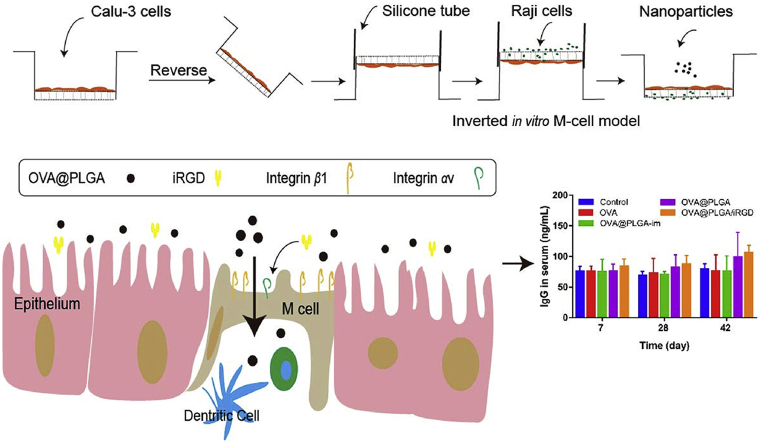
Abstract
In order to better evaluate the transport effect of nanoparticles through the nasal mucosa, an in vitro nasal cavity-mimic model was designed based on M cells. The differentiation of M cells was induced by co-culture of Calu-3 and Raji cells in invert model. The ZO-1 protein staining and the transport of fluorescein sodium and dexamethasone showed that the inverted co-culture model formed a dense monolayer and possessed the transport ability. The differentiation of M cells was observed by up-regulated expression of Sialyl Lewis A antigen (SLAA) and integrin β1, and down-regulated activity of alkaline phosphatase. After targeting M cells with iRGD peptide (cRGDKGPDC), the transport of nanoparticles increased. In vivo, the co-administration of iRGD could result in the increase of nanoparticles transported to the brain through the nasal cavity after intranasal administration. In the evaluation of immune effect in vivo, the nasal administration of OVA-PLGA/iRGD led to more release of IgG, IFN-γ, IL-2 and secretory IgA (sIgA) compared with OVA@PLGA group. Collectively, the study constructed in vitro M cell model, and proved the enhanced effect of targeting towards M cell with iRGD on improving nasal immunity.
Key words: M cells, In vitro, Cell model, iRGD peptide, Nasal administration, Mucosal vaccination
Graphical abstract
Calu-3 and Raji cells were co-cultured in inverted model to construct in vitro nasal cavity mimic M-cell model. iRGD targets integrin receptors on the surface of M cells to enhance the uptake of antigens by M cells. iRGD is co-administrated for nasal immunization to improve the immune response.
1. Introduction
Nasal administration typically delivers drug through the absorption from nasal mucosa to blood circulation and other tissue to exert a local or systemic therapeutic effect. Compared with other administration routes, nasal administration exhibits some unparalleled advantages, such as its rapid absorption, the avoidance of first-pass effect in the liver, high bioavailability, and direct entry into brain, etc1, 2, 3, 4, 5. Nasal administration is especially promising in vaccine immunity, due to the existence of nasal mucosa-related lymphoid tissue, which contains abundant immune cells, such as B cells, CD4+ and CD8+ lymphocytes, and dendritic cells6, 7, 8, 9, 10. After the uptake and delivery of antigen via M cells, the antigens are immediately processed and presented by dendritic cells. Mucosal lymphocytes then emigrate from the nasal-associated lymphoid tissue (NALT), circulate through the bloodstream and home to distant mucosal effector sites to induce immune response. Compared with intramuscular and subcutaneous injections, nasal administration can stimulate not only the systemic immunity, but the mucosal immune response, resulting in more complete immune protection11, 12, 13.
Due to enzyme degradation when exposed to the nasal environment, nano delivery system is adopted for the nasal administration to improve the stability of immunogens. Therefore, simple and effective in vitro evaluation model is urgently needed for nasal preparation to conduct various studies on the nasal absorption, metabolic characteristics and toxicity of the drug delivery system. The in vitro models are often presented by isolating primary mucosal epithelial cells or using a type of nasal epithelial cancer cell line14, 15, 16, but these models cannot mimic the overall nasal mucosa due to the complexity of the nasal environment.
An in-depth study of the nasal mucosa revealed that M cells, distributed in the nasal mucosa, play a critical role in the translocation of antigen and drug, which is similar to that found in the Payer's Patch of intestinal epithelium17, 18, 19. M cells are characterized by irregular shape and an absent brush border. Basolateral membrane of M cells is deeply caved, with a pocket-like shape to host some lymphocytes20,21. Given that structure of M cells, nanoparticles and antigens have easier access to M cells rather than other cells in nasal mucosa. Therefore, the establishment of an in-vitro nasal model based on M cell differentiation can more accurately simulate the drug transport in the nasal mucosa.
To date, the in vitro M cell model has been obtained by co-culturing Raji lymphoma cells and Caco-2 colon cancer cells to induce differentiation of M cells22, 23, 24, 25. Moreover, the researchers improved the M-cell induction efficiency by developing the inverted co-culture model26,27. However, this model is still not suitable for in vitro evaluation of nasal administration, mainly because Caco-2 cells lack the function of secreting mucus and expression of some ion channels, which is quite different from the physiological state of nasal cavity. Therefore, we chose to replace Caco-2 cells with Calu-3 cells to construct a nasal M cell model built on the inverted co-culture model. Apart from the properties of forming polar monolayer membrane and tight junctions similar to Caco-2 cells, Calu-3 cells possess mucus-secreting ability so that this M cells model should better reflect the true state of the nasal M cells28, 29, 30, 31.
The outstanding characteristic of M cells is that they can efficiently transport macromolecule drugs or particles. However, the low distribution ratio of M cells limits the transport efficiency. Thus, it is of profound significance to design an M cell-targeting delivery system. Noting that some specific receptors overexpressed on M cells, such as α-l-fucose32,33, claudin 4 protein and integrin β134, 35, 36, ligand-modified nanoparticles have proved the targeting ability toward M cells (for instance, Aleuria aurantia lectin, CKS9 peptide and RGD)20,37, 38, 39, 40. Yet, complicated preparation still remains an inevitable challenge to be addressed. iRGD, as a cell membrane penetrating peptide, can bind to the αv integrin receptor expressed on the cell surface by the contained RGD motif to promote the nanoparticle uptake via co-administration41,42. M cells in intestinal mucosa were found to express integrin αv receptor on the surface, which is essential for invasion of some pathogens43. Therefore, we speculated that the co-administration of iRGD could combine with integrin receptor on the surface of M cells and promote the uptake of nanoparticles or antigens.
In this study, the co-culture inverted model of Calu-3 and Raji cells was established to evaluate the nasal transport and mechanism of nanoparticles. In addition, the targeting effects of iRGD were evaluated at the cellular and animal level. M cell-targeting enhanced the immune response of nasal administration, as proved by in vivo immune experiment. The research will provide an alternative model for in vitro evaluation of nasal delivery system, and help promote the further development of nasal delivery system.
