Abstract
We herein report a 52-year-old man with multiple hypoechoic lesions in the body and tail of the pancreas detected during a screening ultrasound. Computed tomography (CT) showed no lesions other than those in the pancreas and peripheral lymph nodes. Contrast-enhanced CT identified hypovascular tumors in the pancreas. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) demonstrated partial fibrosis and noncaseating granulomas with Langhans giant cells. To our knowledge, this is the first report of isolated pancreatic sarcoidosis diagnosed by EUS-FNA. Although pancreatic sarcoidosis is very rare, clinicians should be aware of this possibility in patients presenting with multiple hypovascular pancreatic tumors.
Keywords: noncaseating granuloma, Langhans giant cells, isolated pancreatic sarcoidosis, endoscopic ultrasound-guided fine-needle aspiration
Introduction
Sarcoidosis is a chronic systemic disease of unknown origin characterized by the presence of noncaseating granulomas in the affected tissues (1,2). The disease commonly affects young and middle-aged adults and can involve multiple organ systems, typically the hilar lymph nodes (often bilaterally), lung, heart, liver, spleen, eyes, kidney, other lymph nodes, salivary glands, nervous system, muscles, and bones (3,4). However, other organs are rarely involved.
Pancreatic sarcoidosis is particularly rare and often not diagnosed before death. It is found in 1-5% of patients with systemic sarcoidosis in autopsy studies (1,3,5-8), and isolated pancreatic sarcoidosis is rarer. Consequently, the radiological appearance of pancreatic sarcoidosis has not yet been described in detail. Only a few reports have described the presenting characteristics of pancreatic sarcoidosis on computed tomography (CT), magnetic resonance imaging (MRI) (9-11), or endoscopic ultrasonography (EUS) (7).
Furthermore, the clinical and radiological findings of pancreatic sarcoidosis sometimes resemble those of malignancy, making a diagnosis more challenging. Most reports have described cases of pancreatic sarcoidosis diagnosed by a surgical biopsy (12,13). Only a few case reports have described the diagnosis of pancreatic sarcoidosis using endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) (14-16).
We herein report the first case of isolated pancreatic sarcoidosis diagnosed by EUS-FNA.
Case Report
A 52-year-old man was referred to our hospital for the diagnostic examination of a pancreatic mass that had been incidentally detected by ultrasonography (US) during an evaluation of diabetes. He denied any symptoms, such as a fever, appetite or weight loss, or abdominal pain. He had suffered from a perianal abscess two months earlier. At that time, he had also been diagnosed with type 2 diabetes mellitus. He smoked two packs of cigarettes and drank 350 mL of beer and 1,000 mL of cocktails per day.
His axillary and inguinal lymph nodes were enlarged bilaterally. The histology of one of the inguinal lymph nodes was typical of lymphadenitis without granulomas. Laboratory examinations on admission showed elevated levels of alkaline phosphatase (430 U/L), gamma-glutamyl transferase (77 U/L), hemoglobin A1c (8.1%), and soluble interleukin-2 receptor (838 U/mL). Amylase, calcium, alanine aminotransferase, aspartate aminotransferase, carcinoembryonic antigen, carbohydrate antigen 19-9, Duke pancreatic monoclonal antigen type 2, and angiotensin-converting enzyme levels were within normal limits, and the T-SPOT.ⓇTB test (Oxford Immunotec, Abingdon, UK) was negative (Table).
Table.
Laboratory Examinations.
