Abstract
Small heat shock proteins (sHsps) are ubiquitous molecular chaperones found in all domains of life, possessing significant roles in protein quality control in cells and assisting the refolding of non-native proteins. They are efficient chaperones against many in vitro protein substrates. Nevertheless, the in vivo native substrates of sHsps are not known. To better understand the functions of sHsps and the mechanisms by which they enhance heat resistance, sHsp-interacting proteins were identified using affinity purification under heat shock conditions. This paper aims at providing some insights into the characteristics of natural substrate proteins of sHsps. It seems that sHsps of prokaryotes, as well as sHsps of some eukaryotes, can bind to a wide range of substrate proteins with a preference for certain functional classes of proteins. Using Drosophila melanogaster mitochondrial Hsp22 as a model system, we observed that this sHsp interacted with the members of ATP synthase machinery. Mechanistically, Hsp22 interacts with the multi-type substrate proteins under heat shock conditions as well as non-heat shock conditions.
Keywords: Small heat shock protein (sHsp), Molecular chaperone, Protein substrates, Hsp22, Drosophila melanogaster
Introduction
Maintaining protein homeostasis (proteostasis) is one of the most important processes for all cellular organisms. Many proteins are working together to efficiently stabilize protein homeostasis (Richter et al. 2010). This process has been implicated in many degenerative diseases in which proteins tend to become insoluble forming aggresomes (Luheshi et al. 2008; Powers et al. 2009; Richter et al. 2010). Molecular chaperones perform an essential role and function as the first line of defense in this process (Hartl et al. 2011; Tyedmers et al. 2010). Stress conditions lead to dramatic increases in the level of protein unfolding, resulting in the accumulation of damaged proteins (Tyedmers et al. 2010). ATP-dependent chaperones including Hsp60, Hsp70, and Hsp90 bind to the partially folded or denatured proteins under these conditions and promote their refolding using the energy of ATP hydrolysis (Richter et al. 2010).
The family of small heat shock proteins (sHsps) are ubiquitous molecular chaperones with polypeptide sizes between 12 and 43 kDa. They are the key players in stress responses and exist in all forms of life (Jakob et al. 1993; Maaroufi and Tanguay 2013; Mymrikov and Haslbeck 2015; Walther et al. 2015). In contrast to the other ATP-dependent Hsps, the sHsps suppress protein aggregation in an ATP-independent fashion and are thought to stabilize early stress affected intermediates (Haslbeck et al. 1999; Horwitz 1992; Tsvetkova et al. 2002). sHsps effectively interact with a large variety of unfolded proteins, ranging from peptides to large proteins, and trigger activation of ATP-dependent chaperones, Hsp70\Hsp40, which assist release of individual proteins from aggregates and facilitate their subsequent refolding to the native form (Fig. 1) (Bepperling et al. 2012; Cashikar et al. 2005; Ehrnsperger et al. 1998; Lee et al. 1997; Lee and Vierling 2000). There are several factors that lead to a shift of the oligomeric distribution of sHsps, which include the increase in temperature, presence of unfolded substrates, changes in pH, and post-translational modifications, especially phosphorylation (Haslbeck et al. 1999) (Fig. 1). Thus, sHsps are central players necessary for inhibition of protein aggregation following acute proteotoxic stresses and act as holdases, as well as disaggregases (Haslbeck and Vierling 2015; Mogk et al. 2018). The ratio of sHsp to substrate, temperature, and characteristics of the substrate determine the composition and size of the sHsp\substrate (Basha et al. 2004b; Stromer et al. 2003). This complex inhibits the exposure of hydrophobic surfaces and prevents the formation of more aggregates; the association of sHsp with its substrate can also facilitate disaggregation in the presence of ATP-dependent chaperones (Ungelenk et al. 2016). Heat shock is a proteotoxic stress that activates the regulation of many signaling pathways, including overexpression of sHsps in various mammalian cells to increase resistance against different stresses especially in the early stress response (Nadeau and Landry 2007). In addition, overexpression of sHsp genes has been shown to increase longevity in Drosophila (Morrow et al. 2004b) and in various mammalian cell models (Morrow et al. 2004a; Salinthone et al. 2007).
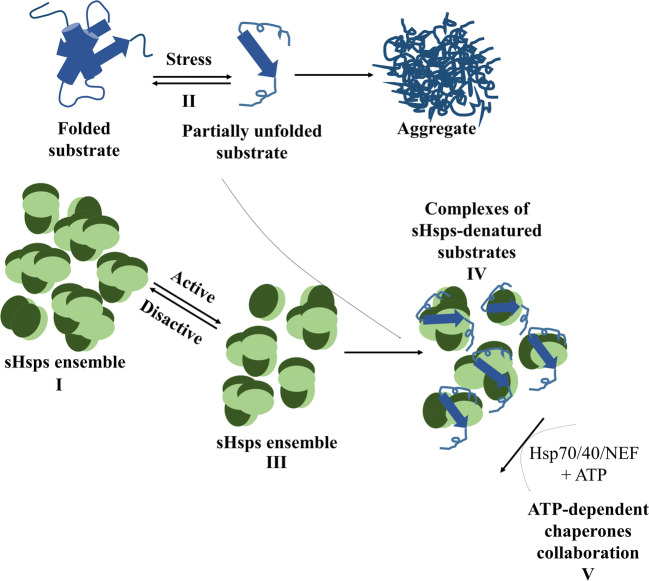
Fig. 1.
