Abstract
The global pandemic of coronavirus disease 2019 (COVID-19) has upended the world with over 6.6 million infections and over 391,000 deaths worldwide. Reverse-transcription polymerase chain reaction (RT-PCR) assay is the preferred method of diagnosis of COVID-19 infection. Yet, chest CT is often used in patients with known or suspected COVID-19 due to regional preferences, lack of availability of PCR assays, and false-negative PCR assays, as well as for monitoring of disease progression, complications, and treatment response. The International Atomic Energy Agency (IAEA) organized a webinar to discuss CT practice and protocol optimization from a radiation protection perspective on April 9, 2020, and surveyed participants from five continents. We review important aspects of CT in COVID-19 infection from the justification of its use to specific scan protocols for optimizing radiation dose and diagnostic information.
Key Points
• Chest CT provides useful information in patients with moderate to severe COVID-19 pneumonia.
• When indicated, chest CT in most patients with COVID-19 pneumonia must be performed with non-contrast, low-dose protocol.
• Although chest CT has high sensitivity for diagnosis of COVID-19 pneumonia, CT findings are non-specific and overlap with other viral infections including influenza and H1N1.
Keywords: COVID-19; Pandemics; Radiation protection; Tomography, X-ray computed
Since its identification in the Hubei province of China in December 2019, the coronavirus disease of 2019 (COVID-19) has exploded into a pandemic with more than 6.6 million confirmed cases and at least 391,000 deaths worldwide [1]. With prolonged shutdowns, the economic fallout may also prove devastating, especially on the most vulnerable sections of the population. To slow and halt the spread of infection, widespread testing of the population with suspected COVID-19 infection followed by isolation is the most critical step [2].
Direct identification of ribonucleic acids (RNA) of the SARS-CoV-2 virus from respiratory tract specimens with reverse-transcription polymerase chain reaction (RT-PCR) assays is the standard for testing of COVID-19 infection [3, 4]. However, chest radiography, computed tomography (CT), and ultrasonography are commonly used in the diagnosis of COVID-19 pneumonia [5, 6]. Out of concern over the use of CT and associated radiation doses to patients with suspected or known COVID-19 infection, the International Atomic Energy Agency (IAEA) organized a survey and a webinar to discuss CT practice and protocol optimization for COVID-19 pneumonia on April 9, 2020. We review important aspects of CT in COVID-19 infection from the justification of its use to specific scan protocols for optimizing radiation dose and diagnostic information. We present the results of the survey of 137 registrants from 84 sites across five continents and answer pertinent queries related to the use of imaging in COVID-19 infection. Participants attending the live webinar were given the opportunity to share their experience and ask questions.
RT-PCR, immunoassays, and imaging in diagnosis of COVID-19
PCR is a simple and commonly used molecular biology method used for the detection of specific sequences of DNA and RNA. The SARS-CoV-2 is an RNA virus. The RT-PCR assays start from the messenger RNA (mRNA) as a template for producing a complementary, single-strand DNA (cDNA) in a process called reverse transcription. Then, cDNA is converted to double-strand DNA and then amplified to indirectly detect the presence of specific mRNA sequences in the tested sample. Although RT-PCR assays have high specificity, their sensitivity depends on the quality and content of the original sample. Studies suggest that nasopharyngeal samples (sensitivity of 70%) are more sensitive than the oropharyngeal swabs (sensitivity of 60%) for detection of COVID-19 infection [7–9]. Although sputum samples have reported positive rates of 75–90%, less than one-third of patients produce sputum [10]. While real-time PCR for COVID-19 infection takes up to 5 min for a positive result, immunoassays can provide diagnostic information in 10–15 min [11]. The latter exploits the antibody-antigen recognition (using either monoclonal antibodies or cloned viral antigens) to provide evidence of prior viral exposure with reported sensitivities of 88–95% and specificities of 90–100% [11]. In early infection (days 4–10), a lower sensitivity is noted (up to 70%), which increases substantially with time.