| Peripheral blood | Immunology | ||||||||||||
| WBC | 6,100 | /μL | AST | 13 | U/L | IgA | 175.4 | mg/dL | |||||
| RBC | 543×105 | /μL | ALT | 9 | U/L | IgG | 9,261 | mg/dL | |||||
| Hb | 17.8 | /μL | LDH | 182 | U/L | IgM | 36.2 | mg/dL | |||||
| PLT | 19.1×105 | /μL | ALP | 430 | U/L | IgG4 | 6.6 | mg/dL | |||||
| Serum | Glu | 185 | mg/dL | CA19-9 | 24 | U/mL | |||||||
| CRP | 0.2 | mg/dL | BUN | 12 | mg/dL | sIL-2R | 838 | U/mL | |||||
| TP | 7.0 | g/dL | Cr | 0.59 | % | DUPAN-2 | <25 | U/mL | |||||
| Alb | 4.5 | g/dL | T-Bil | 0.7 | mg/dL | ACE | 23.6 | U/L | |||||
| AMY | 128 | U/L | Ca | 8.6 | mg/dL | T-SPOT | negative | ||||||
| HbA1c | 8.1 | % | |||||||||||
WBC: White blood cell, RBC: Red blood cell, Hb: Hemoglobin, PLT: Platelet, CRP: C-reactive protein, Alb: Albumin, AST: Aspartate aminotransferase, ALT: Alanine amino-transferase, LDH: Lactate dehydrogenase, Glu: Blood glucose, BUN: Blood urea nitrogen, Cr: Creatinine, T-Bil: Total bilirubin, Ca: Calcium, sIL-2R: soluble IL-2 receptor, DUPAN-II: duke pancreatic monoclonal antigen type 2, ACE: angiotensin I converting enzyme
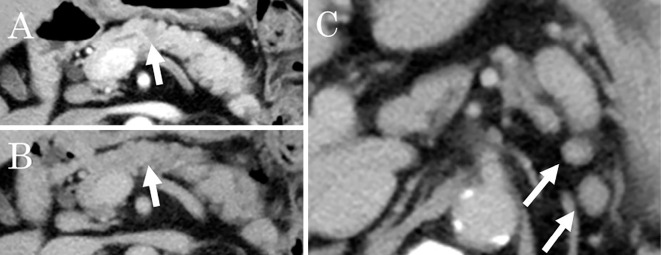
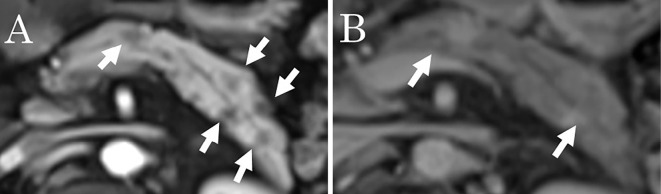
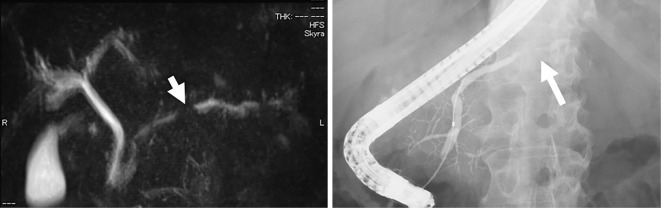
Abdominal US showed hypoechoic, poorly demarcated, solid masses measuring up to 20 mm in diameter in the pancreatic body and tail (Fig. 1). Contrast-enhanced CT revealed hypoattenuating masses in the pancreatic body during the pancreatic phase that became isodense with the surrounding pancreatic parenchyma in the delayed phase (Fig. 2). Hypovascular masses were also revealed around the pancreatic tail and identified as lymph nodes. CT did not show any lesions in the lungs. Multiple pancreatic masses of low signal intensity were detected during the T1 phase of MRI, changing to a slightly high intensity during the T2 phase. Contrast-enhanced MRI with gadolinium revealed hypoattenuated masses in the pancreatic phase that became isoattenuated compared to the surrounding pancreatic parenchyma in the later phase (Fig. 3). Magnetic resonance cholangiopancreatography (MRCP) showed stenosis of the main pancreatic duct (MPD) in the pancreas body and dilatation of the caudal MPD (Fig. 4). Endoscopic retrograde pancreatography (ERCP) was performed to assess the MPD in more detail and revealed stenosis of the MPD in the body (as in the MRCP) but no dilatation of the caudal MPD.
Figure 1.
Ultrasound findings in a 52-year-old man with multiple lesions in the body and tail of the pancreas. A: Hypoechoic, poorly-demarcated, solid masses measuring up to 20 mm in diameter are visible in the pancreatic body (arrow). B: Similar lesions are also visible in the tail (arrow).
Figure 2.
Contrast-enhanced computed tomography findings in a 52-year-old man with multiple lesions in the body and tail of the pancreas. A: Hypoattenuating masses are visible in the pancreatic body during the pancreatic phase (arrow). B: The lesions became isodense compared with the surrounding pancreatic parenchyma in the later phase (arrow). C: Enlarged lymph nodes can be seen around the pancreas (arrow).
Figure 3.
Contrast-enhanced magnetic resonance imaging findings in a 52-year-old man with multiple lesions in the body and tail of the pancreas. A: Pancreatic phase, B: Later phase. Hypoattenuated masses in the pancreatic phase became isoattenuated compared to the surrounding pancreatic parenchyma in the later phase (arrow).
Figure 4.
Magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde pancreatography (ERCP) findings in a 52-year-old man with multiple lesions in the body and tail of the pancreas. A: MRCP showing the stenosis of the main pancreatic duct (MPD) in the body (arrow) and dilatation of the caudal MPD. B: ERCP showing the stenosis of the MPD in the body (arrow).