Mechanism of chaperone function of sHsps. sHsps exist in a wide variety of oligomers with different sizes which exchange their subunits consequently (I). In response to stress, a large variety of substrates are destabilized and form partially unfolded substrate (II). This causes activation of sHsps through formation of a population which can capture early denatured substrates by their substrate-binding sites (III). The activated species bind to the large range of non-native substrate proteins which sometimes are integrated in the oligomeric structure and prevent them from undergoing irreversible aggregation by the formation of sHsps-substrate complexes (IV). Finally, the bound substrates can be released from the complexes and refolded with the help of the ATP-dependent Hsp70/40/NEF chaperone system (V)
It has been more than 20 years since the chaperone-like activity of sHsps has been reported (Horwitz 1992). Most of these studies were done in vitro, where chaperone function of sHsps was measured using different model protein substrates (Basha et al. 2012; Jaya et al. 2009; Morrow et al. 2006; Salinthone et al. 2007). One important question which remained unanswered was the nature of protein substrates protected in vivo. Thus, the identification of in vivo protein substrates of sHsps, as well as their characterization, substrate-binding sites, and structural features of different sHsp/substrates, is needed to clarify the question of specificities or structural preferences of protein domains. Mammalian HspB4 and HspB5, mouse Hsp25, and human HspB1 and HspB8 are involved in diverse cellular functions including cell differentiation, apoptosis, and longevity, and disruption of their functions has been associated with the development of numerous diseases (Favet et al. 2001; Salinthone et al. 2007; Morrow et al. 2004b; Sanbe et al. 2013; Irobi et al. 2004).
The highly conserved alpha-crystallin domain (ACD) is the characteristic feature of all sHsps and is flanked by the highly diverse N-terminal domain and short C-terminal extension (Haslbeck and Vierling 2015; Kriehuber et al. 2010; Mogk et al. 2003). Most members of the sHsp family form large oligomeric structures, using dimers as the building blocks (Haley et al. 1998; Narberhaus 2002). The huge diversity in the expression patterns and biochemical properties of sHsps in organisms suggests a wide variety of substrates and in vivo functions, which needs more investigation as well as comparative functional studies to decipher detailed information (Carra et al. 2013). sHsps integrate tightly with denatured proteins to keep them in soluble form. However, the increasing ratio of substrate to sHsp causes the formation of larger complexes and eventually of insoluble complexes (Mogk et al. 2003). This paper summarizes the various sHsps substrates described in the current literature. The data presented here can help in providing critical insights into the substrates of sHsps in cells and should be useful for understanding the mechanism of chaperone function and other functions performed by members of the sHsps family.
Substrate spectra of sHsps
Several investigations have reported the diversity of substrates interacting with sHsps using proteomic approaches. Co-immunoprecipitation and/or immunoaffinity conjugation lead to the identification of interaction spectra indicating the general properties and functional classifications of substrates. A detailed analysis was performed to explain more precisely the biophysical nature of such protein-protein interactions. Nevertheless, it is still unclear how exactly they recognize the different substrates. It seems that the interactions are mostly dependent on the charge, size, and hydrophobicity of substrates (Basha et al. 2012). Apparently, activation of sHsps needs a shift in the equilibrium toward the formation of smaller populations representing active form oligomers (Basha et al. 2012; Delbecq and Klevit 2013; Haslbeck and Vierling 2015; Haslbeck et al. 1999). For example, 13 and 37 proteins were identified associated with Hsp16.6 from Cyanobacterium Synechocystis and IbpA from E. coli in heat-shocked cells, respectively (Basha et al. 2004a; Butland et al. 2005). We have recently identified a total 137 and 72 proteins of HeLa cells interacting in vivo with Hsp22 from Drosophila melanogaster (DmHsp22) in two independent immunoaffinity conjugations (IAC) in which 60 proteins were common (Dabbaghizadeh et al. 2018). Eighty-nine and 100 proteins were identified as natural substrate proteins for bacterium Deinococcus radiodurans Hsp20.2 and E. coli IbpB, respectively, in heated conditions (Bepperling et al. 2012; Fu et al. 2013b). It seems that IbpB tends to bind a wide range of cellular proteins (Basha et al. 2004a). It has also been reported that Hsp20.2 and IbpB are involved in energy metabolism with 38 and 66 protein substrates involved in this process. Mymrikov and coworkers determined substrate spectra of human sHsps through mass spectrometry following heat shock on cell lysate suspensions (Mymrikov et al. 2016). A total of 1100 proteins were precipitated upon incubation of lysates at elevated temperature (Mymrikov et al. 2016). Among the human sHsps, HspB1 and HspB5 bound to about 100 substrates with highest efficiency, while almost no interactions were detected for HspB6 and HspB7. Immunoprecipitation analysis determined 39 out of 168 proteins which directly or indirectly interact with chloroplast Hsp22E/F with high confidence (Rutgers et al. 2017). Five of these proteins co-precipitated with Hsp22E/F through immunoblot analysis in both heat shock and non-heat shock conditions (Rutgers et al. 2017). Two cytoplasmic chaperones Hsp22A and Hsp70A were detected as interactors with Hsp22E/F probably due to the fusion of chloroplast and cytosolic aggregates during the cell lysis process or due to the cross-interaction of antibody with cytosolic chaperones (Rutgers et al. 2017). Thirty-six and 16 proteins were respectively identified as plant C1 and CII class-associated proteins following heat exposure (McLoughlin et al. 2016). These are directly or indirectly involved in translation such as initiation factor Etf4A, three isoforms of RNA helicase, and three subunits of elongation factor eEF1B (McLoughlin et al. 2016). Forty-two proteins potentially interact in vivo with Hsp16.6 but only under heat-shocked condition in Cyanobacterium Synechocystis (Basha et al. 2004a). A protective effect of Hsp16.6 on serine esterase following exposure to heat treatment at 46 °C for 60 min has been reported (Basha et al. 2004a). Elongation factor Ts (a GDP/GTP exchange factor) and S1 are heat-sensitive substrates of Hsp16.6, and both are involved in translation regulation (Basha et al. 2004a). Ubiquitin-conjugating enzyme, proteasomal subunit, F-box protein, myotonic dystrophy protein kinase, initiation factor 4G, actin, and intermediate filament proteins have been reported as partners of sHsps in different organisms (Basha et al. 2004a). The inhibition of sHsp26 expression reduces the expression level of ATP synthase subunits α and β, but not γ under stress condition. Heat shock reduces ATP synthase subunits activity not only in DmHsp22 but also in maize chloroplast sHsp26 (Dabbaghizadeh et al. 2018; Hu et al. 2015).
