Abstract
We present new data on the effects of HBOT on human kidney (HK-2) cell metabolism using a SeaHorse XF Analyzer to evaluate separately the state of mitochondrial and glycolytic energy metabolism. The data are discussed in the context of the concept of cellular caloristasis networks. The information on the changes in cellular energy metabolism stimulated by HBOT presented here provides new insights into the cellular energy state and mitochondrial environment in which sHSPs function. These data will be useful in forming testable hypotheses about the functions of translocated sHSPs in human mitochondria responding to stressors.
Keywords: HBOT, Hyperbaric oxygen therapy, Small HSPs, Mitochondria, Proteostasis, Caloristasis, Diabetic kidney disease, Oxidative stress, Cytoprotection
Introduction
Deep history of stress bioenergetics: metabolic versus proteotoxic stress
Ferruccio Ritossa reported his discovery of the heat shock response (HSR) of Drosophila in 1962(Ritossa 1962). The title of the paper included not only temperature shock but also 2,4-dinitrophenol (DNP) as inducers, indelibly linking the HSR with cellular energy metabolism. As Ferruccio later reflected (Ritossa 1996), he found a small book by Albert Szent-Gyorgyi titled Bioenergetics in which the Nobel Laureate proposed that electron flow along the respiratory chain in mitochondria was facilitated by an ice-like crystalline structure of water molecules hydrating the transport chain. Ferruccio wondered if heat could destroy such a structure resulting in uncoupling of oxidative phosphorylation. This idea led him to test whether DNP and other uncouplers such as salicylate and recovery from anoxia could induce the heat shock puffs (heat shock gene activation). This was the first attempt to create a testable hypothesis about the functional significance of the heat shock response. Indeed, the uncouplers induced the same puffs, and Ferruccio pursued the hypothesis that the HSR is directly correlated with changes in cellular metabolism, specifically cellular energy production. He commented in his reflections article that “It does not matter if this interpretation was true or false; it was a working link between imagination and reality, like love”(Ritossa 1996). Ferruccio had the analytical mind of a scientist and the restless spirit of an artist. He continued to think of the response as having general importance as an organismal response to an environmental signal. Many colleagues did not share this view, and they considered the response a laboratory artifact, an attitude that Ferruccio found very discouraging.
In his full article that followed his 1962 brief communication, Ferruccio added to the list of inducers sodium azide and dicumarol (Ritossa 1964). He chose inducers known to increase cellular ADP and inorganic phosphate and decrease concentrations of ATP. In the concluding remarks, he foreshadowed the more recent concepts of caloristasis networks and cellular oxygen sensing. He thought that a newly synthesized compound was needed for chromatin puffing to occur and that even though metabolic changes might create the inducing stimulus, puffing required a functional energy-producing system.
The current view
Ferruccio lived long enough to see the resurgence of cellular energy metabolism as a key component of cellular stress responses. His opinion was that the HSR, as he preferred to call it, likely held more surprises for future investigators. Most investigators now call the HSR the proteotoxic stress response (PSR), recognizing that heat is one of the long list of stressors that induce molecular chaperones by damaging proteins, i.e., proteotoxicity (Hightower 1991). In 2015, a remarkable paper by Dai and coworkers (Dai et al. 2015) described how the PSR and the metabolic stress response (MSR) worked in opposition to create a new mechanism to maintain cellular homeostasis. We suggest that this third mechanism, a combination of PSR and MSR, is the caloristasis network. The proximal integrator of these responses is HSF1, its DNA binding activity induced in Dai’s study by heat stress to produce the PSR. A key player in the new mechanism is adenosine monophosphate kinase (AMPK), a key sensor and master regulator of cell nutritional status. The PSR is facilitated by reduced AMPK activity in cells. Low cellular AMPK activity indicates the ATP sufficient, nutritionally replete cellular state capable of supporting the energetically expensive tasks of the PSR. Dai and colleagues found that the PSR can inhibit AMPK activation and thus diminish cells’ ability to mount a MSR. In contrast, cell nutrient deprivation activates AMPK. Activated AMPK phosphorylates HSF1 on Ser121 reducing its DNA binding activity (negative regulation) and blocking the induction of the energetically demanding PSR in cells experiencing reduced levels of ATP and attempting to respond with the MSR to restore cellular homeostasis. This effect may be cell type dependent but holds for a human kidney cell line HEK293 used in the Dai study. A human kidney (HK-2) cell line was used in our HBOT study described herein.
Hyperbaric oxygen therapy: a cellular stress response modulator
The use of compressed air for therapeutics was first noted in 1662 by Henshaw. The discovery of oxygen in 1775 opened a new chapter for the improvement of HBOT. From 1789 through the early 1880s, there was a growing disinclination toward the use of HBOT due to possible toxicity from the excess oxygen (Edwards 2010). However, physicians and researchers around the world started to appreciate the possible therapeutic benefits of HBOT which led to the manufacture of several hyperbaric oxygen chambers for use in human therapy. HBOT is now used primarily for the therapy of decompression illness (patients affected by scuba diving), carbon monoxide poisoning, infections, and wounds seen in diabetic profile patients. Our recent findings indicate that HBOT may even be advantageous for the treatment of type 2 diabetes (Harrison et al. 2018; Verma et al. 2015). The therapeutic actions of HBOT are the result of elevating both the partial pressure of oxygen and the hydrostatic pressure. Henry’s law adapted to our example states that the amount of oxygen dissolved in a liquid at constant temperature is directly proportional to the partial pressure of oxygen in equilibrium with blood plasma. Dalton’s law of partial pressures states that the pressure of a mixture of gases is the sum of the pressures of the individual gases (Choudhury 2018; Thom 2009). The oxygen-carrier protein hemoglobin, nearly fully occupied under normal conditions, becomes saturated with bound oxygen under these conditions. Increased hydrostatic pressure is essential for stimulation of the cell and for tissue-level changes in gene expression responsible for the action of HBOT as a cellular stress response modulator and as an inducer of acquired cytoprotection.