2. Materials and methods
2.1. Materials
NH2‒PEG‒SH (MW = 3400) was purchased from Laysan Bio, Inc. (Arab, USA). Bilirubin was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Albumin from chicken egg white lyophilized powder (≥98%) and poly (vinyl alcohol) (PVA) were purchased from Sigma‒Aldrich Inc. (St. Louis, USA). Poly (d,l-lactide-co-glycolide) polymer (PLGA-50:50: inherent viscosity 0.55–0.75 dL/g) was purchased from LACTEL Absorbable Polymers (Birmingham, USA). The Bradford protein assay kit, BCA protein assay kit and Alkaline Phosphatase assay kit were purchased from Beyotime Biotechnology (Shanghai, China). iRGD was synthesized by Shanghai Dechi Biosciences Co., Ltd. (Shanghai, China). Coumarin-6, fluorescein sodium, dexamethasone, propranolol, chlorpromazine, filipin, sodium azide, and nocodazole were purchased from Dalian Meilun Biotech Co., Ltd. (Dalian, China). Matrigel™ Basement Membrane Matrix was obtained from Becton, Dickinson and Company (Franklin Lakes, USA). Anti-ZO-1 tight junction protein antibody and anti-integrin β1 antibody were purchased from Abcam (Cambridge, UK). CA19-9 monoclonal antibody was gained from Thermo Fisher Scientific (Waltham, USA). Human lung adenocarcinoma cell line (Calu-3) and lymphoma cell line (Raji) were purchased from the Chinese Academy of Science cells Bank (Shanghai, China).
Female BALB/c mice (18 ± 2 g) and Sprague‒Dawley rats (150‒200 g) were purchased from Dashuo Biotechnology Co., Ltd. (Chengdu, China). All animal experiments were performed under the guidelines approved by the Experiment Animal Administrative Committee of Sichuan University (Chengdu, China).
2.2. Construction of an in vitro M cell model
The normally oriented M cell model was obtained following the protocol as mentioned in the literature26. Simply, 12-well transwell inserts were precoated with 300 μL/well Matrigel™ Basement Membrane Matrix, which were diluted in pure DMEM into a final protein concentration of 100 μg/mL, and placed in carbon dioxide cell culture chamber. After 1 h, the remaining liquid was removed and each transwell was washed with preheated 500 μL DMEM +1% (v/v) PEST. Then, Calu-3 cells were seeded on the upper side of insert at a density of 5 × 105 cells per well and cultured. After 7 days, Raji cells were suspended in DMEM+1% (v/v) PEST, and added to the basolateral side of insert about 5 × 105 cells per well. The co-cultures situation was lasted for 4–5 days. The upper chamber medium was replaced every two days. For the inverted model, the inserts were inverted after seeding Calu-3 cells for 3–5 days. A 1.5 cm-long silicone tube was placed at the end of the insert and filled with 500 μL medium. After another 5-day incubation, Raji cells were co-cultured in the basolateral compartment at same cell density as above. Five days later, the models were ready for use. The inverted mono-culture of Calu-3 cells was set as control.
2.3. Measurement of transepithelial electrical resistance (TEER)
TEER was measured every two days during the construction of cell model culture. Briefly, cell monolayers were washed twice in Hank's Balanced Salt Solution (HBSS), and filled with preheated medium. After 15‒30 min of equilibration in the incubator, the TEER was measured by Millicell-ERS ohmmeter (Millipore, Boston, USA). The resistance of the cell culture insert without cells was considered as blank resistance and subtracted. The change in transmembrane resistance during cell growth was recorded.
2.4. Verifying the expression of ZO-1 protein
The cells were fixed in 4% paraformaldehyde for 20 min, washed with PBS for 3 times, and then blocked with 10% goat serum for 2 h. Then the rabbit anti-humanZO-1 antibody was diluted and incubated with cells at 4 °C for 12 h. The cells were washed 3 times with PBS, and incubated with Cy3-labeled donkey anti-rabbit secondary antibody for 2 h. After washing 3 times with PBS, the cells were stained with 0.5 μg/mL DAPI for 5 min, and the pieces were observed by confocal microscopy (A1R+, Nikon, Tokyo, Japan). Sialyl Lewis A antigen (SLAA) and integrin β1 were stained and observed by the same method.
2.5. Transportation of sodium fluorescein and dexamethasone
Sodium fluorescein and dexamethasone were formulated into a final concentration of 500 μg/mL and 1 mg/mL in HBSS, respectively. Take 100 μL of sodium fluorescein or dexamethasone solution into the apical chamber of M-cell model, and 600 μL of HBSS into basolateral chamber. Then, the plate was placed in a cell culture incubator. At 0.5, 1 and 2 h, 100 μL of liquid was sampled from the basolateral chamber and the same volume of blank HBSS was added. The concentrations of sodium fluorescein and dexamethasone were measured to calculate the apparent permeability coefficient [Papp, Eq. (1)]. The fluorescence intensity of sodium fluorescein was measured with microplate reader (Thermo Scientific Varioskan Flash, Waltham, USA) at an Ex/Em of 480 nm/530 nm. The dexamethasone was detected by high performance liquid chromatography (HPLC, Shimadzu, Kyoto, Japan). The mobile phase consisted of acetonitrile and water (45:55). The eluent was monitored at 240 nm. The flow rate was 1.0 mL/min and the column temperature was 40 °C.
| (1) |
where dQ/dt is the drug transport amount per unit time (μg/s); A is the area of the transport membrane (1.12 cm2 for each well of 6-well plate, and 0.33 cm2 for 12-well plate); C0 is the initial concentration of sodium fluorescein or dexamethasone.
2.6. Detection of alkaline phosphatase activity
The medium in the apical and basolateral chamber was collected and centrifuged at 3000×g for 10 min, and the supernatant was taken and detected with an alkaline phosphatase activity kit. At the same time, the sample was used to quantify the protein concentration by BCA kit, and the enzyme activity/mg protein was calculated to compare the alkaline phosphatase activity of the monolayer membrane and the co-culture membrane.
2.7. Quantitative analysis of Raji cells on co-culture membrane
According to the description in the literature26, Raji cells were labeled yellow–green fluorescent with a Cell Trace™ CFSE Cell Proliferation Kit according to the instructions at 3 days before co-cultures. Briefly, Cell Trace™ CFSE stock solution was diluted in PBS to 10 μmol/L, and prewarmed in 37 °C. Raji cells were collected, suspended in prepared solution at a density of 10 × 106 cells per tube and incubated at 37 °C. After 15 min, the cells were centrifuged and suspended in fresh prewarmed medium, and then incubated for another 30 min and washed. Three days later, CFSE-labeled Raji cells were cocultured with Calu-3 monolayer. After 5 days, the co-cultured cells were detected by flow cytometry (Becton, Dickinson and Company, Franklin Lakes, USA).
2.8. Model markers transportation evaluation
Propranolol and albumin were formulated into 10 μg/mL and 2 mg/mL solution in HBSS, respectively. The method of the transport experiment is the same as that of previous transport experiment of fluorescein sodium and dexamethasone. In albumin transportation, a group of inserts were pre-incubated with 2.5 mmol/L EGTA (dissolved in HBSS, pH 7.4) twice for 15 min at 37 °C. EGTA (2.5 mmol/L) was set up to open the tight connection. The concentration of propranolol was determined by HPLC, and the concentration of albumin was determined by Bradford protein assay kit.
2.9. Transportation of cou@BRNPs
Nanoparticles were prepared by solvent diffusion method as described in the literature44. The amphiphilic compounds formed by conjugation of bilirubin and PEG (Br-PEG) were synthesized. Afterwards, Br-PEG self-assembled in water to form micelles to carry hydrophobic molecules in the core (BRNPs). Briefly, coumarin-6 (10 μL, 10 mg/mL) was mixed with Br-PEG (200 mg/mL, 20 μL). The mixture was dripped into 2 mL water and stirred for 10 min, then the solution was centrifuged (6000×g, 10 min) to remove unencapsulated coumarin-6 and concentrated by ultrafiltration to prepare a concentration of 50 μg/mL of nanoparticle solution. 300 μL of cou@BRNPs solution or cou@BRNPs with 4.33 μg/mL iRGD was added apically to the M-cell model, and 1 mL of blank HBSS was added to the lower chamber and the plate was placed into incubator. At 0.25, 0.5, 1 and 2 h, 100 μL of the liquid was taken out from the lower chamber and the same volume of blank HBSS was added. The concentration of nanoparticles was measured using a microplate reader (Thermo Scientific Varioskan Flash).