Functional characteristics of proteins interacting with sHsps
Proteins interacting with sHsps are involved in a huge range of cellular functions including metabolism, translation, folding, and signal transduction. They possess chaperone activity as one of their distinct properties, which change depending on the sHsps and the model substrate (Mymrikov et al. 2016). Some sHsps tightly bind to certain proteins without suppressing their aggregation, which suggests that substrate binding and prevention of aggregation are two independent concepts and substrates may bind stably or transiently to sHsps (Mymrikov et al. 2016). PANTHER classification tool revealed the involvement of detected proteins in different metabolic processes including RNA, DNA, and protein metabolisms and translation processes including tRNA metabolic processes and protein turnover such as protein folding, protein complex assembly, and biogenesis. Furthermore, regulatory proteins especially of the translational machinery are among the most abundant interactors of sHsps (Fu et al. 2013c). General hydrophobicity of a protein is not an important factor for substrate recognition (Mymrikov et al. 2016). Translation-related proteins such as amino-acyl tRNA synthases and translation factors, as well as metabolic enzymes, are some examples of proteins interacting with sHsps. TufB, TufA, and MDH are among the substrates detected for IbpB (Fu et al. 2013c). Ef-Tu has been detected as the IbpB substrate at 50 °C but not at 30 °C, while the expression level is the same at both temperatures (Fu et al. 2013c). While metabolic enzymes prefer to bind to the N-terminal region specially Phe-16 and Asn-25, translation-related proteins interact with both N-terminal and ACD of IbpB, which suggests the possible interaction of each functional class of substrate protein to certain positions in sHsps (Fu et al. 2013c). This suggests that sHsps preferentially bind to proteins involved in translation and transcription machineries during stress conditions and to their immediate refolding by ATP-dependent chaperones (Haslbeck and Vierling 2015). IbpA from Acholeplasma laidlawii reduces the toxic content of denatured proteins in E. coli by interacting with certain proteins during the stress process. It may act indirectly and stabilizes many unfolded proteins upon stress at 46 °C for 30 min. Twelve unique proteins were discovered in the pull-down assay, and these mostly belong to enzymes participating in protein biosynthesis and energy metabolism. ATP synthase F1, β subunit, and transcriptional elongation factor Tu (EF-Tu) were among the detected proteins (Kayumov et al. 2017). Hsp70 (HspA4) interacts with HspB1, HspB2, HspB3, and HspB5, and DNAJB1, a member of Hsp40 family, interacts with HspB4. This indicates that many chaperones work together in a network to rescue aggregation-prone proteins.
Mitochondrial DmHsp22-associated proteins are involved in metabolic and cellular processes according to their classification in molecular pathways. Catalytic activity and transporter proteins are among the most abundant proteins detected relating to the DmHsp22. Many ATP synthase subunits were identified as substrates of DmHsp22 in heat shock and non-heat shock conditions, and the ATP synthase pathway is the most abundant pathway in which mitochondrial partners of DmHsp22 are involved, implicating involvement of DmHsp22 in the energy homeostasis of mitochondria (Dabbaghizadeh et al. 2018). Together, it appears that DmHsp22 could protect proteins involved in quality control of subunits of ATP synthase machinery during the process of ATP production in mitochondria. Hsp16.6-associated proteins mostly participate in translation, transcription, secondary metabolism, and cell signaling (Basha et al. 2004a). Heme oxygenase, serine esterase, and shikimate kinase are among the Hsp16.6-associated proteins detected through mass spectrometry. Ferredoxin-NADP+ reductase, an essential enzyme involved in electron transport pathways, as well as ATP synthase subunit beta which is essential for energy metabolism was detected abundantly as the partners of Hsp16.6 activities (Basha et al. 2004a).
In summary, together these data may suggest new functions of sHsps, acting in complex with HSP70 and HSP40 chaperones. sHsps confer cytoprotection that will be essential to restore proteostasis-requiring proteins such as mitochondrial proteins along with translation and transcription factors.
Cytoskeletal proteins as interactors of sHsps
Small Hsps interact with intermediate filaments, and this interaction is important to maintain individuality of the intermediate filaments and their interactions (Perng et al. 1999). Several cytoskeletal proteins including alpha-tubulin and alpha-centractin, dynein-1, and kinesis-1 were detected as partners of HspB1-HspB5. Eukaryotic sHsps interact with cytoskeletal proteins in cytoplasm and protect them under stress conditions (Verschuure et al. 2003). Human Hsp27 was closely associated with F-actin at lateral cell boundaries and with aggregated actin within the cell body in renal epithelia (Van Why et al. 2003). In addition, binding of HspB1 to tubulin and microtubules was enhanced in Charcot-Marie-Tooth-causing HspB1 mutants leading to a reduction in microtubule dynamics (Almeida-Souza et al. 2011). Chaperone complex BAG3-HspB8 possesses an important role in actin-based spindle positioning of mitotic structures (Fuchs et al. 2015). Different types and isoforms of actin, tubulin, and myosin form a significant part of DmHsp22 partners working in cell extract, as well as mitochondrial suspension. This suggests possible involvement of DmHsp22 in mitochondrial trafficking and positioning in specific subcellular compartments (Dabbaghizadeh et al. 2018). Actin, tubulin, and myosin are among the proteins upregulated in flies overexpressing DmHsp22, which is another observation consistent with the participation of this sHsp in mitochondrial positioning (Morrow et al. 2016b). Another possibility is the involvement of DmHsp22 in intermediate filament assembly.