False-negative results, as well as limited access or availability to RT-PCR and immunoassays for COVID-19, have increased the use of imaging, in particular CT, in patients with suspected COVID-19 infection. Several studies have reported on the use of chest CT in diagnosis as well as in the evaluation of severity and complications of COVID-19 pneumonia [6, 12–16]. These studies reported high sensitivity for CT in COVID-19 pneumonia, earlier detection of COVID-19 pneumonia with CT in patients with negative RT-PCR assays, and ability to differentiate COVID-19 pneumonia from other viral pneumonia on chest CT [12–18]. Conversely, others have opined that chest CT findings in COVID-19 pneumonia are non-specific; overlap with other infections such as influenza, H1N1, SARS, and MERS; and further limited due to co-occurrence of COVID-19 pandemic in the current flu season [19]. A more recent study with 1014 patients reported positive rates of 88% for chest CT and 59% for RT-PCR assay for the diagnosis of suspected COVID-19, and a sensitivity of 97% for CT with RT-PCR as a reference standard [20]. Another study with 51 patients who had chest CT and RT-PCR assay within 3 days reported 98% sensitivity for chest CT and 71% for RT-PCR [21, 22].
Chest radiographs have a high false-negative rate, especially in early and mild disease [23]. A few studies have reported on the successful use of lung ultrasonography (US) for diagnosis of COVID-19 infection [24–26]; however, its application remains limited in many countries.
Justification for CT
A recent study from the New England Journal of Medicine demonstrated that 86% of chest CT examinations were abnormal among patients at the time of admission, and about 18% of patients with non-severe disease had no radiographic or CT abnormalities [27]. Likewise, a multicenter study from China in patients with chest CT at the time of initial presentation with COVID-19 reported a 53% specificity and 42% negative predictive value [19].
The United States Center for Disease Control (CDC) does not recommend the use of chest radiographs or CT for diagnosis of COVID-19 [28] since imaging findings are non-specific and share commonalities with other infections such as influenza and H1N1. On March 11, 2020, the American College of Radiology (ACR) recommended that CT should not be used to screen or diagnose COVID-19 and that its use should be reserved for specific indications in hospitalized, symptomatic subjects. Update on March 22, 2020, acknowledged that “locally constrained resources” may play a role in determining the need for CT for diagnosis of COVID-19 infection [29]. The Royal College of Radiologists (RCR) reiterated “no current role for CT” in suspected COVID-19 on March 12, 2020. It later restated that chest CT may be used “in the absence of rapid access” to other COVID-19 tests in the acutely ill patients requiring an abdominal CT, and possibly needing emergency surgery [30]. Guidelines from Spain recommend that imaging can be considered in emergency patients when RT-PCR assays are either limited or suspected of being false negative [31]. Although recommendations from China and Italy suggested the use of imaging (chest radiographs and/or CT) with RT-PCR assay, the World Health Organization (WHO) advised against the use of imaging as the only mean of first-line diagnosis [2, 32]. In areas with insufficient RT-PCR and immunoassays, or with high prevalence of COVID-19, CT can be considered as a diagnostic method. This was suggested in the interim guidance for diagnosis and treatment of COVID-19 pneumonia from the Chinese General Office of National Health Committee [33].
The multinational consensus statement from the prestigious Fleischner Society on the role of chest imaging in COVID-19 stated that “imaging is not indicated” in suspected COVID-19 infection with mild clinical features. The statement supports the use of imaging in COVID-19 patients with worsening respiratory status as well as in those with suspected COVID-19 and moderate to severe presentation with a high pre-test probability of infection [34].