HBOT and the caloristasis network
Swan and Sistonen (Swan and Sistonen 2015) have provided a useful summary of the complex, data-rich paper of Dai et al. (2015). Taken together, they provide an excellent example of the value of the caloristasis network concept, first publicly presented by L. Hightower at the CSSI Turku Congress (Bonorino et al. 2018). This concept emphasizes the integrative regulatory interactions needed to understand cellular energy homeostasis. In this paper, we present new data about the changes in energy metabolism in human cells exposed to HBOT, oxygen provided at about 2.4 atm for 60 min followed by a recovery period. We have long argued that HBOT should not be considered an oxidative stress because the small amount of reactive oxygen species (ROS) generated in cells is sensed as a signal to activate the transcription factor Nrf-2, the master regulator of expression of genes involved in cellular antioxidant defenses.
In the Dai study and in Fig. 1 (HSF1 integrates multiple stress responses) of the Swan and Sistonen summary, it is clear that HBOT should be placed alongside the anti-diabetes drug metformin and not with heat shock and oxidative stress. Metformin blocks mitochondrial ROS production by inhibiting respiration, leading to activation of AMPK (Foretz et al. 2014). It is significant that Swan and Sistonen included both heat shock and oxidative stress on the PSR side of their figure and metformin on the MSR side. Combination of PSR and MSR is one of the most frequently encountered stressor pairings that organisms encounter in the real world outside of the research laboratory. Two examples in mammals are responses to tissue damage and inflammation including infections. Both involve a combination of heat stress and oxidative stress, provoking cell and tissue responses orchestrated primarily by the activation of HSF1 and Nrf-2, respectively. The PSR and MSR appear to be controlled by the cellular equivalent of a continuously adjustable rheostat rather than two on/off switches as these regulators respond to rapidly changing states of proteostasis and caloristasis.
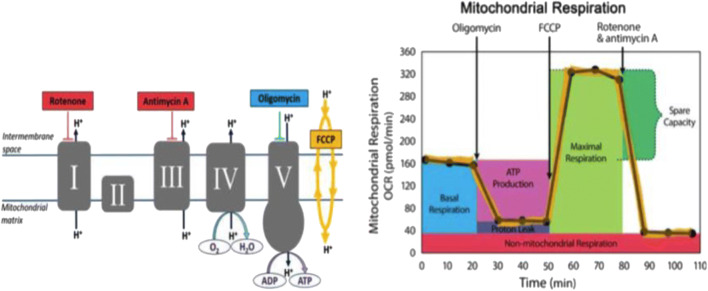
Fig. 1.
XFp mito stress test modulators of electron transport chain (ETC). The target of action of all compounds (oligomycin, FCCP, rotenone, and antimycin A) injected into the reagent ports enabling the measurements of basal respiration, ATP production, maximal respiration, spare capacity, and non-mitochondrial respiration. (Images used with permission from Agilent SeaHorse Technologies, Inc.)
HBOT as a cytoprotective response
The effects of HBOT on a cellular level have been studied in terms of its antioxidant pathway-activating mechanisms associated with cytoprotection (Verma et al. 2015). Cellular stressors, including injury, infection, and chronic diseases, trigger a stress response that results in the production of antioxidants (Giudice et al. 2010). Nrf2-Keap1 plays the key role in regulating the expression of antioxidant genes in the stressed cells. Under stress-free conditions, Nrf2 is found in a ubiquitinated state degraded in the proteasome, whereas in the presence of an electrophilic stressor, Nrf2 is stabilized and transported to the nucleus where it activates antioxidant response element-regulated genes (Giudice et al. 2010; Godman et al. 2010a; Godman et al. 2010c; Verma et al. 2015). Post-HBOT expression levels of Nrf2 in human endothelial cells (HMEC-1) showed an increase, indicating antioxidant pathway activation as an immediate response to HBOT, thus implying a cytoprotective effect of HBOT. We tested for protection by allowing HBOT-treated HMEC-1 cells to recover for 24 h before challenging them with the oxidant tert-butyl-hydroperoxide. Relative to untreated control cells, the HBOT-treated cells were significantly more resistant to the hydroperoxide (Godman et al. 2010a; Godman et al. 2010c).
In our previous study, Db/Db mice (type 2 diabetes model lacking leptin receptor activity) were used to study the effect of periodic HBOT on suppressing renal injury. Several markers of kidney damage were reduced significantly by HBOT (Verma et al. 2015). Expression of the stress response genes Nrf2 and HMOX1 was also reduced after periodic exposure to HBOT. These findings suggest that HBOT triggers non-cytotoxic oxidative stimuli, ultimately reducing oxidative stress and increasing antioxidant levels. We anticipated that ROS generated by 100% oxygen would act as a non-cytotoxic signal since moderate doses of ROS stimulate cytoprotection by preconditioning effects and induce cellular defense pathways (Bilban et al. 2008). However, another key factor that has roles in cytoprotection, cellular energy metabolism, has not been investigated in detail using HBOT.
