 mg twice daily and prednisone at 5 mg. We also reviewed the literature on patients who developed tuberculosis during treatment with ruxolitinib. There are 13 case reports of patients who developed tuberculosis during treatment with ruxolitinib, including our case. Disseminated tuberculosis manifestations were observed in 84.6 % of the patients and 50 % of them died. Although the interferon-gamma release assay was performed for seven of the patients with six positive results at the time of tuberculosis diagnosis, none were tested before the commencement of ruxolitinib. We suggest taking a history of tuberculosis and screening for and treating latent tuberculosis before administering ruxolitinib, especially in areas where the risk of tuberculosis is high.
Introduction
Primary myelofibrosis (PMF) is a myeloproliferative disorder characterized by bone marrow fibrosis, splenomegaly, and anemia. Ruxolitinib, a selective Janus kinase (JAK) 1 and JAK 2 inhibitor, is an oral drug that is used to treat intermediate and high-risk myelofibrosis and polycythemia vera, with excellent efficacy in improving constitutional symptoms [1]. However, the inhibition of JAK/signal transducers and activators of the transcription (STAT) pathway has been reported to exert negative effects on both T cell and dendritic cell functions, resulting in the dysfunction of cellular immunity [2]. It has also been reported that ruxolitinib therapy can result in predisposition to opportunistic infections such as the reactivation of herpes zoster virus, Pneumocystis jirovecii infection, and tuberculosis [3]. Herein, we report the case of disseminated tuberculosis in a patient with PMF treated with ruxolitinib and prednisolone. We also carried out a literature review on patients who developed tuberculosis during treatment with ruxolitinib.
Case report
An 80-year-old man was diagnosed with PMF 3 years prior to hospital admission, as confirmed by bone marrow biopsy, expressing the JAK2 V617 F mutation but with no mutations in MPL exon 10 and CALR. His medical history included herpes zoster at 79 years of age, sick sinus syndrome controlled by a permanent pacemaker, and pulmonary tuberculosis, which was partially treated at 18 years of age because isoniazid and pyrazinamide was not approved in Japan at that time, and rifampin and ethambutol had not been discovered. He did not recently have contact with patients with tuberculosis or have family or friends with a tuberculosis history. He was treated with 20 mg ruxolitinib twice daily 10 months prior to admission. Ruxolitinib induced rapid symptom relief and therefore was reduced to 15 mg twice daily, and prednisolone at 20 mg daily was started 8 months prior to admission. As his general condition improved, the prednisolone dose was gradually reduced from 20 to 5 mg. Two weeks prior to admission, he had a fever, back pain, and multiple nodules in the left femur, inguinal node, and armpit. An unenhanced chest and spine computed tomography scan demonstrated the nodules, as well as bilateral miliary shadows, in the lungs (Fig. 1) and bone destruction in thoracic vertebrae 8/9 (Fig. 2). Laboratory findings on admission were as follows: white blood cell count, 6900/μL; C-reactive protein, 7.6 mg/dL; creatinine, 1.19 mg/dL; blood urea nitrogen, 22.6 mg/dL; aspartate aminotransferase, 52 U/L; alanine aminotransferase, 45 U/L; alkaline phosphatase, 318 U/L; γ-glutamyl transpeptidase, 62 U/L; total bilirubin, 1.17 mg/dL; glucose, 138 mg/dL; HbA1c, 5.4 %. The interferon-gamma release assay (IGRA; Interferon-QuantiFERON-TB) test on admission was positive. He was hospitalized for suspected disseminated tuberculosis. Microbiological examination of his sputum showed positive acid–alcohol resistant bacilli, and polymerase chain reaction and culture were both positive for Mycobacterium tuberculosis, which was susceptible to all anti-tuberculosis drugs. We diagnosed disseminated tuberculosis, including tuberculous spondylitis, pulmonary tuberculosis, and skin tuberculosis. The standard tuberculosis treatment (300 mg isoniazid, 450 mg rifampin, and l500 mg ethambutol once daily) was started, and his general condition gradually improved. We did not administer pyrazinamide, because he was over 80 years of age and his laboratory results showed elevated liver enzymes. Four weeks later, he had fever diagnosed as an initial aggravation, and thus, pyrazinamide at 1200 mg once daily was added to the anti-tuberculosis treatment. Ruxolitinib was discontinued gradually over 4 months. Three months after the commencement of treatment, a follow-up sputum culture was negative for M. tuberculosis and the lower respiratory symptoms and skin nodules were resolved. Five months after treatment, thoracic vertebrae debridement surgery was performed to control his back pain. On post-operation day 5, however, the patient presented with acute heart failure and was diagnosed with takotsubo cardiomyopathy. He died on post-operation day 13.