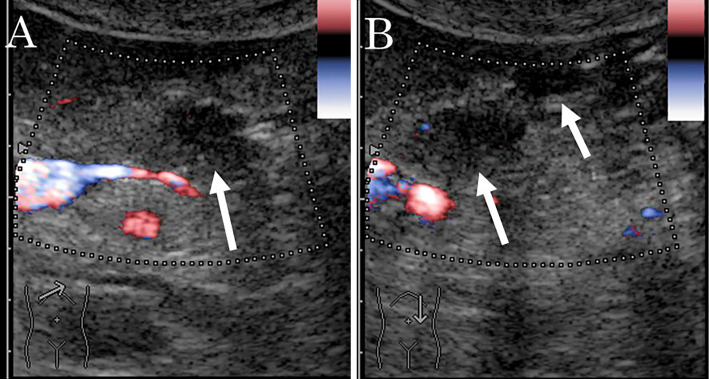
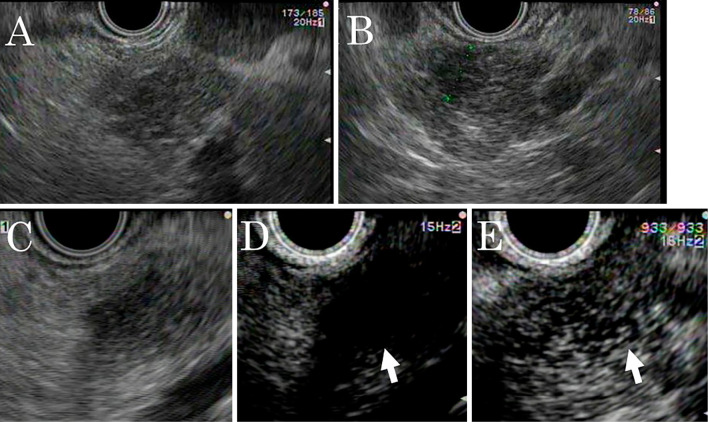
Brush cytology was performed in the stenotic part. The pathological examination revealed no malignant cells but failed to identify the cause of the stenosis. Conventional EUS revealed several isodense, ill-defined, poorly demarcated, hypoechoic lesions in the pancreatic body and tail and enlarged lymph nodes around the pancreas. Subsequently, we performed enhanced EUS using an ultrasound contrast agent (SonazoidⓇ; Daiichi-Sankyo, Tokyo, Japan). Twenty seconds post-injection, the lesions were less enhanced than the surrounding pancreatic parenchyma; however, after 60 seconds, the lesions became isoenhanced and ill-demarcated from the pancreatic parenchyma (Fig. 5).
Figure 5.
Endoscopic ultrasonography (EUS) findings in a 52-year-old man with multiple lesions in the body and tail of the pancreas. A, B: Conventional EUS reveals several isodense, ill-defined, poorly-demarcated, hypoechoic lesions in the pancreatic body and tail. A: Pancreatic body, B: Pancreatic tail. C, D, E: Enhanced EUS using an ultrasound contrast agent. C: Pre-injection. A low-echoic lesion. D: Twenty seconds post-injection. The lesions are less enhanced than the pancreatic parenchyma (arrow). E: After 60 s, the lesions became isoenhanced and ill-demarcated from the pancreatic parenchyma (arrow).
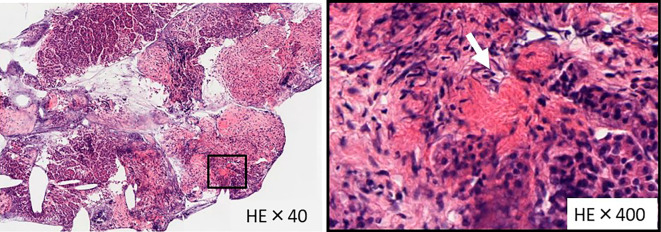
EUS-FNA using a disposable 22-gauge needle (ExpectⓇ; Boston Scientific, Marlborough, USA) was performed. A histological examination revealed partial fibrosis and noncaseating granuloma formation with Langhans giant cells (Fig. 6). There were no atypical epithelial cells. Special staining (Periodic acid-Schiff, Grocott, Zieh-Neelsen) did not reveal a pathogenic microorganism, and immunostaining [cluster of differentiation (CD)38, immunoglobulin G (IgG), IgG4] showed hardly any IgG4-positive cells. Based on these findings, we diagnosed the patient with pancreatic sarcoidosis.
Figure 6.
Histopathological findings. Hematoxylin and Eosin staining was performed. Histology revealed partial fibrosis and noncaseating granulomas with Langhans giant cells (arrow). A: 40-fold magnification. B: 400-fold magnification.