Thus, there is substantial evidence on when the use of CT in patients with suspected or known COVID-19 infection may be justified. When RT-PCR and immunoassays are available, and patients have mild disease, most organizations do not support diagnostic imaging. When these assays have limited availability, diagnostic imaging (chest radiographs or CT) can be used in patients with at least moderate to severe clinical features supportive of COVID-19 pneumonia. Finally, although CT has been used in assessing the severity of COVID-19 pneumonia, its routine use is not recommended [30, 31].
Along with the justification of diagnostic imaging, radiology services must ensure strict infection control measures to prevent infection transmission across imaging personnel and other patients without known or suspected COVID-19 infections [34, 35]. These measures include thorough cleaning and disinfection of imaging equipment after each use and personal protective equipment such as masks, face shields, gloves, and/or isolation gowns for imaging personnel.
Scanning techniques in COVID-19 pneumonia
There are less clarity and guidance on specific CT techniques and protocols for imaging of patients with suspected or known COVID-19 pneumonia. However, most publications on CT in suspected or known COVID-19 pneumonia report a single-phase, non-contrast chest CT without the need for contrast injection or post-contrast series (Fig. 1) [36–38]. Because the findings in non-complicated COVID-19 pneumonia are limited to lungs with rare involvement of pleural and mediastinum, there is little use of post-contrast CT images. In subjects with suspected pulmonary embolism or necrotizing pneumonia from superimposed bacterial infection, direct post-contrast arterial phase CT can be performed. There is no evidence to support the use of routine multiphase chest CT in patients with COVID-19 pneumonia. When possible, chest CT must be performed with an inspiratory breath-hold, extending from the lung apex to the lung base without the need to cover the adrenals. Patients must be given clear instructions on breath-holding before their scan.
Fig. 1.
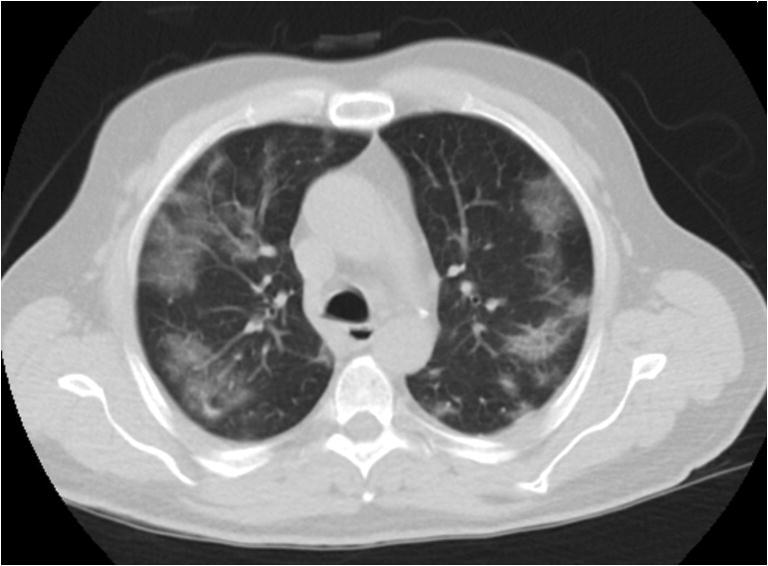
Transverse, low-dose, non-contrast chest CT image demonstrates typical peripheral, bilateral ground-glass opacities in a 65-year-old man with RT-PCR positive COVID-19 pneumonia (CTDIvol 2.2 mGy)
When selecting the scan parameters for chest CT protocol in patients with COVID-19 pneumonia, one must remember that a substantial proportion of patients are either short of breath or have coughing (Fig. 2). Therefore, protocols with faster scanning should be preferred in these patients with the use of faster gantry rotation time (0.5 s or less) and higher pitch values (greater than 1:1). The ability to implement faster scanning depends on the type and make of the CT scanner as well as the patient’s body habitus. Nonetheless, because most patients need a single-phase, low-dose CT acquisition, regardless of patient body habitus, users can apply faster scanning to avoid motion artifacts in patients who cannot hold their breath during image acquisition. This will help minimize suboptimal imaging and reduce the need for repeat scanning.
