Abstract
Dysregulation of mTORC1/mTORC2 pathway is observed in many cancers and mTORC1 inhibitors have been used clinically in many tumor types; however, the mechanism of mTORC2 in tumorigenesis is still obscure. Here, we mainly explored the potential role of mTORC2 in esophageal squamous cell carcinoma (ESCC) and its effects on the sensitivity of cells to mTOR inhibitors. We demonstrated that RICTOR, the key factor of mTORC2, and p-AKT (Ser473) were excessively activated in ESCC and their overexpression is related to lymph node metastasis and the tumor-node-metastasis (TNM) phase of ESCC patients. Furthermore, we found that mTORC1/ mTORC2 inhibitor PP242 exhibited more efficacious anti-proliferative effect on ESCC cells than mTORC1 inhibitor RAD001 due to RAD001-triggered feedback activation of AKT signal. Another, we demonstrated that down-regulating expression of RICTOR in ECa109 and EC9706 cells inhibited proliferation and migration as well as induced cell cycle arrest and apoptosis. Noteworthy, knocking-down stably RICTOR significantly suppresses RAD001-induced feedback activation of AKT/PRAS40 signaling, and enhances inhibition efficacy of PP242 on the phosphorylation of AKT and PRAS40, thus potentiates the antitumor effect of RAD001 and PP242 both in vitro and in vivo. Our findings highlight that selective targeting mTORC2 could be a promising therapeutic strategy for future treatment of ESCC.
Key words: RICTOR, AKT, RAD001, pp242, Esophageal squamous cell carcinoma
Abbreviations: AKT, protein kinase B (PKB); ESCC, esophageal squamous cell carcinoma; 4EBP-1, E binding protein-1; FDA, U.S. Food and Drug Administration; H&E staining, hematoxylin and eosin staining; IC50, half maximal inhibitory concentration; mTOR, mammalian target of rapamycin; mTORC1, mTOR complex 1; mTORC2, mTOR complex 2; PI3K, phosphatidylinositol 3 kinase; p70S6K, p70 ribosomal S6 kinase-1; rapalogs, rapamycin and its analogs; RICTOR, rapamycin-insensitive companion of mTOR; TNM, tumor-node-metastasis; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling
Graphical abstract
This study demonstrated that mTORC2 subunit, RICTOR, and p-AKT (Ser473) have hyperactivity in esophageal squamous cell carcinoma (ESCC) and promotes its development. Moreover, down-regulation of RICTOR can improve the sensitivity of ESCC cells to the pan-mTOR inhibitor PP242 as well as mTORC1 inhibitor RAD001.
1. Introduction
Esophageal cancer (EC) is one of the most common malignancies worldwide, which can be classified into adenocarcinoma (EAC) and squamous cell carcinoma (ESCC) according to its histological and pathological characteristics1,2, and ESCC is the most prevalent in the developing countries3. Although the traditional therapy measures like surgery, radiotherapy and chemotherapy have achieved prominent progress in ESCC treatment, the therapeutic effects are not ideal and the 5-year survival rate of patients with ESCC is only 12%–20%2,4. Thus, exploring the molecular mechanism of tumorigenesis and searching novel targeted therapy methods for ESCC should be significantly important.
