Abstract
Anti-myelin oligodendrocyte glycoprotein (MOG) antibodies have been associated with steroid-responsive cortical encephalitis and comorbid generalized epilepsy. A 44-year-old woman developed repeated epilepsia partialis continua (EPC) without generalized seizures and was anti-MOG antibody-positive. Radiological abnormalities were detected in the bilateral medial frontoparietal cortices, but there were no cerebrospinal fluid abnormalities. She achieved remission with anti-epileptic drugs alone. However, encephalitis recurred four months later when pleocytosis appeared, and steroid therapy was effective. Altogether, EPC without typical cerebrospinal fluid features can be an early sign of anti-MOG antibody-positive encephalitis. Thus, patients with EPC of unknown etiology need to be screened for anti-MOG antibodies.
Keywords: epilepsia partialis continua (EPC), anti-myelin oligodendrocyte glycoprotein (MOG) antibody, cortical encephalitis
Introduction
Anti-myelin oligodendrocyte glycoprotein (MOG) antibodies have recently been documented in a subtype of steroid-responsive cortical encephalitis that is gaining recognition as a new clinical entity (1-10). Patients with anti-MOG antibody-positive encephalitis usually present with generalized epileptic seizures (1,3-9). Cerebrospinal fluid (CSF) tests usually reveal pleocytosis (1-10). However, the potential association between anti-MOG antibodies and epilepsy without these typical features has received little attention.
Epilepsia partialis continua (EPC), a variant of focal status epilepticus characterized by prolonged repetitive muscle jerks with retained consciousness, is sometimes observed in the acute phase of encephalitides (11-13), but it is rarely reported with anti-MOG antibody-positivity. We encountered a patient who experienced repeated episodes of EPC affecting the four limbs at the onset of anti-MOG antibody-positive encephalitis. Brain magnetic resonance imaging (MRI) revealed cortical hyperintensities in the bilateral medial frontoparietal areas on diffusion-weighted imaging and fluid-attenuated inversion recovery (FLAIR) imaging, but a routine CSF analysis showed no abnormalities. EPC and radiological abnormalities were ameliorated by anti-epileptic drugs only. However, dizziness recurred four months later when pleocytosis emerged, which prompted us to check for anti-MOG antibodies and led us to the diagnosis and administration of effective steroid therapy.
This case is unique in that EPC without specific CSF features was an early sign of anti-MOG antibody-positive encephalitis. It thus highlights the need for patients with EPC of unknown etiology to be screened for anti-MOG antibodies. Such screening may facilitate an early diagnosis and the timely initiation of effective immunosuppressant therapy.
Case Report
A 44-year-old Japanese woman was admitted to our hospital with an acute presentation involving episodes of periodic upper and lower limb twitching of alternating laterality. Two earlier episodes of twitching in her right limbs had occurred over the past three weeks, and each episode had lasted a few hours. She had no history of epilepsy, and neither preceding infection nor recent vaccination was noted. On admission, a neurological examination revealed left-side repetitive muscle jerks and Todd's paresis, which was bilaterally present, but most severely affected her right limbs. However, her consciousness was unimpaired. She experienced no generalized seizures. She was given peroral levetiracetam (1,000 mg/day) and repeated diazepam injections (5 mg each), but the involuntary movements were not ameliorated and continued over the next five days.