Purpose of this paper
We present new data on the effects of HBOT on human kidney (HK-2) cell metabolism using a SeaHorse XF Analyzer to evaluate separately the state of mitochondrial and glycolytic energy metabolism. We now have three different lines of evidence linking HBOT to changes in mitochondrial functions that we summarize in the “Discussion” section. Currently, our hypothesis linking the changes in cellular energy metabolism caused by HBOT to sHSPs in our work is speculative but firmly grounded in published studies. We did detect HSPB1 gene expression in a microarray study of gene expression in HBOT-treated HMEC-1 cells (Godman et al. 2010a; Godman et al. 2010c). However, HBOT did not significantly affect HSPB1 (Hsp27) gene expression. Unlike Drosophila and plant species that have been studied, mammalian cells do not have a sHSP isoform that is primarily located in mitochondria. However, there is ample evidence that sHSPs translocate to mitochondria in stressed mammalian cells (Zeng et al. 2013). In their review article, Zeng et al. concluded that several kinds of sHSPs are translocated to mitochondria where they confer protection again stress by maintaining mitochondrial homeostasis. We will limit our remarks to human Hsp27. Zeng et al. summarized studies showing that Hsp27 plays an essential role in the intracellular trafficking of mitochondria during mitophagy (mitochondrial autophagy). Hsp27 knockdown in human cell lines results in fragmentation of mitochondria similar to mitophagy with reduced aerobic respiration and ATP levels. In cytoprotected cells (thermotolerance), HSP27 associates with the mitochondrial fraction, and its loss stimulates cytochrome c release from mitochondria exposed to caspase-dependent apoptotic stimuli (Samali et al. 2001). Hsp27 is also involved in neuroprotection and cardioprotection (Zeng et al. 2013).
The information on the changes in cellular energy metabolism stimulated by HBOT presented here provides new information on the cellular energy state and mitochondrial environment in which these sHSPs function. These data will be useful in forming testable hypotheses about the functions of translocated sHSPs in human mitochondria responding to stressors.
Methods
Cell mito stress test
HK-2 cells were plated on a SeaHorse microwell miniplate treated at 2.4 atm with 100% saturated oxygen for 2 h within the Life Support Technologies Small Round Experimental Hyperbaric Chamber. Then, glycolytic flux and mitochondrial respiration of HK-2 cells were simultaneously assessed by SeaHorse Bioscience extracellular flux analyzer. Glycolysis and mitochondrial stress tests (Figs. 1 and 2) were performed on day 0 and 1 following HBOT treatment. The mitochondrial respiration and glycolysis profiles of the HBOT-treated and non-treated groups were compared by taking the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) standard graphs (Figs. 1 and 2) as a reference.
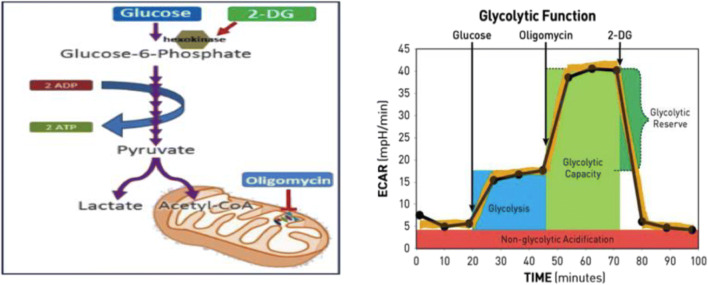
Fig. 2.
XFp glycolysis stress test modulators of glycolysis. The target of action of all compounds (glucose, oligomycin, 2-DG) injected into the reagent ports and measuring glycolysis, glycolytic capacity, glycolytic reserve, and non-glycolytic acidification. (Images used with permission from Agilent SeaHorse Technologies, Inc.)
Mitochondrial respiration (OCR) of HK-2 cells under ± HBOT conditions was evaluated using XF Cell Mito Stress Kit (Agilent SeaHorse). The sensor of the analyzer approached as close as 2 ul above the cells and detected changes in the oxygen concentration of the cells in real time.
Mitochondrial respiration, the oxygen consumption rate, was measured after the injections of oligomycin, FCCP, rotenone, and antimycin A through individual ports into the microwells. The injections provided information on the baseline oxygen consumption (respiratory capacity), ATP-linked and non-ATP-linked oxygen consumption, respiration capacity, and non-mitochondrial respiration.
Following the first injection, oligomycin inhibited ATP synthase (complex V) (increased pH gradient) and consequently lowered the OCR. The second injection of the mitochondrial uncoupler protein FCCP collapsed the pH gradient and increased the OCR. Following the final injection, rotenone and antimycin A blocked electron transport occurring from complex I and III to ubiquinone, respectively. It resulted in the inhibition of mitochondrial respiration. Thus, oxygen consumption via ETC was inhibited. The remaining OCR measurements after the rotenone/antimycin A injection represented only the non-mitochondrial oxygen consumption and respiration.
Glycolysis stress test
ECAR, glycolytic flux, represents the rate of extracellular proton production in real time. The first injection of glucose is added to the wells. The cells convert the supplied source of glucose into pyruvate through glycolysis which results in the production of ATP, NAD+, H2O, and protons. The increase in the proton (H+) concentration causes an increase in the ECAR levels. Secondly, oligomycin is injected to limit the mitochondrial respiration, thus shifting energy metabolism to glycolysis, leading to a steep increase in ECAR. Finally, the injection of a glucose analog, 2-deoxy-glucose (2-DG), targets glucose hexokinase due to its high affinity. The competitive binding of 2-DG to glucose hexokinase results in inhibition of glycolysis which drops ECAR levels. It also suggests that it is the process of glycolysis which regulates ECAR.
Results
Early (day 0) and late (day 1) metabolic responses of HK-2 cells to hyperbaric oxygen therapy (HBOT = experimental group) were analyzed with SeaHorse extracellular flux technology and compared with the response of the control group.
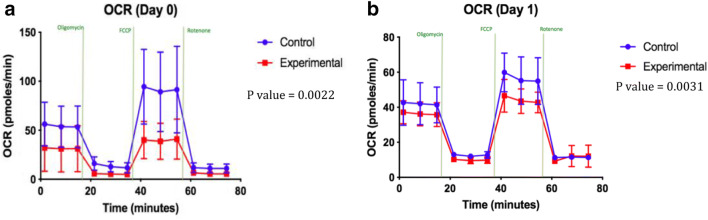
The first and second HBOT were performed on day 0 and 1, respectively. HK-2 cells were exposed to hyperbaric oxygen on both days for 2 h. Measurements were made immediately. Both day 0 and 1 HBOT groups displayed a reduced mitochondrial activity and energy metabolism indicated by lower reads of OCR compared with the non-treated control as shown in Fig. 3 a and b. All of the graphs shown in Figs. 3, 4, 5, 6, and 7 were created within the SeaHorse instrument using Agilent Cell Analysis software. HBOT leads to higher levels of ROS production by the mitochondria; therefore, cells may decrease ROS accumulation by reducing their mitochondrial activity to alleviate the oxidative stress as a protective response.