2.10. Transportation of cou@PLGA nanoparticles
PLGA and coumarin-6 were dissolved in dichloromethane as the organic phase, and 1% PVA was used as the aqueous phase. Two phases were mixed together, sonicated for 5 min, and then the organic phase was removed by rotary evaporation. The remaining liquid was washed and concentrated by ultrafiltration. Nanoparticles transportation experiments were carried out as described above.
2.11. Transportation mechanism of nanoparticles
Co-culture membranes were pre-incubated with clathrin-mediated endocytosis inhibitor chlorpromazine (10 μg/mL), caveola-dependent endocytosis inhibitor filipin (5 μg/mL), energy generation inhibitor sodium azide (NaN3, 2.6 mg/mL), and microtubule inhibitor nacodazole (33 μmol/L) for 3 h at 37 °C. Incubation at 4 °C was used to evaluate the influence of energy generation inhibition. Then, the cells were incubated with cou@BRNPs for 1 h as described previously. The number of transported nanoparticles was measured.
2.12. In vivo distribution of nanoparticle
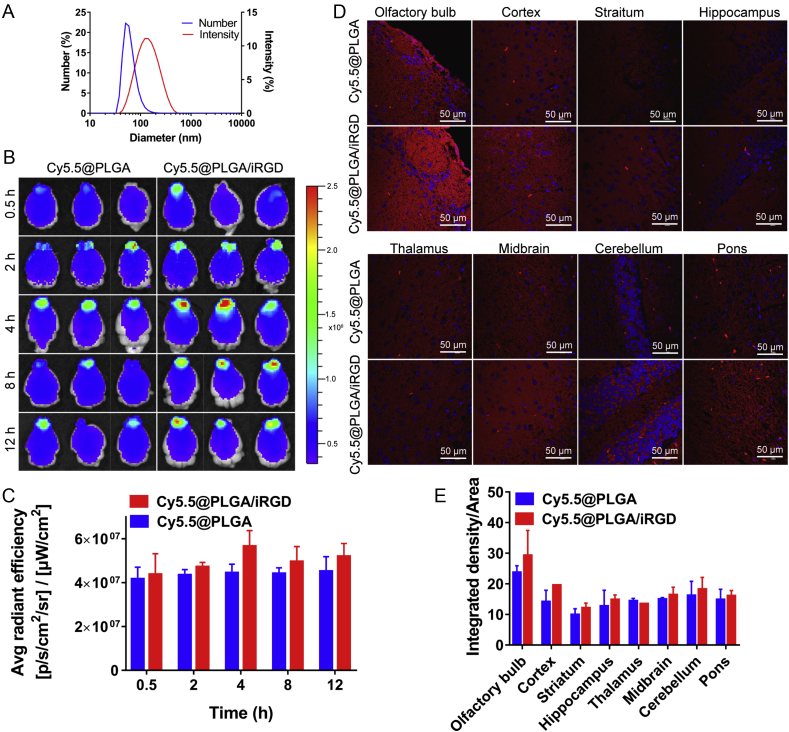
Cy5.5@PLGA nanoparticles were prepared using the same procedure as that of cou@PLGA. Female BALB/c mice were randomly divided into two groups, which were intranasal administrated with Cy5.5@PLGA/iRGD and Cy5.5@PLGA, respectively, at identical Cy5.5 dose of 35 μg/kg and iRGD dose of 4 μmol/kg. Mice were sacrificed at 0.5, 2, 4, 8 and 12 h after administration, and the distribution of nanoparticles was observed by Lumina III imaging system (PerkinElmer, Waltham, USA). Subsequently, the brains were divided into olfactory bulb, cortex, striatum, hippocampus, thalamus, midbrain, cerebellum, and pons for slice preparation. The slices were stained with DAPI, and the fluorescence distribution was observed by confocal microscopy (A1R+, Nikon).
2.13. Nasal immunity studies
OVA@PLGA nanoparticles were prepared by multiple emulsion method. Briefly, OVA was dissolved in 1% PVA solution to form water phase, PLGA was dissolved in dichloromethane to form organic phase, then the two phases were mixed in a volume ratio of 1:10, and W/O emulsion was obtained by probe ultrasonication for 8 min. Then the emulsion was added with 5% PVA with a volume ratio of 1:6 for another 8 min ultrasonication. The organic solvent was evaporated to obtain OVA@PLGA nanoparticles.
Female Sprague‒Dawley rats weighting between 150 and 200 g were used for the immunization studies. Prior to the experiment, rats were anesthetized by intraperitoneal injection of 10% chloral hydrate and animals were divided into 5 groups (9 rats in each group) to receive the following treatments: PBS, OVA@PLGA, OVA@PLGA/iRGD and plain OVA were administered intranasally, while intramuscular injection of OVA@PLGA (OVA@PLGA-im) was set as control. The dose of OVA was 50 μg/animal, and all animals were immunized on Day 0 and 14. On Days 7, 14, 28, and 42 after administration, blood of the rats was collected, and the concentration of OVA-specific IgG in the serum was measured by Rat OVA-IgG ELISA kit (ZCi Bio, Shanghai, China). Saliva and vaginal washes were also collected for determination of secretory IgA (sIgA) content by rat OVA-sIgA ELISA kit. Saliva and vaginal wash were sampled according to the method described in the literature45. At the end of the experiment, the rats were sacrificed and alveolar lavage fluid was collected for sIgA assay. Spleens were isolated and homogenized in tissue lysate reagents. The solution was centrifuged at 3000×g for 20 min, and the endogenous cytokines (IFN-γ and IL-2) in the supernatant were assayed using rat IFN-γ and IL-2 ELISA kits according to the manufacturer's instructions.
3. Results and discussion
3.1. Characterization of the inverted in vitro M-cell model
3.1.1. Evaluation of cell monolayer integrity
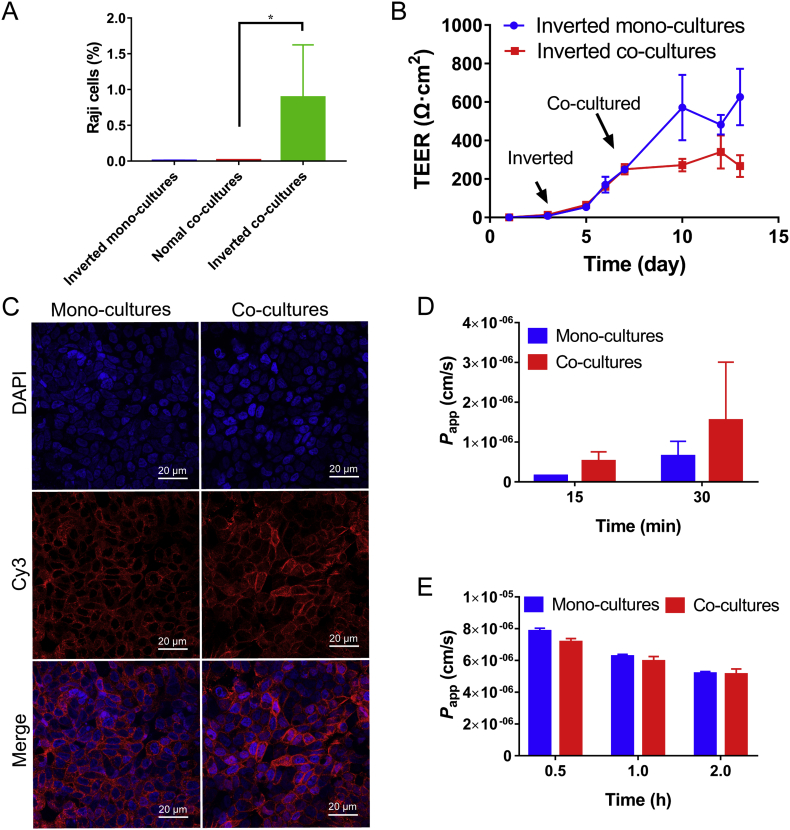
The inverted model was adopted to shorten the distance between the two kinds of cells, and make the Raji cells infiltrating into the compartment membrane and contacting with Calu-3 cells to better induce M cells. After co-culture, the number of Raji cells labeled by CFSE in the cell monolayer was detected by flow cytometry (Fig. 1A). In the normal orientated co-cultures, Raji cells on the cell layer were hardly detected. On the inverted co-cultures, approximately 0.89% of the fluorescent-labeled Raji cells were detected. It indicated that the inverted co-culture provided better contact conditions for M cell differentiation.