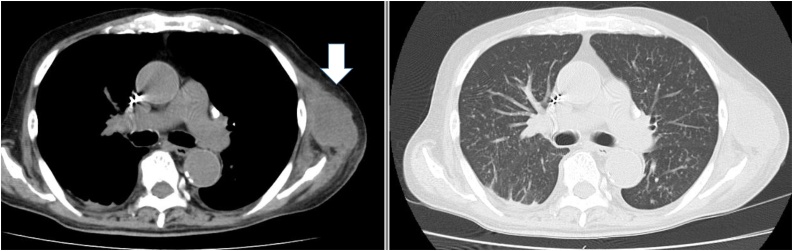
Fig. 1.
An enhanced computed tomography scan of the chest demonstrating a left axial subcutaneous nodule and bilateral miliary nodules.
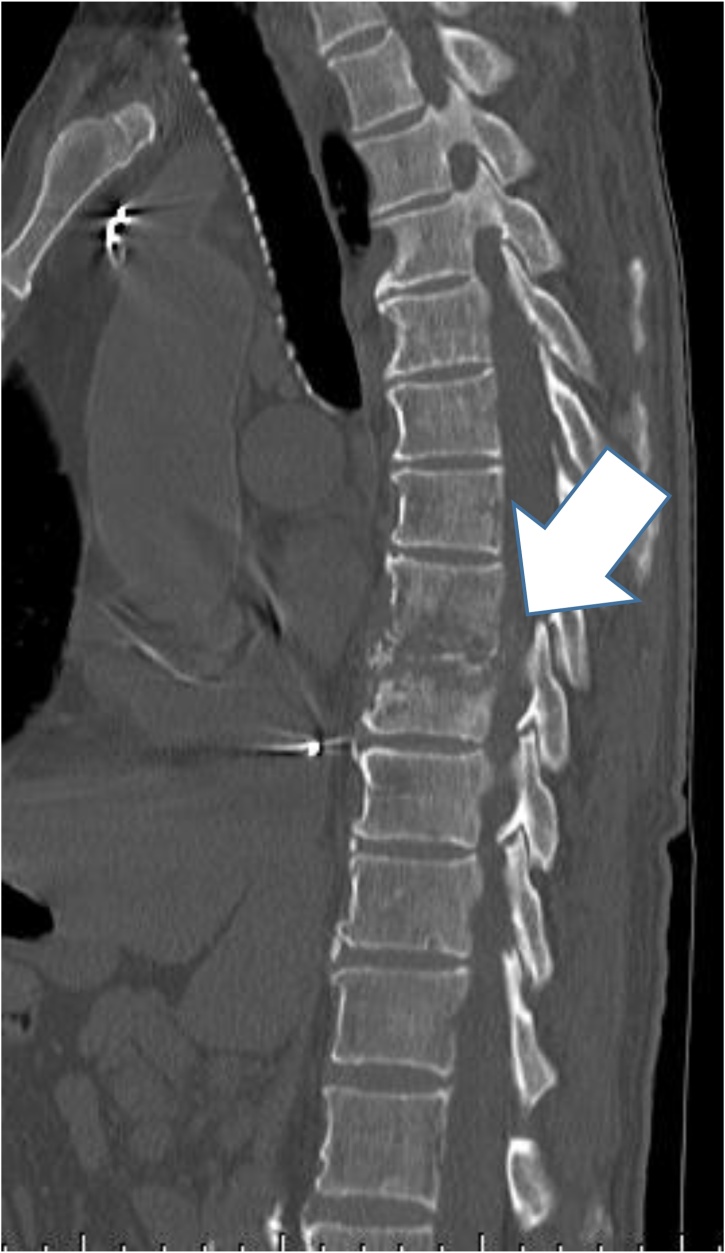
Fig. 2.