General characteristics of proteins interacting with sHsps provide insights into the cellular processes affected by heat stress
Comparative analysis of the size, isoelectric point (pI), and substrate hydrophobicity showed some of the common features of substrates interacting with sHsps under heat shock conditions. First, the aggregation tendency is the principal factor determining the interaction between sHsps and protein substrates during the folding/unfolding process, and sHsps interact with all type of aggregation-prone model substrate proteins under in vitro conditions (Carver et al. 2002; Jakob et al. 1993; Merck et al. 1993). Studies on the natural structural features of protein substrates of sHsps suggested their selective preference to bind the substrates with certain structural features (Bepperling et al. 2012; Fu et al. 2013c). They showed more tendency to interact with higher molecular weight substrates (> 60 kDa) than with smaller proteins (20–30 kDa) (Fu et al. 2014). It seems that larger proteins are more prone to become aggregated/misfolded during stress conditions and they require more protection by sHsps during the folding/unfolding process (Fu et al. 2014; Fu et al. 2013b). Recently published data reported that substructural dimers of two plant sHsps, Ta16.9 and Ps18.1, are more effective chaperones to encounter early denatured substrates and folded dimers considered as functional units for capturing primary unfolded substrates (Santhanagopalan et al. 2018). Proteins containing low percentage of β-sheets and high percentage of α-helixes are the most favorable subunits of sHsps (Fu et al. 2013a). Acidic isoelectric pH is another conserved property of most of the proteins associated with sHsps, and both IbpB and Hsp20.2 significantly prefer to interact with substrates with IP between 5.0 and 5.5 (P < 0.05) (Fleckenstein et al. 2015; Fu et al. 2014; Rutgers et al. 2017). Although hydrophobic interactions are considered important sHsp-substrate interactions under in vitro condition, some other weak interactions including ionic interactions as well as hydrogen bonds play crucial roles in this process. Together, multi-type interactions cause dynamic behavior of sHsps/substrates complexes and facilitate the release and refolding of substrates (Friedrich et al. 2004; Mogk et al. 2003). Some recent investigations revealed sHsps do not tend to interact with substrates containing hydrophobic residues including aliphatic (Leu, Ile, Val) and aromatic (Trp, Phe, Tyr), but they prefer to bind charged (negatively or positively) residue substrates, which shows the importance of ionic interactions rather than hydrophobic interactions (Fu et al. 2014; Fuchs et al. 2010; Matuszewska et al. 2005). Thermolability is another common feature among Hsp22E/F substrates, which become disrupted as they are exposed to the heat stress conditions mostly because they impede their optimal function at the specific growing temperature of organisms (Rutgers et al. 2017). Mymrikov et al. (2016) determined common features of sHsp’s substrates at heat shock conditions by performing a comparative analysis on the size, pI, and distribution of substrates hydrophobicity. Investigations revealed a tendency of sHsps to bind to the substrates with higher content of ß-sheets, as it improves not only substrate recognition occurrence by sHsps but also intermolecular ß-sheet interactions between sHsps and substrates (Fu et al. 2014). Protein substrates containing more α-helix and coil have more chance to be trapped by sHsps (Fu et al. 2014). Thus, IbpB prefers to bind to the multi-domain proteins rather than single-domain proteins according to the analysis of substrates of IbpB with identified 3D structure using domain-classification databases SCOP (Murzin et al. 1995). IbpA selectively protects proteins with high molecular weights; by contrast, Hsp60 prefers to interact with low molecular weight proteins (Kayumov et al. 2017).
Chaperone functions of sHsps under heat shock conditions
Chaperone activity of sHsps is enhanced under elevated temperature (Fu et al. 2003). According to some investigations, the abundance of substrates of sHsps is temperature-dependent and is increased by temperature (Raman et al. 1995). There are many reports about the diversity of substrates and multi-type substrate-binding sites suggesting their involvement in multi-functional processes. sHsps prevent aggregation of denatured proteins by forming complexes with the aggregation-prone partially unfolded forms of the substrate proteins (Lindner et al. 1997; Rajaraman et al. 2001). Interactions are weak with thermally induced early unfolding intermediates that cause refolding of the enzyme to its active form (Rajaraman et al. 2001). Indeed, chaperone function of sHsps consists of both transient and stable interactions based on the nature of intermediate unfolded substrates, which causes reactivation of the target activity or prevents aggregation (Goenka et al. 2001; Rajaraman et al. 2001). ATP binding leads to the suppression of aggregation, as well as enhancing reactivation yield (Muchowski et al. 1999). DmHsp22 is an efficient chaperone in vitro as well as in human cells. In HeLa cells that are transiently expressing mitochondrial-localized DmHsp22, the latter is able to reactivate mitochondrial targeting luciferase. Consequently, cells expressing DmHsp22 were less affected by heat stress during the period of inactivation at 40–46 °C (Dabbaghizadeh et al. 2018). This is most likely due to the fact that high affinity interactions take place between DmHsp22 and aggregation-prone late unfolding intermediates, thereby keeping them in a soluble complex. Finally, target proteins interacting with sHsps in vivo become refolded or are degraded, thus contributing to maintain a functional proteome (Rajaraman et al. 2001).
Short summary on functions of mitochondrial-localized sHsps
Mitochondrial functions of sHsps have been reported in some of the previous investigations. Mitochondrial-localized HspB5 in human lens preserves mitochondrial functions following exposure to an oxidative stress (McGreal et al. 2013; McGreal et al. 2012). Mitochondrial-localized Hsp70 facilitates import of proteins into the different mitochondrial subcompartments and causes the correct folding and assembly of transported proteins (Herrmann et al. 1994). Hsp10, Hsp60, and Hsp70 are involved in the assembly and folding of nuclear- and mitochondrial-encoded subunits of ATP synthase machinery, and they protect mitochondria against apoptosis (Danan et al. 2007; Herrmann et al. 1994). Furthermore, in Drosophila mitochondrial DmHsp22 increases longevity and resistance against oxidative stress (Morrow et al. 2004a). Transgenic mouse models expressing Hsp22 (HspB8) inhibit mitochondrial pathways of apoptosis (Depre et al. 2003; Qiu et al. 2011; Rashed et al. 2015). Mitochondrial-localized Hsp22 in mammalian cells promotes cardiac cell survival, oxidative metabolism, and cardioprotection which is iNOS-dependent (Rashed et al. 2015). Deletion of Hsp22 in vivo significantly impairs respiration which is due to the disruption of cardiac function under stress conditions (Qiu et al. 2011). Human cells expressing DmHsp22 possess higher luciferase activity following heat exposure suggesting that DmHsp22 binds to the heat-denatured luciferase and prevents it from irreversible aggregation and presents substrates for refolding to other ATP-dependent chaperones such as Hsp60 and mtHsp70 (which were detected as mitochondrial partners of DmHsp22 in different experiments). It is worth mentioning that ATP synthase subunits alpha and beta are among the proteins upregulated in flies overexpressing Hsp22 (Morrow et al. 2016a). DmHsp22 could improve the assembly of ATP synthase subunits through its chaperone efficiency and be involved in the aging process by modulating subunits alpha and beta which are affected during aging (Kim et al. 2006). DmHsp22 acts as a molecular carrier for several transporters such as SLC25 family members (Dabbaghizadeh et al. 2018).