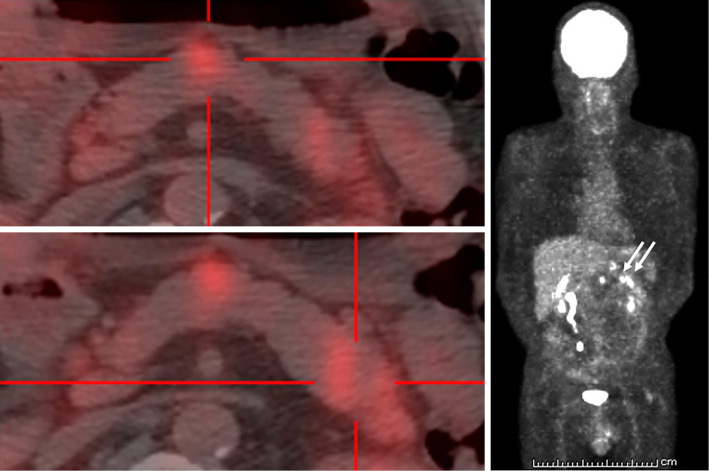
To identify potential effects on other organs, we performed fluorine-18-deoxyglucose (FDG)-positron emission tomography/CT (FDG-PET/CT), echocardiography, and eye and skin examinations. FDG-PET/CT confirmed the lesions in the body and tail of the pancreas, but no accumulation of lesions in other organs, including the heart and lungs, was found (Fig. 7). The electrocardiogram was normal, and echocardiography revealed mild hypokinesis; however, no asynergy, no thickening of the basal interventricular septum, and no morphological changes were found. No eye lesions were identified, and the skin examination showed no eruptions or erythema. Consequently, we diagnosed the case as one of isolated pancreatic sarcoidosis.
Figure 7.
Fluorine-18-deoxyglucose (FDG)-positron emission tomography/CT in a 52-year-old man with multiple lesions in the body and tail of the pancreas. Several lesions in the body and tail of the pancreas are visible (arrow), but no lesions in other organs are seen. A: The accumulation in the pancreatic body. B: The accumulation in the pancreatic tail. C: Coronal section
The superficial lymph node enlargement improved six months after the diagnosis, whereas the pancreatic masses and enlargement of abdominal lymph nodes remained for over three years.
Discussion
Isolated pancreatic sarcoidosis is extremely rare, and to our knowledge, there are fewer than 40 case reports of isolated pancreatic sarcoidosis. Patients with symptomatic pancreatic sarcoidosis present with abdominal pain, weight loss, obstructive jaundice, emesis, and related symptoms. However, patients with pancreatic sarcoidosis may be asymptomatic (12,15). Our patient had no symptoms, and the pancreatic masses were incidentally found during an assessment of diabetes mellitus.
Pancreatic sarcoidosis lesions detected on contrast-enhanced CT showed a lower density than the pancreatic parenchyma in our patient. In patients without a history of pancreatitis, non-contrast-enhance CT may show diffuse pancreatic calcifications (17). On MRI, pancreatic sarcoidosis has a slightly increased signal intensity on T2-weighted images and a slightly decreased signal intensity on T1-weighted images. On contrast-enhanced MRI, the lesions are hypointense compared to the pancreatic parenchyma during the arterial phase and become isointense during the portal or delayed phase (10). The lesions of pancreatic sarcoidosis were described as hypoechoic on EUS and as similar to pancreatic cancer on contrast-enhanced EUS in one report (14). There has been only one report to date describing the findings of pancreatic sarcoidosis on contrast-enhanced EUS. EUS may distinguish pancreatic sarcoidosis from mild pancreatic inflammation (7). Pancreatic sarcoidosis may resemble chronic pancreatitis with fibrosis. The clinical and radiological findings of pancreatic sarcoidosis may sometimes resemble those of malignancy, and the differentiation of pancreatic sarcoidosis from a malignant pancreatic tumor is sometimes difficult. There are cases that require surgery to diagnose the underlying condition correctly (18-21). Only a few reports have described cases where pancreatic sarcoidosis was diagnosed using EUS-FNA, and all of these cases consisted of patients with systemic sarcoidosis that affected the pancreas and other organs (14,15,22). To our knowledge, this is the first case of isolated pancreatic sarcoidosis diagnosed by EUS-FNA.
The diagnosis of sarcoidosis should be based on the clinical symptoms, radiological findings, pathological demonstration of noncaseating granulomas, and systematic exclusion of other diseases with a similar presentation, such as Hodgkin's disease, non-Hodgkin's lymphoma, carcinoma, and pancreatic cancer (16,19,23).