Fig. 3.
a OCR in day 0 of HBOT. b OCR in day 1 of HBOT
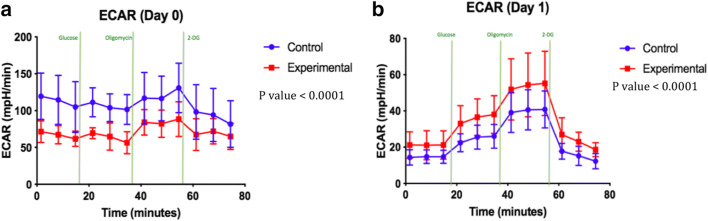
Fig. 4.
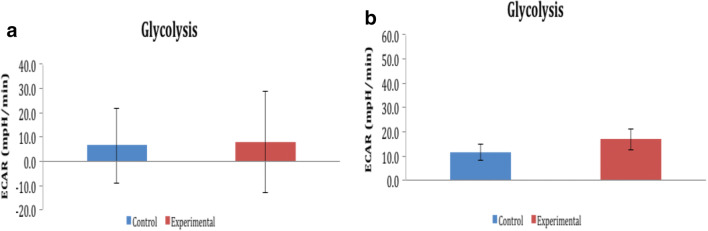
a ECAR in day 0 of HBOT. b ECAR in day 1 of HBOT
Fig. 5.
a Glycolytic activity in day 0 of HBOT. b Glycolytic activity in day 1 of HBOT
Fig. 6.
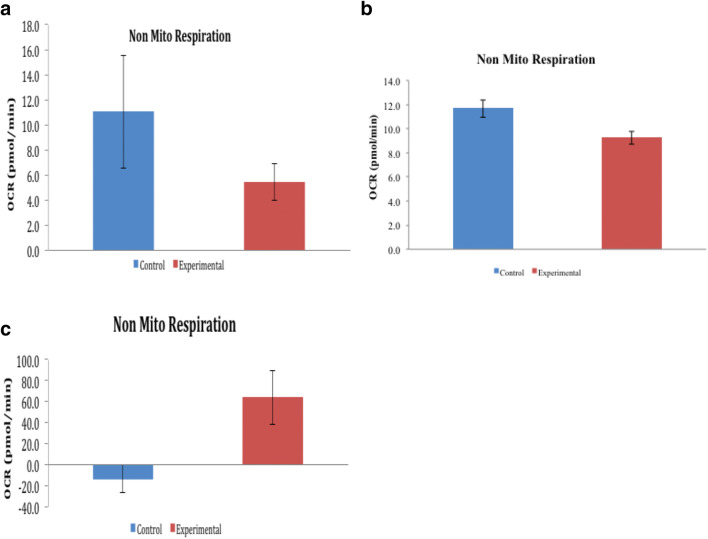
a Non-mitochondrial respiration in day 0 of HBOT. b Non-mitochondrial respiration in day 1 of HBOT. c Non-mitochondrial respiration on day 0 in an independent experiment
Fig. 7.
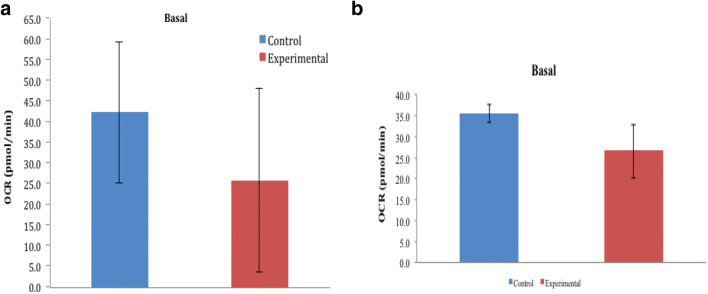
a Basal mitochondrial respiration in day 0 of HBOT. b Basal mitochondrial respiration in day 1 of HBOT
Glycolysis was evaluated using the extracellular acidification rate (ECAR) generated by HK-2 cells upon glucose injection. Figure 4 a and b show the ECAR measurements done after the first and second HBOT. The HBOT group indicated an increased ECAR relative to control values between day 0 and 1.
To compensate for the drop in ATP production and reduced mitochondrial respiration shown in Fig. 4 a and b, HBOT-treated cells shift energy metabolism toward glycolysis as seen in Fig. 5 a and b. Note that the standard deviations of the control and HBOT data overlap almost completely so the small difference in ECAR is not statistically significant on day 0. Day 1 in Fig. 4b represents HBOT-treated cells starting to undergo a recovery phase to produce energy. Glycolytic reduction in day 1 of the HBOT group might be an opportunity to synthesize more glycolytic enzymes and to expand glycolytic capacity to meet bioenergetics demands.
Glycolytic activity of the HBOT group is supported by increased non-mitochondrial respiration which provides the source of NAD+. We also observed an almost twofold increase in the non-mitochondrial oxygen consumption of the HBOT group between day 0 and 1 as seen in Fig. 6 a and b. Control groups on day 0 and day 1 displayed a stable non-mitochondrial respiratory activity (12 pmol/min on day 0 and day 1).
Figure 6c from an independent experiment confirmed the significant ability of the HBOT group to perform non-mitochondrial respiration. HBOT may therefore stimulate cells to perform higher transplasma membrane electron transport (tPMET). tPMET activity may allow cells to maintain energy levels and redox homeostasis by reducing oxidative stress in order to regulate biological functions including cell metabolism, growth, and death (Prata et al. 2010; Stuart et al. 2018).