The main partner of DmHsp22 in pure mitochondrial fractions was the complex I of mitochondrial respiration which is the main source of ROS production (Barja 2014; Genova and Lenaz 2015; Scialo et al. 2013). Twenty-nine and 43 proteins were detected working in 1 mg of mitochondrial suspension, and 66 proteins were detected working in 2 mg of pure mitochondrial fraction. Proteins detected at three independent IACs in suspensions of mitochondrial fractions are summarized in Table 1. Chaperone proteins, especially the mitochondrial 60 kDa heat shock protein, are among the proteins detected with high abundance in two independent immunoaffinity capture experiments, and these remain stably bound during heat shock condition. Gene ontology was used to describe three aspects of differentially transcribed mitochondrial DmHsp22-associated proteins including molecular function, biological process, and protein class, using the PANTHER classification tool (Mi et al. 2005; Thomas et al. 2003). Twenty-seven out of 60 (45%) proteins were involved in catalytic activity (0003824): these were mostly hydrolases (40%), oxidoreductases (28%), ligases (20%), transferases (8%), and lyases (4%). Forty-two were involved in metabolic processes (GO:0008152) such as primary metabolic processes (31%), nitrogen compound metabolic processes (18%), biosynthetic processes (13%), generation of precursor metabolites and energy (12%), phosphate-containing compounds (13%), catabolic processes (9%), and coenzyme metabolic processes (1.5%). Finally, according to the PANTHER protein class, they are mostly transporters (PC00227) (21%) including cation transporters (41%), ion channels (25%), amino acid transporters (7%), ATP-binding cassette transporters (8.3%), and mitochondrial carrier proteins (8%) (Dabbaghizadeh et al. 2018).
Table 1.
Proteins interacting with DmHsp22 which were commonly detected in all three independent IACs in mitochondria fractions. Numbers in the columns represent the number of detected peptides
| Proteins detected | Protein accession | Numbers of detected peptides | ||
|---|---|---|---|---|
| Exp 1 | Exp 2 | Exp 3 | ||
| 60 kDa, heat shock protein, mitochondria | P10809 | 26 | 23 | 46 |
| ATP synthase subunit beta, mitochondrial ATP5B | P06576 | 14 | 16 | 29 |
| ATP synthase subunit alpha, mitochondrial ATP5A1 | P25705 | 14 | 15 | 31 |
| Stress-70 protein, mitochondrial | P38646 | 6 | 10 | 23 |
| Elongation factor Tu, mitochondrial | P49411 | 8 | 9 | 18 |
| NAD(P) transhydrogenase, mitochondrial | Q13423 | 6 | 4 | 19 |
| ATP synthase subunit O, mitochondrial ATP50 | P48047 | 4 | 4 | 11 |
| Trifunctional enzyme subunit beta, mitochondrial | P55084 | 1 | 2 | 10 |
| Heat shock protein 75-kDa, mitochondrial | Q12931 | 4 | 3 | 10 |
Conclusion
Many techniques and experiments have been used to confirm interactions between sHsps and in vivo proteins. These interactions can be weak and easily broken by elevated temperature and are highly dependent on the presence of different stressors. Others are not specific interactions as they are found in controls. sHsp chaperoning interactions with each of the detected proteins and their ability to prevent protein aggregation will have to be examined. It seems that different organisms have a complex network of chaperones and proteins that work together to maintain protein homeostasis. The presence of ACD and especially the diversity of their NTRs and CTRs likely explain the different functions of sHsps and their interaction with a huge range of intra-cellular substrates, each involved in different aspects of cellular functions.
In the case of the mitochondrial DmHsp22, there is now evidence that links DmHsp22 to the UPRmt. One important role of UPRmt is to prevent the accumulation of damaged proteins in mitochondria and maintain mitochondrial protein homeostasis (mitochondrial proteostasis). DmHsp22 is a good candidate to fulfill this role due to its mitochondrial localization and its strong chaperone efficiency. Association of DmHsp22 with ETC subunits is a novel exciting area that certainly deserves further investigations in order to understand the importance of the mitochondrial localization of this sHsp. Also understanding the precise mechanisms of the effects played by Hsp22 in the mitochondria will require more studies.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Almeida-Souza L, Asselbergh B, d'Ydewalle C, Moonens K, Goethals S, de Winter V, Azmi A, Irobi J, Timmermans JP, Gevaert K, Remaut H, van den Bosch L, Timmerman V, Janssens S. Small heat-shock protein HSPB1 mutants stabilize microtubules in Charcot-Marie-Tooth neuropathy. J Neurosci. 2011;31(43):15320–15328. doi: 10.1523/JNEUROSCI.3266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barja G. The mitochondrial free radical theory of aging. Prog Mol Biol Transl Sci. 2014;127:1–27. doi: 10.1016/B978-0-12-394625-6.00001-5. [DOI] [PubMed] [Google Scholar]
- Basha E, Lee GJ, Breci LA, Hausrath AC, Buan NR, Giese KC, Vierling E. The identity of proteins associated with a small heat shock protein during heat stress in vivo indicates that these chaperones protect a wide range of cellular functions. J Biol Chem. 2004;279(9):7566–7575. doi: 10.1074/jbc.M310684200. [DOI] [PubMed] [Google Scholar]
- Basha E, Lee GJ, Demeler B, Vierling E. Chaperone activity of cytosolic small heat shock proteins from wheat. Eur J Biochem. 2004;271(8):1426–1436. doi: 10.1111/j.1432-1033.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- Basha E, O'Neill H, Vierling E. Small heat shock proteins and alpha-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci. 2012;37(3):106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bepperling A, Alte F, Kriehuber T, Braun N, Weinkauf S, Groll M, Haslbeck M, Buchner J. Alternative bacterial two-component small heat shock protein systems. Proc Natl Acad Sci U S A. 2012;109(50):20407–20412. doi: 10.1073/pnas.1209565109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butland G, Peregrín-Alvarez JM, Li J, Yang W, Yang X, Canadien V, Starostine A, Richards D, Beattie B, Krogan N, Davey M, Parkinson J, Greenblatt J, Emili A. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature. 2005;433(7025):531–537. doi: 10.1038/nature03239. [DOI] [PubMed] [Google Scholar]