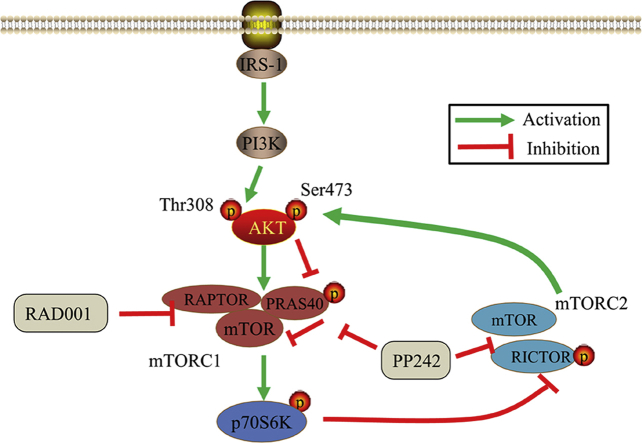
Mammalian target of rapamycin (mTOR), as a serine/threonine kinase, was an essential factor in many pathways associated with growth factor and nutrition. mTOR can regulate various cellular processes, including proliferation, survival, apoptosis, metabolism and autophagy5, 6, 7. Activity of mTOR kinase is associated with sets of different proteins, which is involved in two functionally and structurally distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1, mainly containing mTOR, regulatory-associated protein of mTOR (raptor) and mammalian lethal with Sec13 protein 8 (mLST8), controls protein synthesis as well as cell growth by phosphorylating the p70 ribosomal S6 kinase-1 (p70S6K) and 4E binding protein-1 (4EBP-1) in response to the availability of nutrients and growth factors8. Distinct from mTORC1, mTORC2, formed by mTOR, rapamycin-insensitive companion (RICTOR), mLST8, mammalian stress-activated protein kinase-interacting 1 (mSIN1), and protein observed with RICTOR 1/2 (Protor1/2), is considered to regulate the actin cytoskeleton network through phosphorylating the protein kinase B (AKT), protein kinase C (PKC) and serum/glucocorticoid-activated kinase 1 (SGK1)9, 10, 11, 12.
Rapamycin was firstly identified as a specific allosteric inhibitor of mTORC1 via binding with FKBP12/rapamycin-binding (FRB) domain. Some analogs of rapamycin (rapalogs) like everolimus (RAD001) have been approved by U.S. Food and Drug Administration (FDA) for the treatment of various tumor types5,13, 14, 15. However, these rapalogs are insufficient for achieving a promising curative effect in clinical application because they are mainly cytostatic with poor proapoptotic activity, and they could reactivate AKT signaling through some negative feedback loops by selectively inhibiting mTORC15,16, 17, 18. Compared with rapalogs, mTORC1/mTORC2-selective inhibitors late-discovered to display more powerful anti-proliferative and pro-apoptotic effects because they only block the catalytic domain of mTOR and suppress both mTORC1 and mTORC2 kinase activity, and thus completely inhibit the output of mTOR19, 20, 21. And PP242 is the prototype inhibitor of this class22, the antitumor effects of which were demonstrated in ESCC and acute myeloid leukemia (AML) cells by suppressing mTORC1/2 activity23,24. Additionally, numerous researchers have concentrated in mTORC1, but function of mTORC2 is still not well understood. It has been demonstrated that RICTOR, as a critical player for mTORC2 kinase activity, harbors important function in the development of some cancer types25, 26, 27, 28, 29, 30, but there are little reports about RICTOR in ESCC. Although a recent study has been demonstrated RICTOR was overexpressed and associated with the poor prognosis in ESCC31, the potential role of RICTOR/mTORC2 remains obscure in ESCC.
In the present study, to explore potential function of RICTOR/mTORC2 in ESCC, expression and the clinicopathological significance of RICTOR were analyzed in tissues of ESCC patients. Moreover, the effects of RICTOR-knockdown (RICTOR-KD) on cell proliferation, cell cycle, cell migration, cell apoptosis and tumor growth were investigated both in ESCC cells and xenografts. Most importantly, we demonstrated whether or not inhibition of RICTOR/mTORC2 activity can enhance the sensitivity of ESCC cells to RAD001 and PP242 as well as potential molecular mechanisms.
2. Materials and methods
2.1. Chemicals and antibodies
RAD001 and PP242 were purchased from MedChem Express (Monmouth Junction, NJ, USA). Primary monoclonal antibodies recognizing RICTOR (#9476s), phospho-AKT (Ser473, #4064s), AKT (#2920s), p70S6 kinase (#2708), phospho-PRAS40 (Thr246, #13175), PRAS40 (#2691) and GAPDH (#5174) as well as corresponding secondary antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). Primary monoclonal antibodies of phospho-p70S6 kinase (Thr389) were obtained from Cell Signaling Technology (#9206s) or Santa Cruz (sc-377529, Dallas, TX, USA).