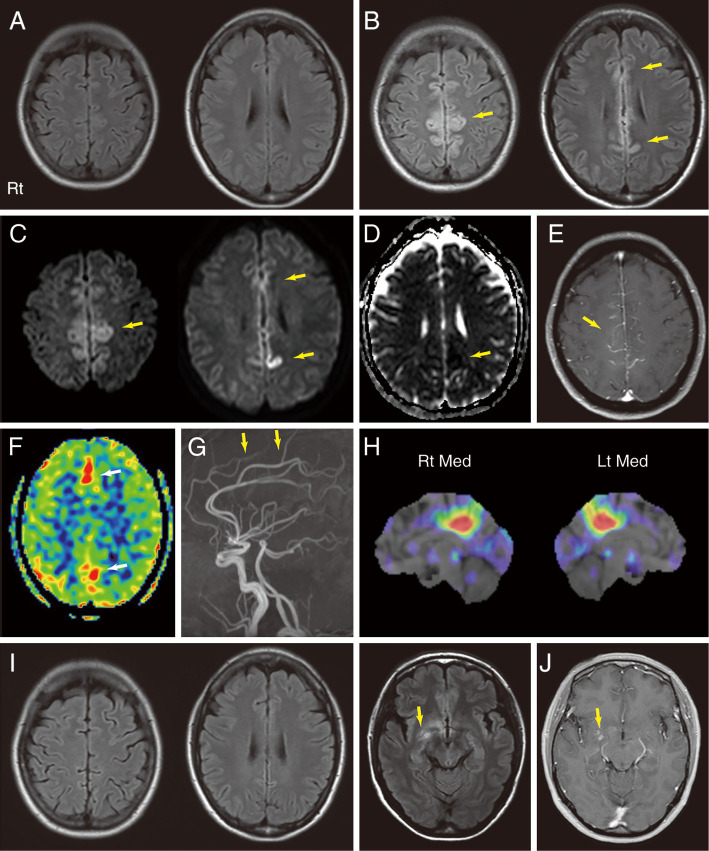
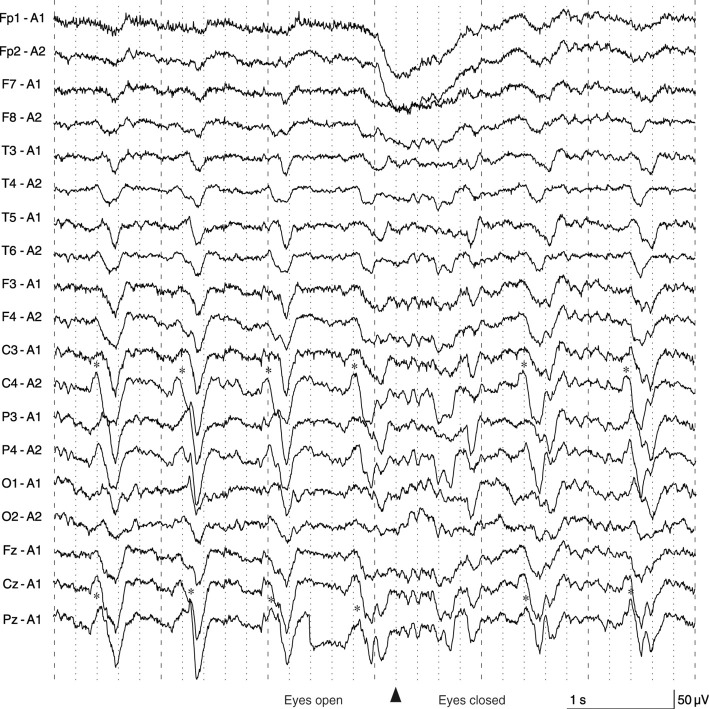
Brain MRI performed two weeks before admission (after the first episode of EPC) detected no apparent abnormalities on FLAIR imaging (Fig. 1A). However, FLAIR (Fig. 1B) and diffusion-weighted imaging (Fig. 1C, D) performed on the fifth day of admission showed hyperintensities extending bilaterally from her mesial frontal cortices to her posterior cingulate cortices. Gadolinium-enhanced T1-weighted imaging showed leptomeningeal enhancement in the same area alongside the cerebral falx (Fig. 1E). Hyperintensities on arterial spin labeling imaging (Fig. 1F) and an increased signal in the anterior cerebral artery territory on magnetic resonance angiography (Fig. 1G) suggested hyperperfusion caused by encephalitis or sustained focal epilepsy (14). Electroencephalography revealed periodic sharp wave complexes in a region extending from the central area to the parietal area (Fig. 2). These findings indicated that the seizures that alternately affected her right and left limbs resulted from focal epileptic involvement of the bilateral parasagittal cortices (3,15,16). A routine CSF analysis revealed normal cell counts and protein levels, a normal IgG index, and nonelevated myelin basic protein (MBP) levels (Table), but a single oligoclonal band absent in the serum was noted. We subsequently diagnosed her with EPC of unknown etiology.