The basal respiration includes the respiration that drives the production of ATP, which can be determined by oligomycin injection, and the respiration that is linked with the processes of proton leakage. The basal respiration excludes the non-mitochondrial respiration rate. Higher levels of mitochondrial oxygen consumption were observed in the control condition compared with HBOT under basal conditions (Fig. 7 a and b). HBOT caused mitochondrial respiration to be stabilized: 25 pmol/min on day 0 and 1.
Discussion
Cellular energy metabolism under the stress of type 2 diabetes mellitus
Type 2 diabetes mellitus leads to cellular stress and tissue damage due to accumulation of ROS that triggers increased glycolysis to meet increased cellular energy requirements. This stress/damage can ultimately result in kidney failure. A healthy kidney primarily uses oxidative phosphorylation to oxidize glucose to CO2 and H2O in the process of generating ATP. However, in the diabetic kidney, glycolysis dominates in a similar chronic trend to the Warburg effect seen in cancer (Wittig and Coy 2008). This is possibly the result of poor circulation/O2 delivery. The shift toward glycolysis is noted as one of the driving factors of diabetic kidney disease along with mitochondrial dysfunction. The diabetic kidney profiles also indicate lower mitochondrial mass, due to mitophagy, which results in lower electron transport chain (ETC) cycle metabolites and mitochondrial activity. One of the possible explanations for the metabolic shift toward the glycolysis seen in type 2 diabetes is that the glucose is metabolized faster via aerobic glycolysis compared with that of oxidative phosphorylation within the mitochondria (Zhang et al. 2018). Studies have also revealed that the alterations in cellular oxidative stress can control the metabolic shift toward glycolysis in the hepatoma cells. This shift indicates an increased cellular ROS microenvironment associated with a higher rate of glycolysis, whereas reduced levels of ROS are associated with the inhibition of glycolysis (Shi et al. 2009). ROS signaling may trigger a similar change in cellular metabolism as a result of a controlled application of HBOT but without the chronic damage caused by type 2 diabetes.
HSF1-deficient mouse hepatocytes and HK-2 cells compared
Carles Canto (Cantó 2017) wrote a very useful summary for another important paper by Qiao and coworkers in the Mivechi laboratory (Qiao et al. 2017) that extends our knowledge of HSF1 as a central regulator between hepatic bioenergetics and protein homeostasis. The key new finding is that HSF1 binds to the Nampt promoter as well as the PSR promoter. This gene encodes nicotinamide phosphoribosyltransferase (Nampt), the critical enzyme in the NAD+ salvage pathway from nicotinamide. The link to mitochondria is that NAD+ is a co-substrate for SIRT1 and SIRT3, deacetylases that play a major role in maintaining functional mitochondria. HSF1 deficiency leads to a reduction of the number of mitochondria and oxidative capacity in mouse hepatocytes via this HSF1-Nampt-NAD + -SIRT axis. Qiao and colleagues used the same type of flux analyzer (Seahorse Bioscience) that we used which facilitated the comparison of mitochondrial complex activities and respiration capacity measured by OCR. The pattern of the OCR data for wild-type hepatocytes is remarkably similar to untreated HK-2 cells in our study, whereas the changes in hsf1-/- hepatocytes were relatively very similar to the HBOT-treated HK-2 cells on day 1, indicating reduced mitochondrial respiratory capacity. The ECAR was slightly higher in hsf1-/- hepatocytes, whereas ECAR was relatively much higher in day 1 HBOT-treated HK-2 cells, indicating a much greater shift to glycolytic metabolism. We interpreted these changes as consistent with cells entering a cytoprotected state in response to HBOT. Interestingly, Qiao et al. found the HSF1-deficient cells had decreased ROS levels in their cytoplasm and mitochondria. They speculated that the changes in mitochondrial dynamics in hsf1-/- mice represented an adaptive or compensatory response to the lower levels of NAD+ and ATP. After normalizing to mitochondrial protein or DNA levels, they suggested that decreases in numbers of mitochondria per cell were the more likely explanation for impaired respiration. So, what about the overall cellular response? Perhaps their most surprising conclusion is that the loss of HSF1 actually increases cellular fitness and maintains the viability of cells by stimulating what they term “an adaptive compensatory response” to compensate for the diminished levels of NAD+ and ATP. They ascribe this change to the attenuation of nutrient-regulated anabolic metabolism in the liver.
In our gene expression studies, it is clear that both HSF1 and Nrf2 transcription factors are active in HBOT-treated vascular endothelial cells and that these cells acquire protection from oxidative stress. In our study, HBOT also downregulated ribosomal protein S6 kinase (S6K) in the mammalian target of rapamycin complex 1 (mTORC1) pathway which would attenuate nutrient-regulated anabolic metabolism. It is possible that Nrf2-regulated gene products are primarily responsible for the changes in energetics in these cells and that HSF1-regulated gene products provided a counterbalance that matches anabolic metabolism with available energy. Support for this idea comes from a report from the Lindquist laboratory describing the outcome of a high-throughput screen for small molecules that inhibit activation of HSF1 (Santagata et al. 2013). The strongest inhibitor was rocaglamide A, an inhibitor of translation initiation. They reported that RHT, an analog of rocaglamide A, increased thioredoxin-interacting protein (TXNIP) at both the protein and RNA levels as well as reduced glucose uptake and lactate production. In effect, rocaglates alter tumor energy metabolism, and HSF1 activation is required to support the anabolic malignant state that encompasses the Warburg effect which is essentially a metabolic state of many cancers marked by increased lactic acid production due to elevated aerobic glycolysis and consequently acidification of the culture medium. The target of RHT which is TXNIP is a key negative regulator of glucose uptake and thus cellular energy status. In cancer cells, lower levels of TXNIP facilitate the Warburg effect. In effect, cancer cells have co-opted cytoprotection and adapted it for their own benefit. Interestingly, a broad range of prokaryotic and eukaryotic cells use aerobic glycolysis during periods of rapid cell proliferation. It has been proposed that the major function of aerobic glycolysis is to produce elevated levels of intermediates of the glycolytic pathway needed to support the high levels of biosynthesis needed to produce new cells (Lunt and Vander Heiden 2011). Following this reasoning, cancer cells are co-opting a normal change in metabolic state that virtually all cells use to proliferate rapidly. As part of a cellular stress response, to oxidative stress, for example, the switch to aerobic glycolysis would allow stressed cells to rapidly replace damaged macromolecules and also produce ATP while lowering the production of ROS in mitochondria.