A computed tomography scan demonstrating bone destruction in the thoracic vertebrae.
Discussion
Ruxolitinib was approved in 2011 for PMF. Its use for patients with PMF prolongs survival time and improves symptoms as compared to those with conventional treatment [1]. However, ruxolitinib inhibits the JAK–STAT signaling pathway and downregulates proinflammatory cytokines resulting in immunosuppression [2]. An association between ruxolitinib and various infections has been reported [3]. The most commonly reported infections are urinary tract infections, pneumonia, sepsis, and viral infections. Ruxolitinib is also associated with an increased risk of other opportunistic infections, such as fungal and mycobacterial infections [3]. A recently published position paper by the European Conference on Infections in Leukemia (ECIL) concluded that ruxolitinib-treated patients should be carefully evaluated for serious infections at the onset of fever [4].
Our search revealed 13 reported cases of tuberculosis that developed during ruxolitinib treatment, including our case (Table 1) [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]]. Eleven of these case reports have been published during the past 3 years. The average age of the patients was 69.5 years and the male-to-female ratio was 10:3. The underlying disease in all cases was PMF. Only three patients took prednisone for PMF, suggesting that ruxolitinib alone can predispose an individual to tuberculosis. In our review, 11 of the 13 patients had disseminated tuberculosis; this is significantly higher than the average rate (3.5 %) of disseminated manifestations among tuberculosis cases [16]. Although IGRA was utilized as a diagnostic tool for tuberculosis in seven cases with six positive results, it was not utilized in any of the cases as a screening tool for tuberculosis before the commencement of ruxolitinib.
Table 1.
Literature review of tuberculosis with ruxolitinib treatment in patients with primary myelofibrosis.
| Year/reference | Country | Age /sex | Culture | Diagnosis (abnormality) | Timing of infection after the start of ruxolitinib | IGRA before ruxolitinib | IGRA after diagnosis of TB | Treatment for LTBI | Other risk of tuberculosis | Ruxolitinib therapy after diagnosis of infection | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Branco (2016) [5] | Born in Algeria but living in France | 78/F | Cervical lymph node | Disseminated (cervical lymph node, T4–T5 spinal disc) | 1.5 years | NA | Positive | None | Diabetes | Continued | Alive |
| Shamil (2015) [6] | UK | 78/F | Cervical lymph node | Disseminated (cervical lymph node, lung) | Unspecified | NA | NA | None | None | Discontinued | Alive |
| Keizer (2015) [7] | Netherland | 72/M | N/A | Disseminated(N/A) | 5 months | Not performed | NA | N/A | N/A | Discontinued | dead |
| Keizer (2015) [7] | Netherland | 68/M | N/A | Pulmonary | 4 weeks | Not performed | NA | N/A | N/A | Discontinued | dead |
| Chen (2015) [9] | Taiwan | 82/M | Sputum | Pulmonary | 2 months | NA | NA | None | PSL 10 mg | Discontinued | Alive |
| Palandri (2015) [10] | Italy | 65/F | Lymph node | Extrapulmonary (subaxillary lymph nodes) | 4 months | Not performed | Negative | None | none | Discontinued | Alive |
| Hopman (2014) [11] | Emigrated from Vietnam to the United States | 62/M | BALF, blood | Disseminated (lung, lymph node) | 7 weeks | NA | NA | none | none | Discontinued | Alive |
| Colomba (2012) [12] | Italy | Unspecified/M | Inguinal lymph node, sputum | Disseminated (inguinal lymph node, lung) | 2 months | NA | Positive | None | none | Unspecified | Unspecified |
| Panda (2016) [13] | India | 35/M | Right psoas abscess | Disseminated (psoas muscle, para-aortic lymph node, lung) | 2 years | NA | NA | None | hydroxyurea | 6 weeks continued, discontinued | Alive |
| Abidi (2016) [14] | India | 69/M | Cervical lymph node | Disseminated (lung, cervical lymph node) | 3 weeks | NA | Positive | None | None | Discontinued | Alive |
| Tsukamoto (2017) [8] | Japan | 73/M | Sputum, gastric lavage fluid, blood, and urine | Disseminated (lung) | 6 months | NA | Positive | None | PSL 5 mg | Discontinued | dead |
| Lescuyer et al. (2019) [15] | France | 73/M | Cerebral biopsy | Disseminated (lung, brain) | 5 months | NA | Positive | None | None | Discontinued | dead |
| Current | Japan | 80/M | Sputum, skin abscess | Disseminated (skin abscess, lung, spine) | 10 months | Not performed | Positive | None | PSL 5 mg | 4 months continued, discontinued | dead |
NA: not available; there was no relevant information in the manuscript.