Figure 1.
Brain MRI and SPECT findings. A: Axial FLAIR images taken two weeks before the first admission (after the first episode of EPC) appeared normal. B-G: MRI scans obtained at the first admission. On the fifth day of the admission, axial FLAIR imaging (B) showed hyperintense lesions (arrows), and diffusion-weighted imaging (C) revealed hyperintense lesions (arrows) extending bilaterally from the mesial frontal cortices to the posterior cingulate cortices. Parts of the same area exhibited low ADCs (arrow) (D). Gadolinium-enhanced T1-weighted imaging (E) showed corresponding leptomeningeal enhancement alongside the cerebral falx (arrow). ASL imaging (F) showed hyperintensities in the same area (arrows). MRA (G) showed an increased signal (arrows) in the anterior cerebral artery territory. H: After the first discharge, three-dimensional stereotactic surface projections of interictal [123I]-iodoamphetamine SPECT data showed an area of focally decreased perfusion in the bilateral posterior cingulate cortices but no areas of increased perfusion. I: FLAIR imaging performed at the second admission showed that the original hyperintensities in the medial frontoparietal areas were no longer visible. New hyperintensities (arrow) emerged in the right mesial frontotemporal areas. J: Part of the same areas exhibited gadolinium enhancement (arrow). ADC: apparent diffusion coefficient, ASL: arterial spin labeling, FLAIR: fluid-attenuated inversion recovery, Lt: left, Med: Medial, MRA: magnetic resonance angiography, MRI: magnetic resonance imaging, Rt: right, SPECT: single-photon emission computed tomography
Figure 2.
Electroencephalogram findings. PSWCs (asterisks) were observed in a region extending from the central area to the parietal area. PSWCs in the right hemisphere preceded those on the left side by approximately 0.1 seconds, which indicated that the PSWCs originated from the right hemisphere and spread to the left hemisphere. Posterior dominant rhythms were slow (8 Hz) on both sides and suppressed when the eyes were open. The patient exhibited left-side EPC during the test, but we did not perform simultaneous recording of electromyography or jerk-locked back averaging. EPC: epilepsia partialis continua, PSWC: periodic sharp wave complex
Table.
Results of Routine CSF Tests and Anti-MOG Antibody Titer Tests at Different Timepoints in the Patient’s Clinical Course.
| CSF | Serum | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cells (/μL) |
Protein (mg/dL) |
IL-6 (pg/mL) |
IgG-index | MBP | MOG Ab titers | MOG Ab titers | ||||||||
| 1st admission (during EPC) |
4 | 25 | NA | 0.59 | Negative | 1:16 | NA | |||||||
| 2nd admission (before treatment) |
21 | 26 | 37.9 | 0.66 | Negative | 1:8 | 1:128 | |||||||
| 2nd admission (after treatment) |
8 | 22 | NA | 0.66 | Negative | Undetectable | 1:512 | |||||||
CSF: cerebrospinal fluid, EPC: epilepsia partialis continua, IL-6: interleukin-6, MBP: myelin basic protein, MOG: myelin oligodendrocyte glycoprotein, MOG Ab: anti-MOG antibody, NA: not analyzed
On the sixth day of the hospitalization, her pharmacotherapy regimen was modified by increasing her levetiracetam dose to 2,000 mg/day and adding carbamazepine (400 mg/day). Her twitches showed amelioration within three days. She gradually recovered from postictal paraparesis and was discharged on the 20th postadmission day. Interictal [123I]-iodoamphetamine single-photon emission computed tomography revealed an area of focally decreased perfusion in her bilateral posterior cingulate cortices (Fig. 1H) but no areas of increased perfusion. Soon after discharge, she developed an intermittent headache and a mild fever (approximately 38℃) but remained seizure-free.