Fig. 2.
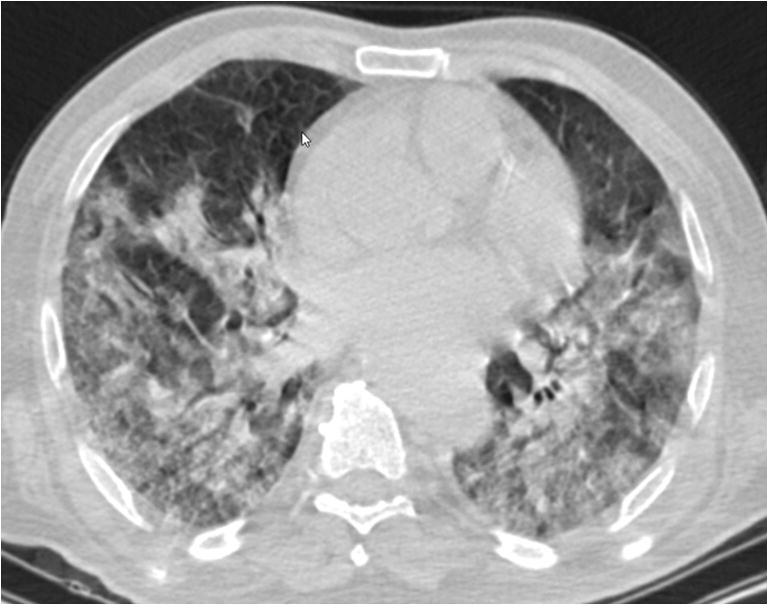
Extensive motion artifacts in transverse, low-dose, non-contrast chest CT image of a 77-year-old man with RT-PCR positive COVID-19 pneumonia (CTDIvol 4.9 mGy). Despite motion artifacts, it is possible to detect multifocal bilateral pulmonary opacities. Although suboptimal, chest CT was not repeated
There are few studies on the evaluation of low-dose CT protocol in patients with known or suspected COVID-19 pneumonia. Kang et al reported adequate assessment of pulmonary opacities related to COVID-19 pneumonia at 100 kV with tin filter (spectral shaping filter, Siemens Healthineers) and iterative reconstruction technique with a volume CT dose index (CTDIvol) of 0.4 mGy versus standard-dose protocol at 3.4 mGy [36]. Another study applied 100 kV with tin filter and 0.6-s exposure time using a high pitch and fast gantry rotation time to acquire chest CT examinations in COVID-19 pneumonia at 0.6 mGy CTDIvol, which were comparable to chest CT at 6.4 mGy [36].
The choice of specific scan parameters depends on the type and make of CT scanners. In general, most low-dose chest CT can be acquired at less than or equal to 100 kV and low tube current. The use of automatic tube current modulation technique should be preferred since it will allow automatic adjustment of tube current based on patient body habitus while accounting for factors that enable faster scanning. Automatic tube current modulation techniques require users to specify an image quality parameter to ensure that low-dose chest CT exams are performed at lower doses when compared with routine chest CT protocols. Fixed, low tube current can also be used for low-dose chest CT but may require some adjustment for patients with very large body habitus. While selecting the tube potential and tube current, users can target a CTDIvol for 3 mGy or less on most scanners for small or average size patients (up to 80 kg), which is recommended for low-dose lung nodule follow-up CT or for lung cancer screening. The radiation dose can be reduced further based on scanner technology or reconstruction method (iterative reconstruction versus filtered back projection). Conversely, for larger patients and patients requiring post-contrast chest CT, a higher radiation dose may be needed.