- Carra S, et al. Different anti-aggregation and pro-degradative functions of the members of the mammalian sHSP family in neurological disorders. Philos Trans R Soc Lond Ser B Biol Sci. 2013;368(1617):20110409. doi: 10.1098/rstb.2011.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver JA, Lindner RA, Lyon C, Canet D, Hernandez H, Dobson CM, Redfield C. The interaction of the molecular chaperone alpha-crystallin with unfolding alpha-lactalbumin: a structural and kinetic spectroscopic study. J Mol Biol. 2002;318(3):815–827. doi: 10.1016/S0022-2836(02)00144-4. [DOI] [PubMed] [Google Scholar]
- Cashikar AG, Duennwald M, Lindquist SL. A chaperone pathway in protein disaggregation. Hsp26 alters the nature of protein aggregates to facilitate reactivation by Hsp104. J Biol Chem. 2005;280(25):23869–23875. doi: 10.1074/jbc.M502854200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbaghizadeh, Morrow G, Amer YO, Chatelain EH, Pichaud N, Tanguay RM. Identification of proteins interacting with the mitochondrial small heat shock protein hsp22 of Drosophila melanogaster: implication in mitochondrial homeostasis. PLoS One. 2018;13:e0193771. doi: 10.1371/journal.pone.0193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danan IJ, Rashed ER, Depre C. Therapeutic potential of H11 kinase for the ischemic heart. Cardiovasc Drug Rev. 2007;25(1):14–29. doi: 10.1111/j.1527-3466.2007.00002.x. [DOI] [PubMed] [Google Scholar]
- Delbecq SP, Klevit RE. One size does not fit all: the oligomeric states of alphaB crystallin. FEBS Lett. 2013;587(8):1073–1080. doi: 10.1016/j.febslet.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depre C, Wang L, Tomlinson JE, Gaussin V, Abdellatif M, Topper JN, Vatner SF. Characterization of pDJA1, a cardiac-specific chaperone found by genomic profiling of the post-ischemic swine heart. Cardiovasc Res. 2003;58(1):126–135. doi: 10.1016/s0008-6363(02)00845-3. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, et al. Stabilization of proteins and peptides in diagnostic immunological assays by the molecular chaperone Hsp25. Anal Biochem. 1998;259(2):218–225. doi: 10.1006/abio.1998.2630. [DOI] [PubMed] [Google Scholar]
- Favet N, Duverger O, Loones MT, Poliard A, Kellermann O, Morange M (2001) Overexpression of murine small heat shock protein HSP25 interferes with chondrocyte differentiation and decreases cell adhesion. Cell Death and Differ 8(6):603–613 [DOI] [PubMed]
- Fleckenstein T, et al. The chaperone activity of the developmental small heat shock protein Sip1 is regulated by pH-dependent conformational changes. Mol Cell. 2015;58(6):1067–1078. doi: 10.1016/j.molcel.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Friedrich KL, Giese KC, Buan NR, Vierling E. Interactions between small heat shock protein subunits and substrate in small heat shock protein-substrate complexes. J Biol Chem. 2004;279(2):1080–1089. doi: 10.1074/jbc.M311104200. [DOI] [PubMed] [Google Scholar]
- Fu X, et al. Small heat shock protein Hsp16.3 modulates its chaperone activity by adjusting the rate of oligomeric dissociation. Biochem Biophys Res Commun. 2003;310(2):412–420. doi: 10.1016/j.bbrc.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Fu W, et al. Apoptosis of osteosarcoma cultures by the combination of the cyclin-dependent kinase inhibitor SCH727965 and a heat shock protein 90 inhibitor. Cell Death Dis. 2013;4:e566. doi: 10.1038/cddis.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, et al. In vivo substrate diversity and preference of small heat shock protein IbpB as revealed by using a genetically incorporated photo-cross-linker. J Biol Chem. 2013;288(44):31646–31654. doi: 10.1074/jbc.M113.501817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, et al. Small heat shock protein IbpB acts as a robust chaperone in living cells by hierarchically activating its multi-type substrate-binding residues. J Biol Chem. 2013;288(17):11897–11906. doi: 10.1074/jbc.M113.450437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Chang Z, Shi X, Bu D, Wang C. Multilevel structural characteristics for the natural substrate proteins of bacterial small heat shock proteins. Protein Sci. 2014;23(2):229–237. doi: 10.1002/pro.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M, et al. Identification of the key structural motifs involved in HspB8/HspB6-Bag3 interaction. Biochem J. 2010;425(1):245–255. doi: 10.1042/BJ20090907. [DOI] [PubMed] [Google Scholar]
- Fuchs M, et al. A role for the chaperone complex BAG3-HSPB8 in actin dynamics, spindle orientation and proper chromosome segregation during mitosis. PLoS Genet. 2015;11(10):e1005582. doi: 10.1371/journal.pgen.1005582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genova ML, Lenaz G. The interplay between respiratory supercomplexes and ROS in aging. Antioxid Redox Signal. 2015;23(3):208–238. doi: 10.1089/ars.2014.6214. [DOI] [PubMed] [Google Scholar]
- Goenka S, et al. Unfolding and refolding of a quinone oxidoreductase: alpha-crystallin, a molecular chaperone, assists its reactivation. Biochem J. 2001;359(Pt 3):547–556. doi: 10.1042/0264-6021:3590547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley DA, Horwitz J, Stewart PL. The small heat-shock protein, alphaB-crystallin, has a variable quaternary structure. J Mol Biol. 1998;277(1):27–35. doi: 10.1006/jmbi.1997.1611. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Vierling E. A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol. 2015;427(7):1537–1548. doi: 10.1016/j.jmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White HE, Chen S, Sajbil HR, Buchner J (1999) Hsp26: a temperature-regulated chaperone. EMBO J 18(23):6744–6751 [DOI] [PMC free article] [PubMed]