Figure 1.
(A) Raji cells in the monolayers were calculated by flow cytometry analysis (means ± SD, n = 3, *P < 0.05). (B) The change of TEER in the culture progress (mean ± SD, n = 3). (C) Confocal observation of ZO-1 in mono- and co-cultures. Cell nuclei were marked with DAPI (blue). Red fluorescence indicated the ZO-1 proteins. The Papp of sodium fluorescein (D) and dexamethasone (E) in mono- and co-cultures at different time points (mean ± SD, n = 3).
TEER was monitored during cell culture. The TEER of the cell monolayer began to grow after 3 days of culture, and the inverted mono-culture TEER reached equilibrium on the 10th day (Fig. 1B). After starting co-cultivation, the inverted co-culture TEER value no longer increased and kept in the range of 250–340 Ω·cm2. In general, TEER greater than 300 Ω·cm2 is considered to be suitable for transport experiments46. The relatively lower TEER than mono-cultures may be due to changes in the cell growth environment after co-culture, or a decrease in TEER value was observed when Calu-3 cells differentiated into M cells26. Moreover, the red fluorescence-labeled tight junction protein ZO-1 was wrapped around nucleus (blue, Fig. 1C), indicating the successful formation of tight junction in cell monolayer. Then the transportation of sodium fluorescein, a marker for the detection of paracellular leakage, was detected (Fig. 1D). The Papp of sodium fluorescein was higher for co-cultures than mono-cultures because the tight junction of co-cultures may not be as tight as the mono-cultures as former analysis. But the Papp values were both around the standard range of 2 × 10−7‒7 × 10−7cm/s in 15 min, indicating that both the co-cultures and the mono-cultures formed dense tight connection. In addition, the transportation of the trans-cellular marker dexamethasone was measured to detect the transportation capacity of the cell monolayer (Fig. 1E). As time elapsed from 0.5 to 2 h, the Papp of the co-cultures and the mono-cultures decreased, probably owing to the effect of the drug on cell viability. But within 2 h, the Papp was greater than 5 × 10−6cm/s, demonstrating that the cell layer kept transport ability. Through the above results, it can be concluded that the co-cultured and mono-cultured cell monolayer formed a tight junction and had good transportability, and can be used for subsequent nanoparticle transportation evaluation.
3.1.2. Verification of the differentiation of M cells
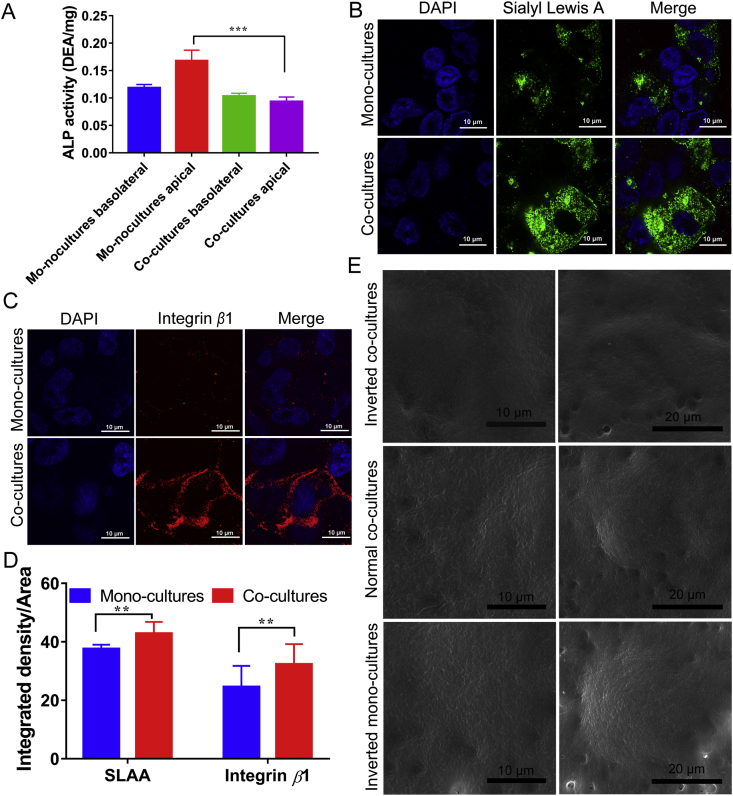
The powerful antigen capture ability of M cells plays an important role in the passage of antigens through the epithelial cell model. Differentiation of M cells is an indicator of successful establishment of the nasal epithelial model. The down regulation of alkaline phosphatase is a feature of M cell differentiation47,48. In the end of culture (Fig. 2A), the alkaline phosphatase activity in the co-culture medium showed a lower level compared to the control group both in the apical and basolateral side medium, indicating the presence of M cell differentiation in co-cultures. Other indicators of M cell differentiation are the up-regulation of the SLAA34,49 and integrin β1 expression. In the immunofluorescence observation of SLAA expression and integrin β1 (Fig. 2B and C), the fluorescence of SLAA and integrin β1 in the co-cultures was stronger than mono-cultures. The mean fluorescence density of SLAA and integrin β1 was counted (Fig. 2D). In co-culture group, the mean fluorescence density of SLAA was 1.14 times of that of monolayer, and the value of integrin β1 was 1.32 times of that of monolayer, indicating the differentiation of the M cells in the co-cultures. The above results were also proved by scanning electron microscope (SEM) analysis (Fig. 2E). The mono-cultures and the normal orientated co-cultures showed dense microvilli on the surface of the cells, while a decreased number of microvilli were observed in inverted co-cultures, indicating that inverted co-culture effectively induced differentiation of M cells.
Figure 2.
(A) The alkaline phosphatase activity in medium after culture (mean ± SD, n = 3, ***P < 0.001). Confocal observation of Sialyl Lewies A antigen (B) and integrin β1 (C) in mono- and co-cultures. The Sialyl Lewies A antigen was labeled with green fluorescence. The integrin β1 was labeled with red fluorescence. Cell nucleus were marked with DAPI (blue). (D) Statistics of mean fluorescence density of Sialyl Lewies A antigen and integrin β1 (mean ± SD, n = 3, **P < 0.01). (E) M cells were observed by SEM analysis.