On some scanners, the choice of section thickness affects scan time (thinner beam collimation and slower table speed) as well as the applied radiation dose. While thin sections (less than or equal to 1.5 mm) are optimal for assessing pulmonary opacities, for patients with trouble holding the breath and higher probability of motion artifacts, thicker sections may be optimal on older scanners where thin sections require longer scan times. When available, iterative reconstruction techniques should be used so that lower radiation doses can be applied without compromising diagnostic quality.
In summary, a single-phase, non-contrast, low radiation dose chest CT is sufficient for the evaluation of most patients with COVID-19 infection. Post-contrast chest CT may be helpful when there is a clinical worsening of cardiorespiratory status or suspicion of pulmonary embolism in patients with COVID-19 pneumonia. Such post-contrast imaging should be performed without a non-contrast or native phase and with a single arterial phase CT. Table 1 summarizes important scan parameters for low-dose chest CT on some CT scanners.
Table 1.
Summary of proposed scan parameters for some multivendor CT scanners for acquiring low-dose chest CT
| Canon | GE | Philips | Siemens | |
|---|---|---|---|---|
| Scan parameters | Aquilion ONE | Revolution | IQon Spectral | Definition Force |
| Scan type | Helical | Helical | Helical | Helical |
| Tube potential | 120 kV | 120 kV | 120 kV | 100Sn |
| Image quality parameter (AEC) | SD 20 (SUREExposure) | NI 20 (SmartmA) | DRI 5 (DoseRight) | QRM 100 (SmartmA) |
| Rotation time | 0.275 s | 0.35 s | 0.4 s | ≤ 0.5 s |
| Pitch | 0.813:1 | 0.992:1 | 1:1 | 1.2:1 |
| D. config (mm) | 80 × 0.5 | 128 × 0.625 | 64 × 0.625 | 96 × 0.6 |
| Thickness (mm) | 1 | Prospective 5 | 1 | 1 |
| (Retro 1.25) | ||||
| Interval (mm) | 0.5 | 0.5 | 0.5 | 0.7 |
| Kernels | Body and lung Std | Lung or bone | YA and A | Br40 and Br64 |
| Reconstruction | AIDR 3D STD | ASIR-V (30) | iDose4 level 5 | ADMIRE level 3 |
AEC automatic exposure control, D. config detector configuration, SD standard deviation, NI noise index, Retro retrospective section thickness, DRI dose right index, 100 Sn 100 kV with tin filter for spectral shaping, QRM quality reference mAs
Survey results on imaging in COVID-19
Of the 1633 registrants from 100 countries, 977 from 84 countries attended the live webinar on COVID-19 and Chest CT: Protocol and Dose Optimization on April 9, 2020. The professions represented included radiologists, radiographers, medical physicists, radiation protection specialists, and students. The registrants were encouraged to respond to a survey questionnaire before the webinar (Europe, n = 76 participants; Asia, n = 23 participants; North America, n = 23 participants; Africa, n = 7 participants; South America, n = 5 participants). Information regarding the country of survey participants was not obtained and may have influenced the survey responses due to cultural and economic factors. Among the 137 responses, 110 registrants (80%) responded that their healthcare sites had received patients with known or suspected COVID-19 infection, while the remaining 7% (10/137) and 12% (17/137) either did not know or had not received such patients. About 51% and 48% of registrants indicated that chest radiograph and CT, respectively, were the most frequently used imaging tests at their site in COVID-19 patients. Most responses (66%, 84/127) suggested that they used CT for diagnosis of COVID-19 pneumonia, followed by evaluation of disease severity (62%, 79/127) and complications (51%, 65/127). Most responders indicated that they often (53%, 69/130) or always (20%, 26/130) use chest CT for diagnosis of suspected COVID-19 pneumonia. A slight majority of responses favored the use of non-contrast chest CT (53%, 62/118) over occasional use of contrast-enhanced chest CT (42%, 49/118) in patients with known or suspected COVID-19 pneumonia. Fortunately, most respondents noted that they always or usually acquired only one chest CT scan series (82%, 101/123), although 18% (22/123) acquired 2–3 scan phases in COVID-19 pneumonia. The non-contrast phase was obtained at most sites (86%, 103/120), although others acquired 1–2 post-contrast phases. A majority of responses indicated that chest CT exams for COVID-19 in their sites were associated with the same dose as a routine chest CT (55%, 64/117; CTDIvol of 5–10 mGy), whereas low-dose (43%, 50/117; CTDIvol < 5 mGy) and high-dose (3%, 3/117; CTDIvol > 10 mGy) CT protocols were used at the remaining sites.