2.2. Cell lines and transfections
Human poor differentiated ESCC cell line EC9706 and TE-1, well differentiated KYSE450, KYSE790 and ECa109 were obtained from Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured as described previously32,33. Human normal esophageal Het-1A cells were obtained from American Type Culture Collection. ECa109 and EC9706 cells, respectively, were transfected with RICTOR-shRNA or Control-shRNA vector (TransOMIC Technologies, Huntsville, AL, USA or GenePharma, Shanghai, China) using Lipofectamine™ 3000 (Invitrogen, Carlsbad, CA, USA) and screened as described before34. The sequence of RICTOR-shRNA and Control-shRNA was GCG AGC TGA TGT AGA ATT AGA and GTT CTC CGA ACG TGT CAC GT, respectively. Cells screened were named RICTOR-KD cells (with RICTOR-shRNA) and control cells (with Control-shRNA).
2.3. Immunohistochemistry
Study about human subjects was performed according to the Code of Ethics of the World Medical Association. Paraffin-embedded ESCC and normal esophageal tissues slides were from 150 ESCC patients (98 male and 52 female with the mean age of 62.3 ± 8.7 years old), who did not suffer chemotherapy or radiotherapy before surgical resection. The informed consent forms for all patients were provided by the Pathology Department of the First Affiliated Hospital of Zhengzhou University, and the use of the samples was permitted by Human Ethic Committee of the First Affiliated Hospital, Zhengzhou University (Zhengzhou, China). Immunohistochemistry (IHC) was performed with antibodies against RICTOR and p-AKT (Ser473) as described before32. The stained sections were evaluated by two pathologists in a blinded manner35. The evaluation criterion is staining intensity (I) (negative: 0; weak: 1; moderate: 2; and strong: 3), positive cells distribution (D) (<10%: 0; 10%–50%: 1; 51%–90%: 2; and >90%: 3) and staining pattern (P) (no staining: 0; sporadic positive: 1; focal positive: 2; and diffuse positive: 3). The total score of every slide was calculated as: I × D × P. Then the total score value = 0 was considered as negative, while the total score value ≥ 1 was considered as positive. Chi-squared (χ2) or Fisher's exact tests were used to assess and represent separated clinicopathologic parameters, association of RICTOR and p-AKT (Ser473) expression level was clarified using the Spearman's rank correlation coefficient.
2.4. Cell proliferation
Cell proliferation was measured by CCK-8 (Beyotime Biotechnology, Shanghai, China), as described before36. Briefly, cells incubated in 96-well plates were treated with RAD001 or PP242, respectively. The absorbance was measured by a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA) at 450 nm after addition of CCK-8 reagent to each well. The IC50 values of RAD001 or PP242 were calculated by non-linear regression analysis by SPSS19.0 software (NIH, Bethesda, MA, USA).
2.5. Colony formation
Colony formation was analyzed as described before36. Briefly, RICTOR-KD and control cells were incubated into 6-well plates, 1000 cells per well. After cultured in medium containing 20 μmol/L of RAD001 or 4 μmol/L of PP242 for 10 days, the clones were fixed using cold methanol, then stained using crystal violet (0.1%). Finally, the number of clones was counted by ImageJ software (NIH, Bethesda, MA, USA).
2.6. Transwell migration
Cell migration was assayed by Transwell chambers (Corning, NY, USA) experiment. In brief, 200 μL (1.5 × 105 cells) of RICTOR-KD or control cell suspension containing 10 μmol/L of RAD001 or 2 μmol/L of PP242 was added into the upper chamber, and culture medium containing 15% FBS was put into basement chamber for 48 h, non-migratory cells in upper chamber were wiped with cotton swabs, and then migratory cells were fixed in cold methanol, then stained with crystal violet (0.1%). Finally, cells were photographed with microscope and the migratory cells in three random fields of each sample were calculated.