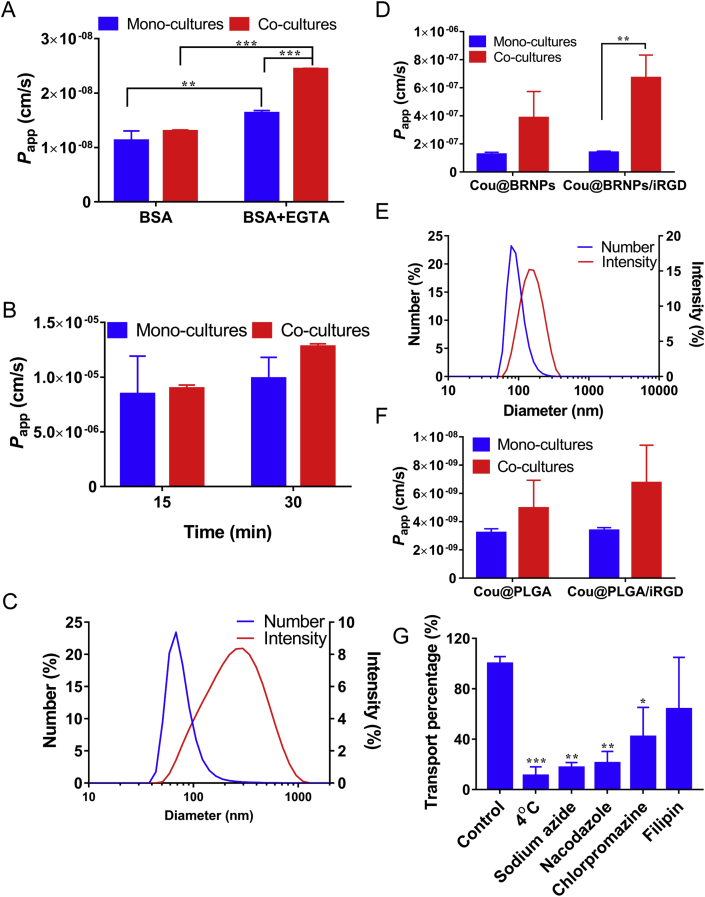
The outstanding feature of M cells is the efficient transport of macromolecules or nanoparticles. To analyze the role of M cells in nasal drug delivery, we evaluated the transport of different substances on the M cell model. Albumin was selected as a representative of macromolecular drug (Fig. 3A). The Papp of albumin was 1.30×10−8 ± 0.02 × 10−8 cm/s in the co-cultures and 1.12×10−8 ± 0.18 × 10−8 cm/s in mono-cultures. With the addition of 2.5 mmol/L EGTA, a calcium chelator to open tight junctions, the Papp of albumin in the co-cultures and mono-cultures were increased to 2.44 × 10−8 ± 0.01×10−8 and 1.63×10−8 ± 0.05 × 10−8 cm/s, respectively. The significant difference between the co-cultures and the mono-cultures may be due to the more loose tight junctions of co-cultures, or it may be due to the strong endocytosis caused by the differentiation of M cells. In a word, increased transport after EGTA addition confirmed the successful formation of tight junctions in cell layers. Then, we chose high-absorbed small molecule propranolol as a representative of trans-cellular marker (Fig. 3B). The Papp of co-cultures and mono-cultures at 15 and 30 min were all around 8 × 10−6 cm/s, which were faster than that of albumin. It is worth indicating that there was no significant difference between co-cultures and mono-cultures, proving that the induction of M cells is more likely to influence the transportation of macromolecules.
Figure 3.
(A)The Papp of BSA with or without EGTA in mono- and co-cultures at transported for 1 h (mean ± SD, n = 3–8, **P < 0.01, ***P < 0.001). (B)The Papp of propranolol in mono- and co-cultures at transported for 15 and 30 min (mean ± SD, n = 3). (C) Size distribution of cou@BRNPs by number and intensity. (D) The Papp of cou@BRNPs in mono- and co-cultures (mean ± SD, n = 3). (E) Size distribution of cou@PLGA by number and intensity. (F) The Papp of cou@PLGA in mono- and co-cultures (mean ± SD, n = 3). (G) The transport percentage calculated in the presence of inhibitors as compared with control (mean ± SD, n = 3, **P < 0.01 and ***P < 0.001 versus control group).
3.2. iRGD promotes the transportation of nanoparticles
3.2.1. Transportation of nanoparticles with iRGD in M-cell model
M cells have strong antigen-capture capabilities. Nanoparticles with M cells-targeting ability will greatly increase transportation efficiency. The size of the cou@BRNPs was 116.4 ± 37.0 nm, measured by the dynamic light scattering (DLS, Fig. 3C). In the transportation experiment, the transportation speed of the co-cultures was faster than that of the mono-cultures, while the Papp of co-cultures was three times higher than that of mono-cultures (Fig. 3D). After co-administration of iRGD, the transportation was increased, and the Papp with iRGD in co-cultures was 1.7 times as high as that without iRGD, indicating that the iRGD could enhance the transportation of nanoparticles. Subsequently, the same experiment was performed with cou@PLGA nanoparticles, whose particle size was 99.4 ± 10.2 nm (Fig. 3E). After giving the iRGD, the transportation of nanoparticles in co-cultures was increased to 1.3 times of that without iRGD as expected (Fig. 3F). The above results suggested that iRGD increased the transportation of nanoparticles.
In order to study the transportation mechanism of nanoparticles in M cell model, different kinds of inhibitors were incubated with the co-cultures during nanoparticle transport (Fig. 3G). Under the action of the energy inhibitor, sodium azide, the transportation percentage decreased to 17% of the control group, and transportation percentage at 4 °C decreased to 11% of the control, indicating that the transportation of nanoparticles was an energy-dependent progress. The transportation percentage of nacodazole group was only 21% of the control group, suggesting pinocytosis was involved in the transport progress. In addition, chlorpromazine inhibited about 59% nanoparticle transportation, indicating that the clathrin-mediated endocytosis was a transportation pathway of nanoparticles. In the filipin group, 36% nanoparticle transportation was inhibited, demonstrating the transport progress was also relevant to caveola-dependent endocytosis. These results suggested that the transportation of the nanoparticles was a complex process, involving energy expenditure, clathrin-mediated endocytosis, and pinocytosis, etc.
3.2.2. In vivo verification of iRGD promoting nanoparticle transportation through nasal mucosa
To evaluate the effect of iRGD in promoting M cells uptake in vivo, live imaging was performed. After intranasal administration of Cy5.5@PLGA nanoparticles with a particle size of 66.3 ± 7.1 nm (Fig. 4A), the nanoparticles were ingested and distributed into the brain (Fig. 4B), mainly concentrating in the olfactory bulb, and reaching peak at 4 h. Compared with the control group, the fluorescence intensity of the iRGD group in the olfactory bulb was stronger than that of the control group. In the semi-quantitative results (Fig. 4C), the fluorescence intensity of the iRGD group was 1.27 times higher than that of the control group at 4 h. Subsequently, the fluorescence distribution in frozen slices was observed (Fig. 4D) and the mean fluorescence density in different brain regions was counted (Fig. 4E), strong red fluorescence of nanoparticles was observed in the olfactory bulb, and the cerebral cortex was second to that of the olfactory bulb. In the striatum, hippocampus and other parts, the fluorescence was relatively weak. In addition, the fluorescence density of nanoparticles in olfactory bulb and cerebral cortex was higher than that in the control group after iRGD administration. It indicated that the iRGD could enhance the transportation of nanoparticles from the nasal cavity to the brain.
Figure 4.
(A) Size distribution of Cy5.5@PLGA by number and intensity. (B) The distribution of Cy5.5@PLGA in mice brain. The fluorescent images were observed at different time post nanoparticles administration by living imaging. (C) Semi-quantitative data of fluorescence intensity in isolated brains (means ± SD, n = 3). (D) Distribution of nanoparticles in different brain regions after 4 h administration. Cell nuclei were stained with DAPI (blue), and red signal indicated the nanoparticles. (E) Statistics of mean fluorescence density of nanoparticles in different brain regions (means ± SD, n = 3).