Pertinent queries on chest CT in COVID-19
In this section, we address some important questions submitted by the registrants on the use of chest CT in patients with COVID-19 pneumonia.
Should we use high-resolution CT (such as for diffuse lung disease with scanning in inspiratory and/or expiratory phases) in patients with suspected COVID-19?
A low-dose, single-phase (inspiratory breath-hold if possible), thin-section, non-contrast CT of the entire chest (apex to the base) is the most frequently applied protocol reported in prior studies [33–35]. To our best knowledge, no previous studies have reported benefits or need for high-resolution CT in COVID-19 pneumonia.
Can radiation dose be reduced when there is an additional need to perform CT angiography (for pulmonary embolism) after the initial non-contrast CT?
For CT pulmonary angiography in patients with known or suspected COVID-19 pneumonia, it is prudent to exclude non-contrast phase CT and directly acquire CT pulmonary angiography using a single post-contrast phase CT protocol. A negative chest CT does not exclude COVID-19 infection, and therefore, in this situation, such exams should be tailored for pulmonary embolism. When present, most findings related to moderate or severe COVID-19 pneumonia can be seen on direct post-contrast images.
Should we consider chest CT or radiography in healthcare workers to diagnose COVID-19 when other recommended tests are limited or unavailable?
There is a growing body of recommendations that imaging (chest CT and radiography) should not be routinely performed for diagnosis of COVID-19 pneumonia. When access or availability to RT-PCR and immunoassays is limited for the healthcare workers, it is prudent to restrict use of imaging for primary diagnosis due to associated radiation exposure as well as lack of sensitivity in early disease. Instead, a regular surveillance (daily or weekly) of COVID-19 symptoms must be performed in all healthcare workers to identify early disease. Imaging should be used with caution in healthcare workers unless they have moderate to severe disease, have other comorbidities, or experience worsening of cardiorespiratory status.
Is it acceptable to perform chest CT with arms by the side of the patient to minimize patient contact with CT technologists or other healthcare workers?
In the absence of contraindications (such as suspected trauma, immobility, and pain at the shoulders), it is not acceptable to perform chest CT with arms by the side of the patient since arms impair the image quality, cause artifacts, and are associated with a substantial increase in radiation dose with an otherwise lower dose chest CT. Ambulatory subjects should be instructed to place their arms over their head for chest CT. For subjects who cannot follow instructions, the operator must manually place the arms over the head using protective gear (gloves).
What is the correct dose for chest CT for patients with COVID-19?
Justification is the most crucial step when it comes to radiation protection. In relation to dose optimization, although there are no recommended dose levels for chest CT in patients with COVID-19 pneumonia, most studies [33–35] report single-phase, low-dose, non-contrast chest CT, which implies CTDIvol < 3 mGy in most small and average size patients.
How do we perform chest CT for suspected COVID-19 in pregnant patients?
Chest CT must be avoided in pregnant patients for the diagnosis of suspected COVID-19 pneumonia. Although there are no specific publications or guidance on this matter, in pregnant patients with suspected complications or worsening respiratory status, a chest CT may be indicated and, when necessary, performed with single-phase, non-contrast, low-dose CT protocol. An exception to this rule is a suspected pulmonary embolism in COVID-19-positive, pregnant patients who will need intravenous contrast and higher dose. In such patients, direct CT angiography must be performed from lung apices to lung bases (without extending the scan into the upper abdomen).