Transplasma membrane electron transport: an underappreciated partner in stress response caloristasis
tPMET systems are found in all mammalian cells examined so far. During a redox-coupled reaction, these systems reduce extracellular electron acceptors while oxidizing cytoplasmic electron donors. Two classes of systems described by Lane and Lawen (2009) can be distinguished mechanistically. The original enzyme-mediated tPMET systems in mammalian cells can be separated into two subclasses, one NADPH dependent and the other NADH dependent. The second system also transfers cytoplasmic reducing equivalents to the extracellular space but without the use of plasma membrane oxidoreductases. Lane and Lawen distinguish these two systems in terms of electron transfer at two different levels, enzyme-mediated tPMET and by transmembrane metabolite shuttling/cycling. The shuttle system is the more specialized one since the fate of the reducing equivalents once they are in the extracellular space depends on the specific redox couple.
According to Lane and Lawen, the tPMET concept dates back to 1924 when the observation was published that cell-impermeant redox dyes can be reduced by tissue slices (Voegtlin et al. 1924). It has been clearly established that tPMET is a crucial part of cellular bioenergetics and that regulation of tPMET is linked to aging and pathologies of humans. Of particular interest to our research and discussion here are the links to diabetic nephropathy (Matteucci and Giampietro 2000) and glycolytic cancer progression (Herst and Berridge 2007). In the stress response literature, tPMET typically has been considered briefly or not at all. A notable exception is a review article by Plácido Navas and colleagues (Navas et al. 2007). They describe this system as the plasma membrane redox system so their paper could be missed in a keyword search using tPMET. It is a particularly relevant paper for us because it points to the tPMET system as crucial to cellular life by preventing membrane damage and regulating apoptotic signaling at the plasma membrane. As such, we consider this system to be part of cellular defense against oxidative and proteotoxic stress, or cytoprotection as we term it. The second reason to focus on the Navas review is that it employs coenzyme Q or ubiquinone (CoQ) as the vehicle the authors use to describe the importance of the tPMET systems in aging and stress responses. They make the bold statement that CoQ is the only lipid-based antioxidant synthesized by all mammalian cells. Also important to our present discussion, CoQ links mitochondria to the plasma membrane. The final reactions of CoQ biosynthesis occur in the mitochondria of yeast and mammalian cells, and some of the CoQ produced is trafficked through the cellular endomembrane system to the plasma membrane (Villalba et al. 1995).
Based on our SeaHorse experiments discussed herein, we hypothesized that HBOT may stimulate cells to substantially increase tPMET activity, thus allowing cells to maintain energy levels (caloristasis) and redox homeostasis by reducing oxidative stress. Navas and coauthors make three points in their paper that taken together support our hypothesis. First, reducing mitochondrial function using ethidium bromide increases CoQ levels in plasma membranes and stimulates tPMET (Gómez-Díaz et al. 1997). Second, re-oxidation of cytosolic NADH is increased which aids to maintain the NAD+/NADH ratio involved in protecting the genome among other crucial roles. In our experiments, the reduction in mitochondrial oxidation viewed as a cell stress response, a beneficial and purposeful cellular change as opposed to ethidium bromide treatment, would be expected to trigger the same response caused by this inhibitor. Finally, increased accumulation of CoQ/CoQ reductases in the plasma membrane could be cytoprotective against ROS by neutralizing them at the cell surface even before they enter the cell. We further showed that cells treated with HBOT switch to aerobic glycolysis and as in the case of a number of cancer cell types, this may involve increased tPMET. The higher level of cytoplasmic NAD+ produced by this increase is needed to sustain elevated aerobic glycolysis that produces reducing equivalents by reducing NAD+ to NADH during the sixth step of glycolysis in a coupled redox reaction carried out by glyceraldehyde phosphate dehydrogenase, reviewed in Xiao et al. (2018). The increase in plasma membrane CoQ is likely provided short term by intracellular reservoirs located in the mitochondria and longer term by increased gene expression of CoQ biosynthetic enzymes. Navas et al. also noted that CoQ levels decreased with age and in elderly non-insulin-dependent diabetes mellitus patients, suggesting that the tPMET system is compromised under these conditions. Interestingly, caloric restriction induces the tPMET system and reduces levels of oxidative stress in aged membranes in several mammalian experimental models for extending life span (see Navas et al. for additional references).