When considering the possibility of tuberculosis, geographic epidemiology is particularly important. Tofacitinib, which inhibits the pan-JAK receptor, is used for rheumatoid arthritis; 81 % of tuberculosis cases during tofacitinib treatment have occurred in countries with a high background tuberculosis incidence rate and the occurrence rate was found to vary with the regional background tuberculosis incidence rate [17]. Baricitinib also predisposes patients to tuberculosis; however, these cases occurred only in pandemic areas [18]. In Japan, the incidence of tuberculosis is the highest among developed countries and is 4.4-fold higher than that in the United States, reaching 64.2 per 100,000 individuals aged 75–84 years, and the expected infection prevalence is 53.1 % among those aged 60–69 years [19]. We agree with the ECIL position paper and Official Statement of the American Thoracic Society, which states that screening and treatment for latent TB should be considered if epidemiological risk factors and medical history are significant [4,20]. Notably, disseminated tuberculosis in patients with a negative IGRA result has been reported; therefore, careful monitoring is necessary during ruxolitinib treatment even if the IGRA result is negative [10,21].
In conclusion, we report a case of disseminated tuberculosis in a patient who received ruxolitinib. When considering the administration of ruxolitinib, screening and treatment for latent tuberculosis is necessary for patients born or living in tuberculosis-endemic areas. Continuous surveillance is needed to evaluate the risk of tuberculosis during treatment with ruxolitinib and other JAK inhibitors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Informed consent to publish this paper was obtained from the patient and his family.
Consent
Informed consent to publish this paper was obtained from the patient and his family.
Author contribution
All authors have made substantial contribution to the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article, or revising it critically for important intellectual content. All authors have provided final approval for submission of the final version. All authors meet the ICMJE authorship criteria.
Declaration of Competing Interest
None.
CRediT authorship contribution statement
Nobuyasu Hirai: Conceptualization, Writing - original draft, Writing - review & editing. Kei Kasahara: Supervision, Conceptualization, Writing - original draft, Writing - review & editing. Shingo Yoshihara: . Tomoko Nishimura: Conceptualization. Keitaro Omori: Conceptualization. Yoshihiko Ogawa: Conceptualization. Taku Ogawa: Conceptualization. Naokuni Hishiya: Conceptualization. Yuki Suzuki: Conceptualization, Investigation. Hisakazu Yano: Investigation. Masahide Yoshikawa: Supervision. Keiichi Mikasa: Supervision.