How to scan children with suspected COVID-19 infection?
Children are more vulnerable than adults to the effects of radiation dose. Like in adults, chest CT in children must be only performed when RT-PCR and immunoassays are not available and/or urgent information is needed in children with severe disease. When indicated, chest CT in children can be performed at CTDIvol as low as 1 mGy (up to 50 kg body weight) with a single-phase, non-contrast acquisition.
Conclusions
Most national and international organizations recommend against routine use of diagnostic imaging for the diagnosis of COVID-19 pneumonia unless there is a lack of availability or access to RT-PCR or immunoassays in patients with moderate to severe disease, worsening respiratory status, or a suspicion of cardiopulmonary complications. When indicated, a chest CT should be performed with a low-dose, single-phase protocol using fast scanning techniques to minimize motion artifacts.
Abbreviations
- ACR
American College of Radiology
- CDC
Center for Disease Control
- cDNA
Single-strand DNA
- COVID-19
Coronavirus disease 2019
- CT
Computed tomography
- CTDI
CT dose index
- DNA
Deoxyribonucleic acid
- IAEA
International Atomic Energy Agency
- kV
Kilovolt
- mRNA
Messenger RNA
- RCR
Royal College of Radiologists
- RNA
Ribonucleic acids
- RT-PCR
Reverse-transcription polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- US
Ultrasonography
- WHO
World Health Organization
Funding information
The authors state that this work has not received any funding related to this project.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Dr. Mannudeep K. Kalra.
Conflict of interest
Our institution received research grants from Siemens Healthineers, Lunit Inc., and Riverain Tech. for unrelated projects.
Statistics and biometry
No statistical methods were necessary for this paper.
Informed consent
Not applicable for a review article.
Ethical approval
Not applicable for a review article.
Methodology
• This is a review article. No prospective or retrospective patient studies were performed. It was a multicenter work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coronavirus Resource Center. John Hopkins’ University. https://coronavirus.jhu.edu/map.html. Accessed on 6.5.2020
- 2.WHO guidelines. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance. Accessed on 4.15.2020
- 3.SUMMARY COVID-19 RT-PCR TEST – FDA. https://www.fda.gov/media/136151/download. Accessed 4.15.2020
- 4.Corman VM, Landt O, Kaiser M et al (2020) Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25(3):2000045. 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed]
- 5.Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi: 10.1016/j.ejro.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wölfel R, Corman VM, Guggemos W et al (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581(7809):465–469. 10.1038/s41586-020-2196-x [DOI] [PubMed]
- 8.Green K, Allen J, Suklan J et al (2020) What is the role of imaging and biomarkers within the current testing strategy for the diagnosis of Covid-19? CEBM, Oxford. Available via https://www.cebm.net/covid-19/what-is-therole-of-imaging-and-biomarkers-within-the-current-testing-strategy-for-the-diagnosis-ofcovid-19/
- 9.Tang YW, Schmitz JE, Persing DH, Stratton CW (2020) Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J Clin Microbiol 58(6):e00512–e00520. 10.1128/JCM.00512-20 [DOI] [PMC free article] [PubMed]
- 10.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheridan C (2020) Fast, portable tests come online to curb coronavirus pandemic. Nat Biotechnol 38(5):515–518. 10.1038/d41587-020-00010-2 [DOI] [PubMed]
- 12.Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;20:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li K, Wu J, Wu F et al (2020) The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Invest Radiol 55(6):327–331. 10.1097/RLI.0000000000000672 [DOI] [PMC free article] [PubMed]
- 14.Yang R, Li X, Liu H, et al. Chest CT severity score: an imaging tool for assessing severe COVID-19. Radiology: Cardiothoracic Imaging. 2020;2:2. doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li K, Fang Y, Li W et al (2020) CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur Radiol 25:1–10. 10.1007/s00330-020-06817-6 [DOI] [PMC free article] [PubMed]