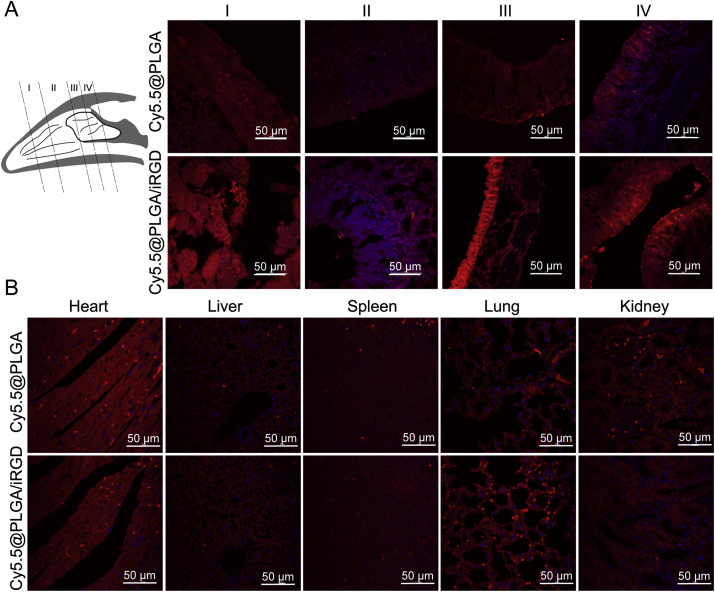
In addition, the nasal cavity of mice was divided into four parts to observe the distribution of nanoparticles in different depths of nasal mucosa (Fig. 5A). In the shallow parts (Ⅰ and Ⅱ), all nanoparticle fluorescence was weak. In the deep part of the nasal cavity (Ⅲ and IV), there was higher nanoparticles distribution of the iRGD group compared with the control group. It was suggested that the application of iRGD may promote the uptake of nanoparticles in the nasal mucosa. Moreover, weak distribution of nanoparticles in the organs (including heart, liver, spleen, lung and kidney) were observed (Fig. 5B). That meant the part of nanoparticles entered the blood circulation and distributed throughout the body.
Figure 5.
(A) Distribution of nanoparticles in different regions of nasal cavity according to the schematic. (B) Distribution of nanoparticles in frozen tissue sections observed by confocal microscopy. Red signal indicated the nanoparticles and the blue one was nucleus stained with DAPI.
3.3. iRGD enhancing in vivo immunity of intranasal administration of OVA@PLGA
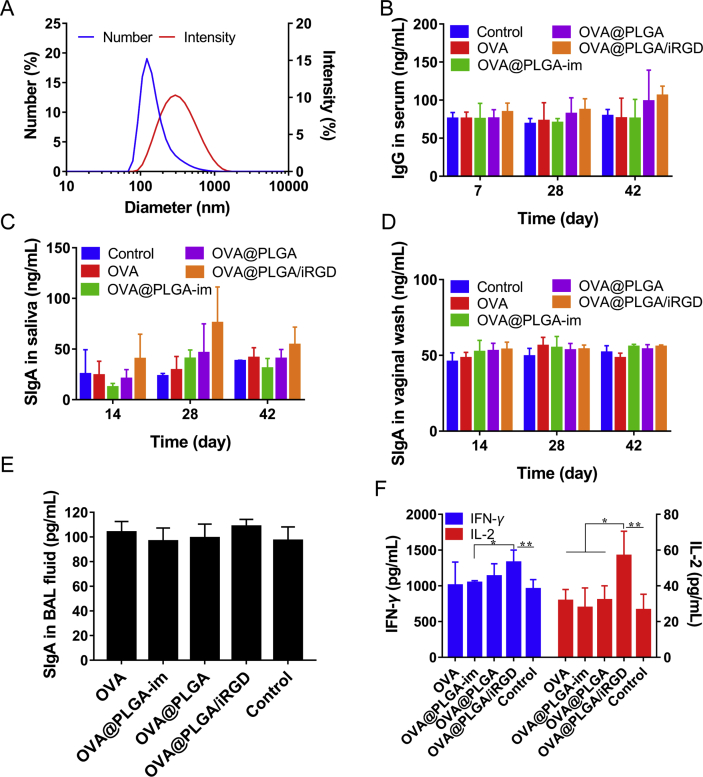
Nasal administration was a better mode of administration that stimulated mucosal immunity. In order to verify the enhanced effect of nasal mucosal immunity after iRGD co-administration, in vivo immune evaluation was carried out. OVA@PLGA nanoparticles were prepared in advance. The number size was 144.12 ± 12.54 nm, and the drug-loading capacity of the nanoparticles was determined to be 3.75% (Fig. 6A).
Figure 6.
(A) Size distribution of OVA@PLGA by number and intensity. The rats were immunized on Days 0 and 14. (B) Determination of OVA specific IgG in serum at Days 7, 28 and 42 after administration of different preparations. Mucosal immune response was evaluated after 2 doses of formulation on Days 0 and 14. Secretory IgA (sIgA) levels were measured in (C) salivary secretion and (D) vaginal wash. (E) In addition, sIgA of bronchoalveolar lavage (BAL) fluid at the end of immunization was determined. The levels of (F) IFN-γ and IL-2 in spleen homogenates of rats at Day 42 after the first dose of the formulations (mean ± SD, n = 3–8, *P < 0.05, **P < 0.01).
To investigate the immune effect of various formulations in vivo, serum collected from rats was used to detect the content of IgG. As shown in Fig. 6B, there was no obvious change in IgG concentration at Days 7 and 14. At Day 42, the level of IgG in plain OVA nasal administration group and OVA@PLGA-im group did not increase significantly compared with the control group. While in the OVA@PLGA/iRGD group, the average IgG concentration was 1.34 times higher than that of the control group.
After nasal immunization with OVA@PLGA, B cells in nasal associated lymphoid tissue were activated and emigrated to bloodstream and home to the region of the respiratory and reproductive tracts. Then, secretory IgA (sIgA) + B cells terminally differentiate into IgA plasma cells for the generation of sIgA50. As shown in Fig. 6C, the sIgA concentration of each group peaked at the 28th day. Compared with the control group, plain OVA and OVA@PLGA-im produced relatively weak immune response. The nasal administration of OVA@PLGA slightly increased. The highest concentration was provided by OVA@PLGA/iRGD group at different time points. The level of sIgA in OVA@PLGA/iRGD group was 1.94 times higher than OVA@PLGA group at Day 14, 1.64 times higher than OVA@PLGA group on Day 28, and 1.34 times higher than OVA@PLGA group on Day 42. At the same time, the sIgA level in the vaginal was measured (Fig. 6D), and the difference between each group and the control group was not obvious, while the iRGD group showed a weak advantage. The weak mucus immune response at distal organs may be due to the low immunogenicity of antigens. It needs the incorporation of immune adjuvant to get better effect in practical application. The same situation occurred in determination of sIgA levels in the bronchoalveolar lavage (Fig. 6E).
The mucosal immune response is divided into Th1 and Th2 types driven by different antigen types or adjuvants. The production of antibody is mainly mediated by Th2 immune response, and Th1 immune response mediates cellular immunity with production of IFN-γ and IL-2. After 42 days, the concentration of cytokines including IFN-γ and IL-2 in spleen homogenate was measured (Fig. 6F). In the measurement of IFN-γ concentration, there was not much difference between control group and plain OVA or OVA@PLGA-im group. In OVA@PLGA group, the concentration was 1.18-fold higher than the control group. In addition, the concentration of OVA@PLGA/iRGD group increased further to 1.34 times of the control group. In the detection of IL-2, more obvious outcome displayed. The level of IL-2 in OVA@PLGA/iRGD was greatly improved to 1.77 times of the OVA@PLGA group. Taken together, the intranasal administration of OVA@PLGA could induce the immune response. Th2 and Th1 type immune responses were further enhanced after the combination of iRGD, which showed the increase of sIgA and IgG secretion, and the increase of IL-2 and IFN-γ secretion. It indicated that the uptake of nanoparticles increased after targeting M cells, which resulted in more effective immune response.