Concluding remarks
Three different experimental approaches have converged to implicate HBOT in changes in mitochondrial dynamics that may facilitate the acquisition of cytoprotection. Over the past 7 years of research into the mechanisms of HBOT, we have uncovered three substantial links between HBOT and mitochondria. First, in our most recent study, a metabolomic approach was applied to the analysis of urine from diabetic patients receiving HBOT for treatment of diabetic foot ulcers (Harrison et al. 2018). The preliminary data show that HBOT reduces biomarkers of renal injury, oxidant stress, and mitochondrial dysfunction. These data suggest that HBOT could be an effective treatment for diabetic kidney disease as well as diabetic foot ulcers (Fig. 8 of Harrison et al. 2018). A number of small organic acids increased in concentration in the urine from diabetic patients receiving HBOT. These included glutarate, 3-methylglutarate/2-methylglutarate, and 3-hydroxybutyrate, all known products of mitochondrial protein and fatty acid catabolism. The increase in these small organic acids in patient urine after HBOT relative to untreated control urine is consistent with improved mitochondrial function. Second, the results of a human genome-wide microarray analysis of gene expression in HMEC-1 cells exposed to HBOT (Godman et al. 2010a) included molecular chaperones and chaperonins associated with mitochondria (Table 1). These were detected 24 h after HBOT and included induced DNAJC proteins involved in mitochondrial protein import and induced mitochondrial matrix chaperonins (HSPE1 and HSPD1). And third, in the present study, HBOT reduced mitochondrial respiration (oxidative phosphorylation) in HK-2 cells during both the initial response phase and the recovery phase of energy production (Table 2). The door is now open for future studies to test detailed hypotheses about the roles of DNAJC and other mitochondrial co-chaperones/molecular chaperones, changes in mitochondrial energy metabolism, and regulatory links between mitochondria and the proteostasis/caloristasis networks.
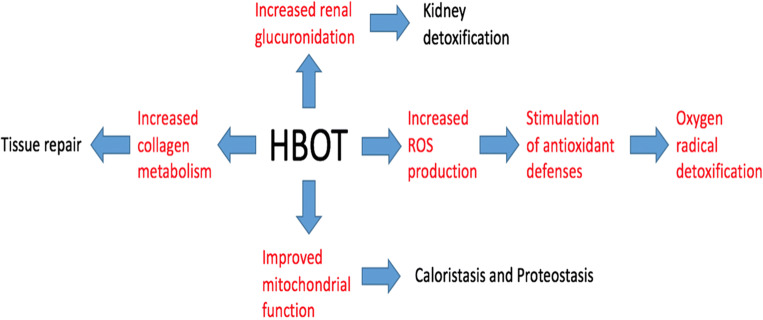
Fig. 8.
Renal protective roles of HBOT on the cellular and tissue levels. Effects elucidated through the metabolomic analysis are shown in red. (Taken from Harrison et al. 2018, with permission from the copyright owner of the Cell Stress Society International)
Table 1.
Molecular chaperones associated with mitochondria are induced by HBOT
| Immediately after HBOT | ||
| Gene symbol | Gene ID | HBOT 0/control 0 |
| DNAJB1 | 3337 | 1.83 |
| HSPA1B | 3304 | 1.81 |
| HSPA1A | 3303 | 1.73 |
| HSPH1 | 10808 | 1.49 |
| HSPA2 | 3306 | 1.34 |
| DNAJA4 | 55466 | 1.32 |
| 24-h after HBOT | ||
| Gene symbol | Gene ID | HBOT 24/control 24 |
| DNAJC11 | 55735 | 2.04 |
| HSPA14 | 51182 | 1.96 |
| HSPE1 | 3336 | 1.96 |
| HSPH2 | 3308 | 1.92 |
| HSPH1 | 10808 | 1.82 |
| HSPC3 | 3326 | 1.72 |
| DNAJC7 | 7266 | 1.59 |
| DNAJC17 | 55192 | 1.54 |
| DNAJC3 | 5611 | 1.54 |
| DNAJC5 | 80331 | 1.47 |
| DNAJC19 | 131118 | 1.45 |
| HSPD1 | 3329 | 1.45 |
| HSPB1 | 3315 | 0.84 |
Table 2.
Summary of HBOT-linked changes in the mitochondrial respiration
| Immediately after HBOT (day 0) | Post-HBOT recovery (24 h) |
|---|---|
| Majority injured by protein damage | DNAJC proteins involved in Mt protein (important induced) |
| Small fold increases | Mt matrix chaperonins induced (HSPE1, HSPD1) |
| No induction of HSPA6 indicates minor protein damage |
Acknowledgments
We wish to acknowledge the Life Support Technologies Group, Tarrytown, NY, and its founder and CEO Glenn J. Butler for generously providing the Small Round Experimental Hyperbaric Chamber, designed for cell cultures, used in these experiments. We thank Jared Fernandez, Agilent Technologies, Seahorse Bioscience, Santa Clara, CA, for helping us to evaluate cellular mitochondrial and glycolytic profiles. We also send our thanks to Carol Norris and Chris O’Connell, University of Connecticut, Flow Cytometry and Confocal Microscopy Facility, for their expert imaging help.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bilban M, Haschemi A, Wegiel B, Chin BY, Wagner O, Otterbein LE. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med (Berl) 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- Bonorino C, Sistonen L, Eriksson J, Mezger V, Santoro G, Hightower LE. The VIII international congress on stress proteins in biology and medicine: täynnä henkeä. Cell Stress Chaperones. 2018;23:171–177. doi: 10.1007/s12192-018-0878-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C. The heat shock factor HSF1 juggles protein quality control and metabolic regulation. J Cell Biol. 2017;216:551–553. doi: 10.1083/jcb.201701093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R. Hypoxia and hyperbaric oxygen therapy: a review. Int J Gen Med. 2018;11:431–442. doi: 10.2147/IJGM.S172460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Tang Z, Cao J, Zhou W, Li H, Sampson S, Dai C. Suppression of the HSF1-mediated proteotoxic stress response by the metabolic stress sensor AMPK. EMBO J. 2015;34:275–293. doi: 10.15252/embj.201489062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards ML. Hyperbaric oxygen therapy. Part 1: history and principles. J Vet Emerg Crit Care. 2010;20:284–297. doi: 10.1111/j.1476-4431.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol. 2010;647:37–74. doi: 10.1007/978-1-60761-738-9_3. [DOI] [PubMed] [Google Scholar]