- 16.Zhao W, Zhong Z, Xie X, Yu Q, Liu J (2020) Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol 3:1–6 [DOI] [PubMed]
- 17.Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020;10:200823. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;12:200343. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen Z, Chi Y, Zhang L et al (2020) Coronavirus disease 2019: initial detection on chest CT in a retrospective multicenter study of 103 Chinese subjects. Radiology: Cardiothoracic Imaging 2(2) [DOI] [PMC free article] [PubMed]
- 20.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;26:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;19:200432. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Liu G, Zhang X, Li H (2020) Chest CT and RT-PCR: radiologists’ experience in the diagnosis of COVID-19 in China. Eur Radiol. Letter to the editor. Available via https://www.europeanradiology.org/opinions/chest-ct-and-rt-pcr-radiologists-experience-in-the-diagnosis-of-covid-19-in-china/
- 23.Wong HYF, Lam HYS, Fong AH, et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2019;27:201160. doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poggiali E, Dacrema A, Bastoni D, et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID-19) pneumonia? Radiology. 2020;13:200847. doi: 10.1148/radiol.2020200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buonsenso D, Pata D, Chiaretti A (2020) COVID-19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med 8(5):e27. 10.1016/S2213-2600(20)30120-X [DOI] [PMC free article] [PubMed]
- 26.Soldati G, Smargiassi A, Inchingolo R et al (2020) Is There a Role for Lung Ultrasound During the COVID-19 Pandemic? J Ultrasound Med. 10.1002/jum.15284 [DOI] [PMC free article] [PubMed]
- 27.Guan WJ, Ni ZY, Hu Y et al (2020) China medical treatment expert group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed]
- 28.Centers for Disease Control and Prevention. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed on 4.16.2020
- 29.ACR recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed on 4.16.2020
- 30.Coronavirus (COVID-19) clinical radiology resources. https://www.rcr.ac.uk/college/coronavirus-covid-19-what-rcr-doing/clinical-information/rcr-position-role-ct-patients. Accessed on 4.16.2020
- 31.Clinical Management of COVID-19 (Spanish). Spanish guidelines, https://seram.es/images/site/Recomendaciones_imagen_SERAM_COVID_19.pdf. Accessed on 4.16.2020
- 32.Nicastri E, Petrosillo N, Bartoli TA, et al. National Institute for the Infectious Diseases “L. Spallanzani”, IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep. 2020;12(1):8543. doi: 10.4081/idr.2020.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.General Office of National Health Committee. Office of state administration of traditional Chinese medicine. Notice on the issuance of a programme for the diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (trial fifth edition). 2020. https://bgs.satcm.gov.cn/zhengcewenjian/2020-02-06/12847.html. Accessed 13 May 2020
- 34.Rubin G, Ryerson C, Haramati L, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Radiology. 2020;7:201365. doi: 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mossa-Basha M, Meltzer CC, Kim DC, et al. Radiology department preparedness for COVID-19: radiology scientific expert panel. Radiology. 2020;16:200988. doi: 10.1148/radiol.2020200988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang Z, Li X, Zhou S (2020) Recommendation of low-dose CT in the detection and management of COVID-2019. Eur Radiol 19:1–2. 10.1007/s00330-020-06809-6 [DOI] [PMC free article] [PubMed]
- 37.Agostini A, Floridi C, Borgheresi A, et al. Proposal of a low-dose, long-pitch, dual-source chest CT protocol on third-generation dual-source CT using a tin filter for spectral shaping at 100 kVp for coronavirus disease 2019 (COVID-19) patients: a feasibility study. Radiol Med. 2020;125(4):365–373. doi: 10.1007/s11547-020-01179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caruso D, Zerunian M, Polici M, et al. Chest CT features of COVID-19 in Rome, Italy. Radiology. 2020;3:201237. doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]