2.7. Cell cycle assay
Cell cycle phase was analyzed by the Cell Cycle Analysis Kit (Beyotime Biotechnology). In brief, after cells were inoculated in 6-well plates (6 × 105 cells per well) and treated using RAD001 (10 μmol/L) or PP242 (2 μmol/L) for 48 h, cells were gathered and fixed with 70% iced ethanol and incubated at 4 °C. Twenty-four hours later, cells were washed with phosphate-buffered saline (PBS) for two times and hatched in the dark with 50 μg/mL of propidium iodide and 50 μg/mL of RNase A for 30 min at 37 °C. Cells (1 × 104) were gathered and cell cycle phase was analyzed using a flow cytometer (BD Accuri™ C6, Piscataway, NJ, USA).
2.8. Cell apoptosis
Cell apoptosis was detected by Annexin V-FITC/PI Apoptosis Detection Kit (Roche, USA) as described before36. Briefly, cells treated with RAD001 (20 μmol/L) or PP242 (4 μmol/L) for 48 h were collected and washed with iced PBS. After incubated with annexin V-FITC staining solution, PI solution was put into cell suspension and cell apoptosis was detected using a flow cytometer (BD Accuri™ C6).
2.9. Tumor xenograft experiments
All the animal studies complied with the ARRIVE guidelines and all animal procedures were permitted by the Animal Ethics Committee, Zhengzhou University. Thirty athymic BALB/c nude mice (male, 4–6 weeks) were obtained from Human Silikejingda Experimental Animal Ltd. (Changsha, China). The housing conditions of animals were described as before36. ECa109 RICTOR-KD or control cells were collected, washed, and resuspended with PBS, 200 μL of cell suspension (4 × 106 cells) was injected subcutaneously into mouse right flank. When the volume of tumor reached 60–80 mm3, the mice were administered RAD001 intragastrically (3 mg/kg) or injected PP242 intraperitoneally (5 mg/kg) every other day for 14 days. Tumors were measured per day and the volume was counted according to Eq. (1)37:
| (1) |
2.10. In situ TUNEL assay and H&E staining
Tumor tissues from nude mice fixed with 4% paraformaldehyde buffer were embedded with paraffin, and produced 4 μm tissue slides for H&E staining36. Meanwhile, in vivo cell apoptosis in the tissue sections was explored using in situ Cell Death Detection Kit (Roche, Oceanside, CA, USA) as described before32,38.
2.11. Western blot
Western blot assay was processed according to the previous description32,38. Briefly, equivalent amounts of proteins (30 μg) extracted from ESCC cells or tumor tissues were separated with 10% SDS-PAGE, then electro-transferred onto a 0.22 μm nitrocellulose membrane. After blocked with 5% skimmed milk for 2 h, the membrane were hatched with indicated primary antibodies (1:1000) at 4 °C overnight, followed by being incubated with HRP-linked secondary antibodies (1:8000) for 2 h. The protein band was investigated with enhanced chemiluminescence (ECL) reagent (Thermo Fisher Scientific, Waltham, MA, USA) and quantitative analyzed by ImageJ software.
2.12. Statistical analysis
The experimental in vitro and Western blot results obtained from no less than three repeated independently experiments were analyzed by independent sample t test or one-way analysis of variance (ANOVA) using SPSS19.0 software (Rhode Island, RI, USA). Data are shown as mean ± SD, and the value of P < 0.05 or less is considered statistically significant.