4. Conclusions
In this study, we co-cultured Calu-3 cells and Raji cells to construct an in vitro M-cell model that can be used to simulate the nasal cavity. Through co-culture of Calu-3 and Raji cells, a tightly connected monolayer was formed and M cells were induced to differentiate. The differentiation of M cells increased the nanoparticle transportation efficiency of membrane. At the same time, the transport of nanoparticles was further enhanced after targeting M cells by iRGD. In addition, the systemic immune effect was enhanced, and a more advantageous mucus immunity was produced in vivo. The study provides a more nasal-like alternative to current M-cell model in vitro, and some insights into how to enhance the therapeutic effect of nasal administration by targeting M cells.
Acknowledgments
The work was supported by National Natural Science Foundation of China (81603057), Research Funds of Sichuan Science and Technology Department (2019YJ0048 and 19YYJC2250, China), the Fundamental Research Funds for the Central Universities (China), and 111 Project (B18035, China).
Author contributions
Xiaotong Yang, Xianchun Chen, Ting Lei, Lin Qin, Chuan Hu, Yang Zhou were mainly responsible for experimental design, data acquisition and analysis, and paper writing. Huile Gao and Qingfeng Liu put forward the hypothesis, supervised and guided experiments, reviewed and approved the manuscript for publication.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Qingfeng Liu, Email: 155135562@qq.com.
Huile Gao, Email: gaohuile@scu.edu.cn.
References
- 1.Türker S., Onur E., Ózer Y. Nasal route and drug delivery systems. Pharm World Sci. 2004;26:137–142. doi: 10.1023/b:phar.0000026823.82950.ff. [DOI] [PubMed] [Google Scholar]
- 2.Khan A.R., Liu M., Khan M.W., Zhai G. Progress in brain targeting drug delivery system by nasal route. J Contr Release. 2017;268:364–389. doi: 10.1016/j.jconrel.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6:268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C., Wang J., Wang Y., Gao H., Wei G., Huang Y. Recent progress in drug delivery. Acta Pharm Sin B. 2019;9:1145–1162. doi: 10.1016/j.apsb.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou X., Hao Y., Yuan L., Pradhan S., Shrestha K., Pradhan O. Nano-formulations for transdermal drug delivery: a review. Chin Chem Lett. 2018;29:1713–1724. [Google Scholar]
- 6.Park J., Seo K.W., Kim S.H., Lee H.Y., Kim B., Lim C.W. Nasal immunization with M cell-targeting ligand-conjugated ApxIIA toxin fragment induces protective immunity against Actinobacillus pleuropneumoniae infection in a murine model. Vet Microbiol. 2015;177:142–153. doi: 10.1016/j.vetmic.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 8.Wang J., Wang Y., Zhang E., Zhou M., Lin J., Yang Q. Intranasal administration with recombinant Bacillus subtilis induces strong mucosal immune responses against pseudorabies. Microb Cell Factories. 2019;18:103. doi: 10.1186/s12934-019-1151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiyono H., Fukuyama S. NALT-versus PEYER'S-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernocchi B., Carpentier R., Betbeder D. Nasal nanovaccines. Int J Pharm. 2017;530:128–138. doi: 10.1016/j.ijpharm.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Hagenaars N., Mastrobattista E., Verheul R.J., Mooren I., Glansbeek H.L., Heldens J.G. Physicochemical and immunological characterization of N,N,N-trimethyl chitosan-coated whole inactivated influenza virus vaccine for intranasal administration. Pharm Res (N Y) 2009;26:1353–1364. doi: 10.1007/s11095-009-9845-y. [DOI] [PubMed] [Google Scholar]
- 12.Ichinohe T., Tamura S.I., Kawaguchi A., Ninomiya A., Imai M., Itamura S. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J Infect Dis. 2007;196:1313–1320. doi: 10.1086/521304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan X., Su Q., Qiu F., Yi Y., Shen L., Jia Z. Intranasal inoculate of influenza virus vaccine against lethal virus challenge. Vaccine. 2018;36:4354–4361. doi: 10.1016/j.vaccine.2018.05.075. [DOI] [PubMed] [Google Scholar]
- 14.Dimova S., Brewster M.E., Noppe M., Jorissen M., Augustijns P. The use of human nasal in vitro cell systems during drug discovery and development. Toxicol Vitro. 2005;19:107–122. doi: 10.1016/j.tiv.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Gonçalves V.S., Matias A.A., Poejo J., Serra A.T., Duarte C.M. Application of RPMI 2650 as a cell model to evaluate solid formulations for intranasal delivery of drugs. Int J Pharm. 2016;515:1–10. doi: 10.1016/j.ijpharm.2016.09.086. [DOI] [PubMed] [Google Scholar]
- 16.Kreft M.E., Jerman U.D., Lasič E., Rižner T.L., Hevir-Kene N., Peternel L. The characterization of the human nasal epithelial cell line RPMI 2650 under different culture conditions and their optimization for an appropriate in vitro nasal model. Pharm Res (N Y) 2015;32:665–679. doi: 10.1007/s11095-014-1494-0. [DOI] [PubMed] [Google Scholar]
- 17.Lin S.F., Jiang P.L., Tsai J.S., Huang Y.Y., Lin S.Y., Lin J.H. Surface assembly of poly(I:C) on polyethyleneimine-modified gelatin nanoparticles as immunostimulatory carriers for mucosal antigen delivery. J Biomed Mater Res B Appl Biomater. 2019;107:1228–1237. doi: 10.1002/jbm.b.34215. [DOI] [PubMed] [Google Scholar]
- 18.Lamichhane A., Azegami T., Kiyono H. The mucosal immune system for vaccine development. Vaccine. 2014;32:6711–6723. doi: 10.1016/j.vaccine.2014.08.089. [DOI] [PubMed] [Google Scholar]
- 19.Fujimura Y. Evidence of M cells as portals of entry for antigens in the nasopharyngeal lymphoid tissue of humans. Virchows Arch. 2000;436:560–566. doi: 10.1007/s004289900177. [DOI] [PubMed] [Google Scholar]
- 20.Mabbott N.A., Donaldson D.S., Ohno H., Williams I.R., Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Gusti V., Saraswati A., Lo D.D. Convergent and divergent development among M cell lineages in mouse mucosal epithelium. J Immunol. 2011;187:5277–5285. doi: 10.4049/jimmunol.1102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.H., Seo K.W., Kim J., Lee K.Y., Jang Y.S. The M cell-targeting ligand promotes antigen delivery and induces antigen-specific immune responses in mucosal vaccination. J Immunol. 2010;185:5787–5795. doi: 10.4049/jimmunol.0903184. [DOI] [PubMed] [Google Scholar]
- 23.Des Rieux A., Ragnarsson E.G., Gullberg E., Préat V., Schneider Y.J., Artursson P. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur J Pharmaceut Sci. 2005;25:455–465. doi: 10.1016/j.ejps.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Chaikhumwang P., Nilubol D., Tantituvanont A., Chanvorachote P. A new cell-to-cell interaction model for epithelial microfold cell formation and the enhancing effect of epidermal growth factor. Eur J Pharmaceut Sci. 2017;106:49–61. doi: 10.1016/j.ejps.2017.05.054. [DOI] [PubMed] [Google Scholar]