- Herrmann JM, et al. Mitochondrial heat shock protein 70, a molecular chaperone for proteins encoded by mitochondrial DNA. J Cell Biol. 1994;127(4):893–902. doi: 10.1083/jcb.127.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89(21):10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, et al. Protein sHSP26 improves chloroplast performance under heat stress by interacting with specific chloroplast proteins in maize (Zea mays) J Proteome. 2015;115:81–92. doi: 10.1016/j.jprot.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Irobi J, Van Impe K, Seeman P, Jordanova A, Dierick I, Verpoorten N, Michalik A, De Vriendt E, Jacobs A, Van Gerwen V, Vennekens K, Mazanec R, Tournev I, Hilton-Jones D, Talbot K, Kremensky I, Van Den Bosch L, Robberecht W, Van Vandekerckhove J, Van Broeckhoven C, Gettemans J, De Jonghe P, Timmerman V (2004) Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat Genet 36(6):597–601 [DOI] [PubMed]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268(3):1517–1520. [PubMed] [Google Scholar]
- Jaya N, Garcia V, Vierling E. Substrate binding site flexibility of the small heat shock protein molecular chaperones. Proc Natl Acad Sci U S A. 2009;106(37):15604–15609. doi: 10.1073/pnas.0902177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayumov AR, et al. Recombinant small heat shock protein from Acholeplasma laidlawii increases the Escherichia coli viability in thermal stress by selective protein rescue. Mol Biol (Mosk) 2017;51(1):131–141. doi: 10.7868/S0026898417010086. [DOI] [PubMed] [Google Scholar]
- Kim KB, Lee JW, Lee CS, Kim BW, Choo HJ, Jung SY, Chi SG, Yoon YS, Yoon G, Ko YG. Oxidation-reduction respiratory chains and ATP synthase complex are localized in detergent-resistant lipid rafts. Proteomics. 2006;6(8):2444–2453. doi: 10.1002/pmic.200500574. [DOI] [PubMed] [Google Scholar]
- Kriehuber T, et al. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010;24(10):3633–3642. doi: 10.1096/fj.10-156992. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Vierling E. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 2000;122(1):189–198. doi: 10.1104/pp.122.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GJ, et al. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16(3):659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner RA, Kapur A, Carver JA. The interaction of the molecular chaperone, alpha-crystallin, with molten globule states of bovine alpha-lactalbumin. J Biol Chem. 1997;272(44):27722–27729. doi: 10.1074/jbc.272.44.27722. [DOI] [PubMed] [Google Scholar]
- Luheshi LM, Crowther DC, Dobson CM. Protein misfolding and disease: from the test tube to the organism. Curr Opin Chem Biol. 2008;12(1):25–31. doi: 10.1016/j.cbpa.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Maaroufi H, Tanguay RM. Analysis and phylogeny of small heat shock proteins from marine viruses and their cyanobacteria host. PLoS One. 2013;8(11):e81207. doi: 10.1371/journal.pone.0081207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewska M, Kuczyńska-Wiśnik D, Laskowska E, Liberek K. The small heat shock protein IbpA of Escherichia coli cooperates with IbpB in stabilization of thermally aggregated proteins in a disaggregation competent state. J Biol Chem. 2005;280(13):12292–12298. doi: 10.1074/jbc.M412706200. [DOI] [PubMed] [Google Scholar]
- McGreal RS, et al. alphaB-crystallin/sHSP protects cytochrome c and mitochondrial function against oxidative stress in lens and retinal cells. Biochim Biophys Acta. 2012;1820(7):921–930. doi: 10.1016/j.bbagen.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreal RS, et al. Chaperone-independent mitochondrial translocation and protection by alphaB-crystallin in RPE cells. Exp Eye Res. 2013;110:10–17. doi: 10.1016/j.exer.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin F, Basha E, Fowler ME, Kim M, Bordowitz J, Katiyar-Agarwal S, Vierling E. Class I and II small heat shock proteins together with HSP101 protect protein translation factors during heat stress. Plant Physiol. 2016;172(2):1221–1236. doi: 10.1104/pp.16.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck KB, Groenen PJ, Voorter CE, de Haard-Hoekman WA, Horwitz J, Bloemendal H, de Jong WW. Structural and functional similarities of bovine alpha-crystallin and mouse small heat-shock protein. A family of chaperones. J Biol Chem. 1993;268(2):1046–1052. [PubMed] [Google Scholar]
- Mi H, et al. The PANTHER database of protein families, subfamilies, functions and pathways. Nucleic Acids Res. 2005;33(Database issue):D284–D288. doi: 10.1093/nar/gki078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Deuerling E, Vorderwülbecke S, Vierling E, Bukau B. Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol Microbiol. 2003;50(2):585–595. doi: 10.1046/j.1365-2958.2003.03710.x. [DOI] [PubMed] [Google Scholar]
- Mogk A, Bukau B, Kampinga HH. Cellular handling of protein aggregates by disaggregation machines. Mol Cell. 2018;69(2):214–226. doi: 10.1016/j.molcel.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Morrow G, et al. Decreased lifespan in the absence of expression of the mitochondrial small heat shock protein Hsp22 in Drosophila. J Biol Chem. 2004;279(42):43382–43385. doi: 10.1074/jbc.C400357200. [DOI] [PubMed] [Google Scholar]
- Morrow G, Samson M, Michaud S, Tanguay RM. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J. 2004;18(3):598–599. doi: 10.1096/fj.03-0860fje. [DOI] [PubMed] [Google Scholar]
- Morrow G, Heikkila JJ, Tanguay RM. Differences in the chaperone-like activities of the four main small heat shock proteins of Drosophila melanogaster. Cell Stress Chaperones. 2006;11(1):51–60. doi: 10.1379/CSC-166.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G, et al. Changes in Drosophila mitochondrial proteins following chaperone-mediated lifespan extension confirm a role of Hsp22 in mitochondrial UPR and reveal a mitochondrial localization for cathepsin D. Mech Ageing Dev. 2016;155:36–47. doi: 10.1016/j.mad.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Morrow G, Le Pecheur M, Tanguay RM. Drosophila melanogaster mitochondrial Hsp22: a role in resistance to oxidative stress, aging and the mitochondrial unfolding protein response. Biogerontology. 2016;17(1):61–70. doi: 10.1007/s10522-015-9591-y. [DOI] [PubMed] [Google Scholar]