While pancreatic sarcoidosis is very rare, clinicians should be aware of this possibility when confronted with multiple hypovascular pancreatic tumors.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank Hiroe Itami and Chiho Obayashi for reporting the pathological findings.
References
- 1.Harder H, Büchler MW, Fröhlich B, et al. . Extrapulmonary sarcoidosis of liver and pancreas: a case report and review of literature. World J Gastroenterol 13: 2504-2509, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mony S, Patil PD, English R, Das A, Culver DA, Panchabhai TS. A rare presentation of sarcoidosis as a pancreatic head mass. Case Rep Pulmonol 2017: 7037162, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwai K, Tachibana T, Hosoda Y, Matsui Y. Sarcoidosis autopsies in Japan. Sarcoidosis 5: 60-65, 1988. [PubMed] [Google Scholar]
- 4.Schauer RJ, Völker U, Kreuzmayr A. An unorthodox pancreatic lesion in a young man presenting with jaundice. Gastroenterology 141: 1563, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am 3: 277-297, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shukla M, Hassan MF, Toor V, Kaur J, Solomon C, Cohen H. Symptomatic pancreatic sarcoidosis. Case report and review of literature. JOP 8: 770-774, 2007. [PubMed] [Google Scholar]
- 7.Romboli E, Campana D, Piscitelli L, et al. . Pancreatic involvement in systemic sarcoidosis. A case report. Dig Liver Dis 36: 222-227, 2004. [DOI] [PubMed] [Google Scholar]
- 8.McCormick PA, O'Donnell M, McGeeney K, FitzGerald O, McCormick DA, FitzGerald MX. Sarcoidosis and the pancreas. Ir J Med Sci 157: 181-183, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Bonhomme A, Dhadamus A, De Bie P, Van Hoe L, Baert AL. Pancreatic involvement in systemic sarcoidosis: CT findings. J Belge Radiol 80: 116-117, 1997. [PubMed] [Google Scholar]
- 10.Baroni RH, Pedrosa I, Tavernaraki E, Goldsmith J, Rofsky NM. Pancreatic sarcoidosis: MRI features. J Magn Reson Imaging 20: 889-893, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics 31: 993-1015, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Caceres M, Sabbaghian MS, Braud R, Wilks S, Boyle M. Pancreatic sarcoidosis: unusual presentation resembling a periampullary malignancy. Curr Surg 63: 179-185, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Al-Sukhni E, Qiu J, Gabriel E, Hochwald S. Sarcoidosis presenting with primary pancreatic manifestations: a case report and review of the literature. J Mol Biomark Diagn 7: 290, 2016. [Google Scholar]
- 14.Azemoto N, Kumagi T, Koizumi M, et al. . Diagnostic challenge in pancreatic sarcoidosis using endoscopic ultrasonography. Intern Med 57: 231-235, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuura S, Mochizuka Y, Oishi K, et al. . Sarcoidosis with pancreatic mass, endobronchial nodules, and miliary opacities in the lung. Intern Med 56: 3083-3087, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brincker H. Sarcoid reactions in malignant tumors. Cancer Treat Rev 13: 147-156, 1986. [DOI] [PubMed] [Google Scholar]
- 17.Folz SJ, Johnson CD, Swensen SJ. Abdominal manifestations of sarcoidosis in CT studies. J Comput Assist Tomogr 19: 573-579, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Siavelis HA, Herrmann ME, Aranha GV, Garcia G, Eubanks T, Reyes CV. Sarcoidosis and the pancreas. Surgery 125: 456-461, 1999. [PubMed] [Google Scholar]
- 19.Bacal D, Hoshal VL Jr, Schaldenbrand JD, Lampman RM. Sarcoidosis of the pancreas: case report and review of the literature. Am Surg 66: 675-678, 2000. [PubMed] [Google Scholar]
- 20.Ohana G, Melki Y, Rosenblat Y, Kravarusic D, Weil R. Pancreatic sarcoidosis mimicking a malignant tumour. Eur J Surg 168: 513-515, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Alizadeh L, Alizadeh AHM. Pancreatic sarcoidosis: a literature review. JOP. J Pancreas (Online) 17: 566-573, 2016. [Google Scholar]
- 22.Mony S. A rare presentation of sarcoidosis as a pancreatic head mass. Case Rep Pulmonol 2017: 7037162, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YT. Tissue diagnosis for carcinoma of the pancreas and periampullary structures. Cancer 49: 1035-1039, 1982. [DOI] [PubMed] [Google Scholar]