- 25.Zhuang J., Wang D., Li D., Yang Y., Lu Y., Wu W. The influence of nanoparticle shape on bilateral exocytosis from Caco-2 cells. Chin Chem Lett. 2018;29:1815–1818. [Google Scholar]
- 26.Des Rieux A., Fievez V., Théate I., Mast J., Préat V., Schneider Y.J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur J Pharmaceut Sci. 2007;30:380–391. doi: 10.1016/j.ejps.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Beloqui A., Brayden D.J., Artursson P., Préat V., Des Rieux A. A human intestinal M-cell-like model for investigating particle, antigen and microorganism translocation. Nat Protoc. 2017;12:1387–1399. doi: 10.1038/nprot.2017.041. [DOI] [PubMed] [Google Scholar]
- 28.Grainger C.I., Greenwell L.L., Lockley D.J., Martin G.P., Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm Res (N Y) 2006;23:1482–1490. doi: 10.1007/s11095-006-0255-0. [DOI] [PubMed] [Google Scholar]
- 29.Dong W., Ye J., Zhou J., Wang W., Wang H., Zheng X. Comparative study of mucoadhesive and mucus-penetrative nanoparticles based on phospholipid complex to overcome the mucus barrier for inhaled delivery of baicalein. Acta Pharm Sin B. 2019 doi: 10.1016/j.apsb.2019.10.002. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George I., Vranic S., Boland S., Courtois A., Baeza-Squiban A. Development of an in vitro model of human bronchial epithelial barrier to study nanoparticle translocation. Toxicol Vitro. 2015;29:51–58. doi: 10.1016/j.tiv.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Stentebjerg-Andersen A., Notlevsen I.V., Brodin B., Nielsen C.U. Calu-3 cells grown under AIC and LCC conditions: implications for dipeptide uptake and transepithelial transport of substances. Eur J Pharm Biopharm. 2011;78:19–26. doi: 10.1016/j.ejpb.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 32.Gupta P.N., Khatri K., Goyal A.K., Mishra N., Vyas S.P. M-cell targeted biodegradable PLGA nanoparticles for oral immunization against hepatitis B. J Drug Target. 2007;15:701–713. doi: 10.1080/10611860701637982. [DOI] [PubMed] [Google Scholar]
- 33.Malik B., Goyal A.K., Markandeywar T.S., Rath G., Zakir F., Vyas S.P. Microfold-cell targeted surface engineered polymeric nanoparticles for oral immunization. J Drug Target. 2012;20:76–84. doi: 10.3109/1061186X.2011.611516. [DOI] [PubMed] [Google Scholar]
- 34.Gullberg E., Leonard M., Karlsson J., Hopkins A.M., Brayden D., Baird A.W. Expression of specific markers and particle transport in a new human intestinal M-cell model. Biochem Biophys Res Commun. 2000;279:808–813. doi: 10.1006/bbrc.2000.4038. [DOI] [PubMed] [Google Scholar]
- 35.Gullberg E., Keita Å.V., Salim S.Y., Andersson M., Caldwell K.D., Söderholm J.D. Identification of cell adhesion molecules in the human follicle-associated epithelium that improve nanoparticle uptake into the Peyer's patches. J Pharmacol Exp Therapeut. 2006;319:632–639. doi: 10.1124/jpet.106.107847. [DOI] [PubMed] [Google Scholar]
- 36.Fievez V., Plapied L., Des Rieux A., Pourcelle V., Freichels H., Wascotte V. Targeting nanoparticles to M cells with non-peptidic ligands for oral vaccination. Eur J Pharm Biopharm. 2009;73:16–24. doi: 10.1016/j.ejpb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Yoo M.K., Kang S.K., Choi J.H., Park I.K., Na H.A., Lee H.C. Targeted delivery of chitosan nanoparticles to Peyer's patch using M cell-homing peptide selected by phage display technique. Biomaterials. 2010;31:7738–7747. doi: 10.1016/j.biomaterials.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 38.Roth-Walter F., Schöll I., Untersmayr E., Ellinger A., Boltz-Nitulescu G., Scheiner O. Mucosal targeting of allergen-loaded microspheres by Aleuria aurantia lectin. Vaccine. 2005;23:2703–2710. doi: 10.1016/j.vaccine.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 39.Garinot M., Fiévez V., Pourcelle V., Stoffelbach F., Des Rieux A., Plapied L. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J Contr Release. 2007;120:195–204. doi: 10.1016/j.jconrel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Su H., Wang Y., Liu S., Wang Y., Liu Q., Liu G. Emerging transporter-targeted nanoparticulate drug delivery systems. Acta Pharm Sin B. 2019;9:49–58. doi: 10.1016/j.apsb.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu C., Yang X., Liu R., Ruan S., Zhou Y., Xiao W. Coadministration of iRGD with multistage responsive nanoparticles enhanced tumor targeting and penetration abilities for breast cancer therapy. ACS Appl Mater Interfaces. 2018;10:22571–22579. doi: 10.1021/acsami.8b04847. [DOI] [PubMed] [Google Scholar]
- 42.Cun X., Chen J., Ruan S., Zhang L., Wan J., He Q. A novel strategy through combining iRGD peptide with tumor-microenvironment-responsive and multistage nanoparticles for deep tumor penetration. ACS Appl Mater Interfaces. 2015;7:27458–27466. doi: 10.1021/acsami.5b09391. [DOI] [PubMed] [Google Scholar]
- 43.Secott T.E., Lin T.L., Wu C.C. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein facilitates M-cell targeting and invasion through a fibronectin bridge with host integrins. Infect Immun. 2004;72:3724–3732. doi: 10.1128/IAI.72.7.3724-3732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X., Hu C., Tong F., Liu R., Zhou Y., Qin L. Tumor microenvironment-responsive dual drug dimer-loaded PEGylated bilirubin nanoparticles for improved drug delivery and enhanced immune-chemotherapy of breast cancer. Adv Funct Mater. 2019;29:1901896. [Google Scholar]
- 45.Thomas C., Rawat A., Hope-Weeks L., Ahsan F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol Pharm. 2011;8:405–415. doi: 10.1021/mp100255c. [DOI] [PubMed] [Google Scholar]
- 46.Ramesh Babu P.B., Chidekel A., Utidjian L., Shaffer T.H. Regulation of apical surface fluid and protein secretion in human airway epithelial cell line Calu-3. Biochem Biophys Res Commun. 2004;319:1132–1137. doi: 10.1016/j.bbrc.2004.05.101. [DOI] [PubMed] [Google Scholar]
- 47.Lügering A., Floer M., Lügering N., Cichon C., Schmidt M.A., Domschke W. Characterization of M cell formation and associated mononuclear cells during indomethacin-induced intestinal inflammation. Clin Exp Immunol. 2004;136:232–238. doi: 10.1111/j.1365-2249.2004.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tyrer P., Ruth Foxwell F.A., Kyd J., Harvey M., Sizer P., Cripps A. Validation and quantitation of an in vitro M-cell model. Biophys Res Commun. 2002;299:377–383. doi: 10.1016/s0006-291x(02)02631-1. [DOI] [PubMed] [Google Scholar]
- 49.Brayden D.J., Jepson M.A., Baird A.W. Keynote review: intestinal Peyer's patch M cells and oral vaccine targeting. Drug Discov Today. 2005;10:1145–1157. doi: 10.1016/S1359-6446(05)03536-1. [DOI] [PubMed] [Google Scholar]
- 50.Yuki Y., Kiyono H. New generation of mucosal adjuvants for the induction of protective immunity. Rev Med Virol. 2003;13:293–310. doi: 10.1002/rmv.398. [DOI] [PubMed] [Google Scholar]