Three months after the original discharge, the patient experienced dizziness and was readmitted to our hospital. FLAIR images revealed a new cortical hyperintensity in her right medial frontal and temporal lobes (Fig. 1I), parts of which showed gadolinium enhancement (Fig. 1J). The original hyperintensities in the bilateral medial frontoparietal areas were no longer visible on FLAIR imaging (Fig. 1I). CSF tests revealed mild mononuclear pleocytosis (monocyte count, 20 μL-1; polycyte count, 1 μL-1) and mildly elevated interleukin-6 levels (37.9 pg/mL; normal range, <4.0 pg/mL) (Table). However, her CSF MBP levels were still nonelevated. The cortical hyperintensity and nonelevated MBP levels indicated cortical encephalitis rather than demyelination. Spinal MRI, somatosensory evoked potential recordings for four limbs, and visual evoked potential recordings revealed no abnormalities.
Cell-based assays detected no anti-N-methyl-D-aspartate receptor antibodies in her CSF. Tests for anti-voltage-gated potassium channel antibodies, anti-aquaporin-4 antibodies, anti-glutamic acid decarboxylase antibodies, anti-thyroid peroxidase antibodies, and anti-thyroglobulin antibodies in her serum all returned negative results, but a cell-based assay detected anti-MOG antibodies in her serum (titer, 1:128) and CSF (titer, 1:8). She was therefore diagnosed with anti-MOG antibody-positive encephalitis and treated with high-dose intravenous methylprednisolone (1,000 mg/day for three days per week). After three weeks of treatment, her symptoms gradually improved. Follow-up gadolinium-enhanced brain MRI showed that the hyperintensity lesion had disappeared, and CSF tests returned negative results for anti-MOG antibodies. The patient was prescribed prednisolone (15 mg/day), and she has experienced no relapses since.
It was later discovered that anti-MOG antibodies were also present (titer, 1:16) in a CSF sample that had been collected and frozen during the patient's first hospitalization.
Discussion
Our patient developed EPC alternately affecting her right and left limbs without generalized seizures at the onset of anti-MOG antibody-positive encephalitis. Although we had not performed the test first because anti-epileptic drugs had improved EPC, a later analysis revealed that anti-MOG antibodies had been present in the patient's CSF.
The routine CSF analysis at the first admission did not show any specific abnormalities in our case, such as pleocytosis. The absence of pleocytosis is not necessarily grounds for ruling out encephalitis, considering that a recent study showed that 35.3% of anti-MOG antibody-positive encephalitis cases did not exhibit pleocytosis (9).
Our findings suggest that EPC can be an initial sign of anti-MOG antibody-positive encephalitis. Given that epilepsy was frequently observed in previously reported cases of anti-MOG antibody-positive encephalitis (1,3-9), we regard our patient's initial EPC presentation and subsequent development of encephalitis four months later as consecutive events. In the present case, initial EPC resulted from focal epileptic involvement of the bilateral medial frontoparietal cortices. Cortical hyperintensities in the parasagittal area alongside the cerebral falx on FLAIR imaging with leptomeningeal enhancement, as seen in our case (Fig. 1B, E), can be common in anti-MOG antibody-positive encephalitis (3,9,10,17). A similar case was reported in which postictal paraparesis resulted from bilateral frontal cortex encephalitis with anti-MOG antibody-positivity, even though that case did not exhibit EPC, but rather secondarily generalized seizures (3). Bilateral EPC with retained consciousness may be often observed in anti-MOG antibody-positive encephalitis, considering several studies have associated the parasagittal area with EPC (15,16).
EPC is often observed in certain encephalitides, such as Rasmussen syndrome (11-13). However, in contrast to cases of Rasmussen encephalitis, in which refractory EPC usually results in severe cognitive impairment and brain atrophy (12), our patient's epilepsy was well controlled and left no sequelae. This good prognosis is consistent with previously reported cases of anti-MOG antibody-positive encephalitis, in which appropriate treatment usually prevented epilepsy relapses (1).
EPC without specific CSF abnormalities presents clinicians with a diagnostic challenge, and the etiology cannot be determined in 19-28% of affected patients (11). Our patient's case suggests that anti-MOG antibodies may be present in some of these seemingly idiopathic EPC cases. Initial-stage antibody screenings can lead to an earlier diagnosis, which in turn facilitates the earlier initiation of immunosuppressant therapies that can prevent both irreversible brain damage caused by prolonged status epilepticus and cortical encephalitis.