- Godman CA, Chheda KP, Hightower LE, Perdrizet G, Shin D-G, Giardina C. Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress Chaperones. 2010;15:431–442. doi: 10.1007/s12192-009-0159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godman CA, Joshi R, Giardina C, Perdrizet G, Hightower LE. Hyperbaric oxygen treatment induces antioxidant gene expression. Ann N Y Acad Sci. 2010;1197:178–183. doi: 10.1111/j.1749-6632.2009.05393.x. [DOI] [PubMed] [Google Scholar]
- Gómez-Díaz C, Villalba JM, Pérez-Vicente R, Crane FL, Navas P. Ascorbate stabilization is stimulated in ρ°HL-60 cells by CoQ10increase at the plasma membrane. Biochem Biophys Res Commun. 1997;234:79–81. doi: 10.1006/bbrc.1997.6582. [DOI] [PubMed] [Google Scholar]
- Harrison LE, Giardina C, Hightower LE, Anderson C, Perdrizet GA. Might hyperbaric oxygen therapy (HBOT) reduce renal injury in diabetic people with diabetes mellitus? From preclinical models to human metabolomics. Cell Stress Chaperones. 2018;23:1143–1152. doi: 10.1007/s12192-018-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herst PM, Berridge MV. Cell surface oxygen consumption: a major contributor to cellular oxygen consumption in glycolytic cancer cell lines. Biochim Biophys Acta. 2007;1767:170–177. doi: 10.1016/j.bbabio.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Lane DJR, Lawen A. Transplasma membrane electron transport comes in two flavors. Biofactors. 2009;34:191–200. doi: 10.1002/biof.5520340303. [DOI] [PubMed] [Google Scholar]
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- Matteucci E, Giampietro O. Transmembrane electron transfer in diabetic nephropathy. Diabetes Care. 2000;23:994–999. doi: 10.2337/diacare.23.7.994. [DOI] [PubMed] [Google Scholar]
- Navas P, Villalba JM, de Cabo R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion. 2007;7:S34–S40. doi: 10.1016/j.mito.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Prata C, Grasso C, Loizzo S, Sega FV, Caliceti C, Zambonin L, Fiorentini D, Hakim G, Berridge MV, Landi L. Inhibition of trans-plasma membrane electron transport: a potential anti-leukemic strategy. Leuk Res. 2010;34:1630–1635. doi: 10.1016/j.leukres.2010.02.032. [DOI] [PubMed] [Google Scholar]
- Qiao A, Jin X, Pang J, Moskophidis D, Mivechi NF. The transcriptional regulator of the chaperone response HSF1 controls hepatic bioenergetics and protein homeostasis. J Cell Biol. 2017;216:723–741. doi: 10.1083/jcb.201607091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Ritossa FM. Experimental activation of specific loci in polytene chromosomes of Drosophila. Exp Cell Res. 1964;35:601–607. doi: 10.1016/0014-4827(64)90147-8. [DOI] [PubMed] [Google Scholar]
- Ritossa F. Discovery of the heat shock response. Cell Stress Chaperones. 1996;1:97–98. doi: 10.1379/1466-1268(1996)001<0097:DOTHSR>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, Paul C, Cotgreave IA, Arrigo AP, Orrenius S. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 2001;6:49–58. doi: 10.1379/1466-1268(2001)006<0049:HPMOTC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagata S, Mendillo ML, Tang YC, Subramanian A, Perley CC, Roche SP, Wong B, Narayan R, Kwon H, Koeva M, Amon A, Golub TR, Porco JA Jr, Whitesell L, Lindquist S. Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state. Science. 2013;341:1238303. doi: 10.1126/science.1238303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D-y, Xie F-Z, Zhai C, Stern JS, Liu Y, S-l L. The role of cellular oxidative stress in regulating glycolysis energy metabolism in hepatoma cells. Mol Cancer. 2009;8:32–32. doi: 10.1186/1476-4598-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart JA et al. (2018) How supraphysiological oxygen levels in standard cell culture affect oxygen-consuming reactions. Oxid Med Cell Longev Article ID 8238459:1–13 doi:10.1155/2018/8238459 [DOI] [PMC free article] [PubMed]
- Swan CL, Sistonen L. Cellular stress response cross talk maintains protein and energy homeostasis. EMBO J. 2015;34:267–269. doi: 10.15252/embj.201490757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol. 2009;106:988–995. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Chopra A, Giardina C, Sabbisetti V, Smyth JA, Hightower LE, Perdrizet GA. Hyperbaric oxygen therapy (HBOT) suppresses biomarkers of cell stress and kidney injury in diabetic mice. Cell Stress Chaperones. 2015;20:495–505. doi: 10.1007/s12192-015-0574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba JM, Navarro F, Córdoba F, Serrano A, Arroyo A, Crane FL, Navas P. Coenzyme Q reductase from liver plasma membrane: purification and role in trans-plasma-membrane electron transport. Proc Natl Acad Sci U S A. 1995;92:4887–4891. doi: 10.1073/pnas.92.11.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegtlin C, Johnson JM, Dyer HA. Quantitative estimation of the reducing power of normal and cancer tissue. J Pharmacol Exp Ther. 1924;24:305–334. [Google Scholar]
- Wittig R, Coy JF. The role of glucose metabolism and glucose-associated signalling in cancer. Perspect Med Chem. 2008;1:64–82. [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Wang R-S, Handy DE, Loscalzo J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid Redox Signal. 2018;28:251–272. doi: 10.1089/ars.2017.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Tan J, Lu W, Lu T, Hu Z. The potential role of small heat shock proteins in mitochondria. Cell Signal. 2013;25:2312–2319. doi: 10.1016/j.cellsig.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Zhang G, Darshi M, Sharma K. The Warburg effect in diabetic kidney disease. Semin Nephrol. 2018;38:111–120. doi: 10.1016/j.semnephrol.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]