References
- 1.Verstovsek S., Kantarjian H., Mesa R.A., Pardanani A.D., Cortes-Franco J., Thomas D.A. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baxter E.J., Scott L.M., Campbell P.J., East C., Fourouclas N., Swanton S. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 3.Lussana F., Cattaneo M., Rambaldi A., Squizzato A. Ruxolitinib-associated infections: a systematic review and meta-analysis. Am J Hematol. 2018;93:339–347. doi: 10.1002/ajh.24976. [DOI] [PubMed] [Google Scholar]
- 4.Maschmeyer G., De Greef J., Mellinghoff S.C., Nosari A., Thiebaut-Bertrand A., Bergeron A. Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL) Leukemia. 2019;33:844–862. doi: 10.1038/s41375-019-0388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branco B., Metsu D., Dutertre M., Marchou B., Delobel P., Recher C. Use of rifampin for treatment of disseminated tuberculosis in a patient with primary myelofibrosis on ruxolitinib. Ann Hematol. 2016;95:1207–1209. doi: 10.1007/s00277-016-2684-0. [DOI] [PubMed] [Google Scholar]
- 6.Shamil E., Cunningham D., Wong B.L., Jani P. Ruxolitinib associated tuberculosis presenting as a neck lump. Case Rep Infect Dis. 2015;2015 doi: 10.1155/2015/284168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keizer S., Gerritsen R., Jauw Y., Janssen J., Koopman B., Bresser P. Fatal tuberculosis during treatment with ruxolitinib. Ned Tijdschr Geneeskd. 2015;159:A8650. [PubMed] [Google Scholar]
- 8.Tsukamoto Y., Kiyasu J., Tsuda M., Ikeda M., Shiratsuchi M., Ogawa Y. Fatal disseminated tuberculosis during treatment with ruxolitinib plus prednisolone in a patient with primary myelofibrosis: a case report and review of the literature. Intern Med. 2018;57:1297–1300. doi: 10.2169/internalmedicine.9165-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Yh, Lee Ch, Pei Sn. Pulmonary tuberculosis reactivation following ruxolitinib treatment in a patient with primary myelofibrosis. Leuk Lymphoma. 2015;56:1528–1529. doi: 10.3109/10428194.2014.963082. [DOI] [PubMed] [Google Scholar]
- 10.Palandri F., Polverelli N., Catani L., Vianelli N. Ruxolitinib-associated tuberculosis: a case of successful ruxolitinib rechallenge. Ann Hematol. 2015;94:519–520. doi: 10.1007/s00277-014-2183-0. [DOI] [PubMed] [Google Scholar]
- 11.Hopman R.K., Lawrence S.J., Oh S.T. Disseminated tuberculosis associated with ruxolitinib. Leukemia. 2014;28:1750–1751. doi: 10.1038/leu.2014.104. [DOI] [PubMed] [Google Scholar]
- 12.Colomba C., Rubino R., Siracusa L., Lalicata F., Trizzino M., Titone L. Disseminated tuberculosis in a patient treated with a JAK2 selective inhibitor: a case report. BMC Res Notes. 2012;5:552. doi: 10.1186/1756-0500-5-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panda A., Chandrashekhara S.H., Nambirajan A., Mishra P. Idiopathic myelofibrosis with disseminated hepatosplenic, mesenteric, renal and pulmonary extramedullary haematopoeisis, portal hypertension and tuberculosis: initial presentation and 2 years follow-up. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-217854. bcr2016217854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abidi M.Z., Haque J., Varma P., Olteanu H., Guru Murthy G.S., Dhakal B. Reactivation of pulmonary tuberculosis following treatment of myelofibrosis with ruxolitinib. Case Rep Hematol. 2016;2016 doi: 10.1155/2016/2389038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lescuyer S., Ledoux M.P., Gravier S., Natarajan-Ame S., Duval C., Maloisel F. Tuberculosis and atypical mycobacterial infections in ruxolitinib-treated patients with primary or secondary myelofibrosis or polycythemia vera. Int J Infect Dis. 2019;80:134–136. doi: 10.1016/j.ijid.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Trends in tuberculosis--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:201–205. [PMC free article] [PubMed] [Google Scholar]
- 17.Winthrop K.L., Park S.H., Gul A., Cardiel M.H., Gomez-Reino J.J., Tanaka Y. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1133–1138. doi: 10.1136/annrheumdis-2015-207319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harigai M., Takeuchi T., Smolen J.S., Winthrop K.L., Nishikawa A., Rooney T.P. Safety profile of baricitinib in Japanese patients with active rheumatoid arthritis with over 1.6 years median time in treatment: an integrated analysis of Phases 2 and 3 trials. Mod Rheumatol. 2019:1–8. doi: 10.1080/14397595.2019.1583711. [DOI] [PubMed] [Google Scholar]
- 19.Toyota M., Sasaki Y. The issue of tuberculosis in the elderly in Japan. Kekkaku. 2010;85:881–894. [PubMed] [Google Scholar]
- 20.American Thoracic Society CDC targeted tuberculin testing and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:S221–47. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 21.Mori T., Harada N., Higuchi K., Sekiya Y., Uchimura K., Shimao T. Waning of the specific interferon-gamma response after years of tuberculosis infection. Int J Tuberc Lung Dis. 2007;11:1021–1025. [PubMed] [Google Scholar]