In conclusion, this report proves that EPC without typical CSF features can be an initial sign of anti-MOG antibody-positive encephalitis. The present findings thus widen the known spectrum of anti-MOG antibody-related diseases. This case also highlights the need for clinicians to screen patients with EPC of unknown etiology for anti-MOG antibodies, as such screenings may lead to an earlier diagnosis and the timely initiation of potentially effective immunosuppressant therapies.
We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This study was partially supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI) and the Health and Labour Sciences Research Grant on Intractable Diseases (neuroimmunologic diseases) from the Ministry of Health, Labour and Welfare of Japan.
Acknowledgement
We are grateful to Dr. Akira Yagishita for his helpful advice concerning this study.
References
- 1.Ogawa R, Nakashima I, Takahashi T, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm 4: e322, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariotto S, Monaco S, Peschl P, et al. MOG antibody seropositivity in a patient with encephalitis: beyond the classical syndrome. BMC Neurol 17: 190, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujimori J, Takai Y, Nakashima I, et al. Bilateral frontal cortex encephalitis and paraparesis in a patient with anti-MOG antibodies. J Neurol Neurosurg Psychiatry 88: 534-536, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima N, Suzuki M, Ogawa R, Hayashi K, Takanashi JI, Ohashi T. A case of anti-MOG antibody-positive multiphasic disseminated encephalomyelitis co-occurring with unilateral cerebral cortical encephalitis. Rinsho Shinkeigaku (Clin Neurol) 57: 723-728, 2017(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 5.Hamid SHM, Whittam D, Saviour M, et al. Seizures and encephalitis in myelin oligodendrocyte glycoprotein IgG disease vs aquaporin 4 IgG disease. JAMA Neurol 75: 65-71, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugimoto T, Ishibashi H, Hayashi M, et al. A case of anti-MOG antibody-positive unilaterally dominant meningoencephalitis followed by longitudinally extensive transverse myelitis. Mult Scler Relat Disord 25: 128-130, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda T, Yamada K, Ogawa R, et al. The pathological features of MOG antibody-positive cerebral cortical encephalitis as a new spectrum associated with MOG antibodies: a case report. J Neurol Sci 392: 113-115, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Adachi H, Ide Y, Takahashi T, Yoneda Y, Kageyama Y. Cerebral cortical encephalitis with anti-myelin oligodendrocyte glycoprotein (MOG) antibody. Rinsho Shinkeigaku (Clin Neurol) 58: 767-770, 2018(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 9.Wang L, ZhangBao J, Zhou L, et al. Encephalitis is an important clinical component of myelin oligodendrocyte glycoprotein antibody associated demyelination: a single-center cohort study in Shanghai, China. Eur J Neurol 26: 168-174, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Numa S, Kasai T, Kondo T, et al. An adult case of anti-myelin oligodendrocyte glycoprotein (MOG) antibody-associated multiphasic acute disseminated encephalomyelitis at 33-year intervals. Intern Med 55: 699-702, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Bien CG, Elger CE. Epilepsia partialis continua: semiology and differential diagnoses. Epileptic Disord 10: 3-7, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Varadkar S, Bien CG, Kruse CA, et al. Rasmussen's encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol 13: 195-205, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mameniškienė R, Wolf P. Epilepsia partialis continua: a review. Seizure 44: 74-80, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Lansberg MG, O'Brien MW, Norbash AM, Moseley ME, Morrell M, Albers GW. MRI abnormalities associated with partial status epilepticus. Neurology 52: 1021-1027, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Weinberger J, Lusins J. Simultaneous bilateral focal seizures without loss of consciousness. Mt Sinai J Med 40: 693-696, 1973. [PubMed] [Google Scholar]
- 16.Bell WL, Walczak TS, Shin C, Radtke RA. Painful generalised clonic and tonic-clonic seizures with retained consciousness. J Neurol Neurosurg Psychiatry 63: 792-795, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katsuse K, Kurihara M, Sugiyama Y, et al. Aphasic status epilepticus preceding tumefactive left hemisphere lesion in anti-MOG antibody associated disease. Mult Scler Relat Disord 27: 91-94, 2019. [DOI] [PubMed] [Google Scholar]