- Muchowski PJ, Hays LG, Yates JR, 3rd, Clark JI. ATP and the core “alpha-crystallin” domain of the small heat-shock protein alphaB-crystallin. J Biol Chem. 1999;274(42):30190–30195. doi: 10.1074/jbc.274.42.30190. [DOI] [PubMed] [Google Scholar]
- Murzin AG, et al. SCOP: a structural classification of proteins database for the investigation of sequences and structures. J Mol Biol. 1995;247(4):536–540. doi: 10.1006/jmbi.1995.0159. [DOI] [PubMed] [Google Scholar]
- Mymrikov EV, Haslbeck M. Medical implications of understanding the functions of human small heat shock proteins. Expert Rev Proteomics. 2015;12(3):295–308. doi: 10.1586/14789450.2015.1039993. [DOI] [PubMed] [Google Scholar]
- Mymrikov EV, Daake M, Richter B, Haslbeck M, Buchner J (2016) The chaperone activity and substrate Spectrum of human small heat shock proteins. J Biol Chem 292(2):672–684 [DOI] [PMC free article] [PubMed]
- Nadeau SI, Landry J. Mechanisms of activation and regulation of the heat shock-sensitive signaling pathways. Adv Exp Med Biol. 2007;594:100–113. doi: 10.1007/978-0-387-39975-1_10. [DOI] [PubMed] [Google Scholar]
- Narberhaus F. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev. 2002;66(1):64–93. doi: 10.1128/MMBR.66.1.64-93.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng MD, et al. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci. 1999;112(Pt 13):2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- Powers ET, et al. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- Qiu H, Lizano P, Laure L, Sui X, Rashed E, Park JY, Hong C, Gao S, Holle E, Morin D, Dhar SK, Wagner T, Berdeaux A, Tian B, Vatner SF, Depre C. H11 kinase/heat shock protein 22 deletion impairs both nuclear and mitochondrial functions of STAT3 and accelerates the transition into heart failure on cardiac overload. Circulation. 2011;124(4):406–415. doi: 10.1161/CIRCULATIONAHA.110.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaraman K, Raman B, Ramakrishna T, Rao CM. Interaction of human recombinant alphaA- and alphaB-crystallins with early and late unfolding intermediates of citrate synthase on its thermal denaturation. FEBS Lett. 2001;497(2–3):118–123. doi: 10.1016/s0014-5793(01)02451-6. [DOI] [PubMed] [Google Scholar]
- Raman B, Ramakrishna T, Rao CM. Temperature dependent chaperone-like activity of alpha-crystallin. FEBS Lett. 1995;365(2–3):133–136. doi: 10.1016/0014-5793(95)00440-k. [DOI] [PubMed] [Google Scholar]
- Rashed E, et al. Heat shock protein 22 (Hsp22) regulates oxidative phosphorylation upon its mitochondrial translocation with the inducible nitric oxide synthase in mammalian heart. PLoS One. 2015;10(3):e0119537. doi: 10.1371/journal.pone.0119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40(2):253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Rutgers M, et al. Substrates of the chloroplast small heat shock proteins 22E/F point to thermolability as a regulative switch for heat acclimation in Chlamydomonas reinhardtii. Plant Mol Biol. 2017;95(6):579–591. doi: 10.1007/s11103-017-0672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinthone S, Ba M, Hanson L, Martin JL, Halayko AJ, Gerthoffer WT. Overexpression of human Hsp27 inhibits serum-induced proliferation in airway smooth muscle myocytes and confers resistance to hydrogen peroxide cytotoxicity. Am J Physiol Lung Cell Mol Physiol. 2007;293(5):L1194–L1207. doi: 10.1152/ajplung.00453.2006. [DOI] [PubMed] [Google Scholar]
- Sanbe A, Marunouchi T, Abe T, Tezuka Y, Okada M, Aoki S, Tsumura H, Yamauchi J, Tanonaka K, Nishigori H, Tanoue A (2013) Phenotype of cardiomyopathy in cardiac-specific heat shock protein B8 K141N transgenic mouse. J Biol Chem 288(13):8910–8921 [DOI] [PMC free article] [PubMed]
- Santhanagopalan I, Degiacomi MT, Shepherd DA, Hochberg GKA, Benesch JLP, Vierling E. It takes a dimer to tango: oligomeric small heat shock proteins dissociate to capture substrate. J Biol Chem. 2018;293:19511–19521. doi: 10.1074/jbc.RA118.005421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialo F, Mallikarjun V, Stefanatos R, Sanz A. Regulation of lifespan by the mitochondrial electron transport chain: reactive oxygen species-dependent and reactive oxygen species-independent mechanisms. Antioxid Redox Signal. 2013;19(16):1953–1969. doi: 10.1089/ars.2012.4900. [DOI] [PubMed] [Google Scholar]
- Stromer T, Ehrnsperger M, Gaestel M, Buchner J. Analysis of the interaction of small heat shock proteins with unfolding proteins. J Biol Chem. 2003;278(20):18015–18021. doi: 10.1074/jbc.M301640200. [DOI] [PubMed] [Google Scholar]
- Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, Diemer K, Muruganujan A, Narechania A. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13(9):2129–2141. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkova NM, et al. Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci U S A. 2002;99(21):13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11(11):777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- Ungelenk S, et al. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat Commun. 2016;7:13673. doi: 10.1038/ncomms13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Why SK, et al. Hsp27 associates with actin and limits injury in energy depleted renal epithelia. J Am Soc Nephrol. 2003;14(1):98–106. doi: 10.1097/01.asn.0000038687.24289.83. [DOI] [PubMed] [Google Scholar]
- Verschuure P, Tatard C, Boelens WC, Grongnet JF, David JC. Expression of small heat shock proteins HspB2, HspB8, Hsp20 and cvHsp in different tissues of the perinatal developing pig. Eur J Cell Biol. 2003;82(10):523–530. doi: 10.1078/0171-9335-00337. [DOI] [PubMed] [Google Scholar]
- Walther DM, Kasturi P, Zheng M, Pinkert S, Vecchi G, Ciryam P, Morimoto RI, Dobson CM, Vendruscolo M, Mann M, Hartl FU. Widespread proteome remodeling and aggregation in aging C. elegans. Cell. 2015;161(4):919–932. doi: 10.1016/j.cell.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]