3. Results
3.1. mTORC2/AKT signaling was excessively activated in ESCC tissues and cells
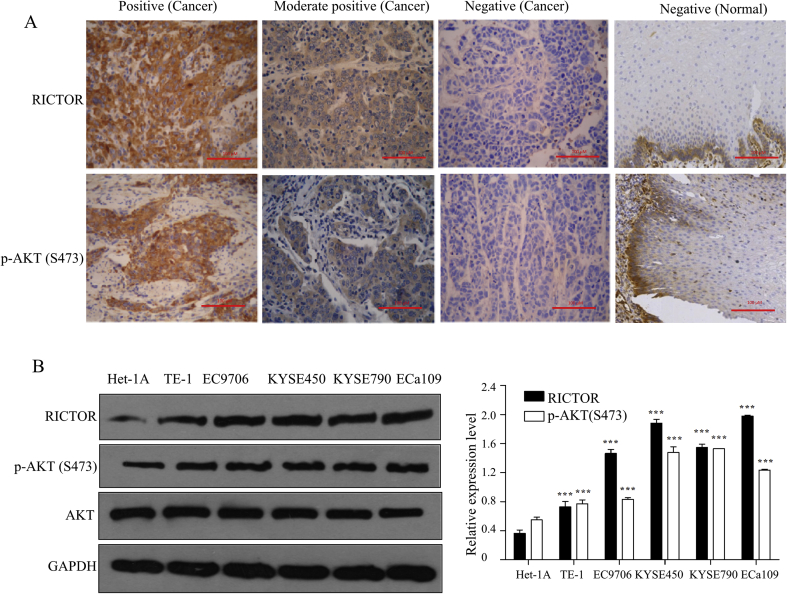
To explore the activated state of mTORC2/AKT in ESCC and its clinical significance, the expressions of RICTOR and p-AKT (Ser473) in ESCC and normal esophageal tissues were examined by immunohistochemistry. As shown in Fig. 1A, both RICTOR and p-AKT (Ser473) displayed cytoplasmic staining (brown-stained particles) in ESCC tissues. Among the 150 ESCC tissues, 92 tissues (61.3%) showed RICTOR-positive staining and 98 tissues (65.3%) showed p-AKT-positive staining. In contrast, among the 150 normal esophageal tissues, 27 tissues (18.0%) showed RICTOR-positive staining and 45 tissues (30.0%) showed p-AKT-positive staining (Table 1). And there are significant statistical differences between the positive expression rates in tumor tissues and normal esophageal tissues (P < 0.01; Table 1), suggesting that mTORC2 and p-AKT (Ser473) are more frequently activated in ESCC tissues than in normal esophageal tissues.
Figure 1.
mTORC2/AKT signaling was activated in ESCC tissues and cells. (A) The expression of RICTOR and p-AKT (Ser473) in 150 ESCC tissues and normal esophageal tissues were detected by immunohistochemistry, and the representative photographs were shown (400 × , scale bar = 100 μm). (B) Total proteins of ESCC cell lines TE-1, EC9706, KYSE450, KYSE790, and ECa109 as well as normal esophageal cell line Het-1A cells were extracted to analyze the expression of RICTOR and p-AKT (Ser473) by Western blot (n = 5), and then semi-quantitative analysis was performed by ImageJ software. Values represent the mean ± SD. ***P < 0.001 versus Het-1A cells.
Table 1.
Expression of RICTOR and p-AKT (Ser473) in ESCC and normal esophageal tissues.
| Tissue type | n | RICTOR |
p-AKT (Ser473) |
||||
|---|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | P | Positive (%) | Negative (%) | P | ||
| Normal | 150 | 27 (18.0) | 123 (82.0) | 0.000 | 45 (30.0) | 105 (70.0) | 0.000 |
| ESCC | 150 | 92 (61.3) | 58 (38.7) | 98 (65.3) | 52 (34.7) | ||
To explore the activated state of mTORC2/AKT in ESCC and their clinical significance, the expressions of RICTOR and p-AKT (Ser473) in 150 ESCC and normal esophageal tissues were examined by immunohistochemistry. The expressions of RICTOR and p-AKT (Ser473) protein were significantly higher in ESCC tissues than that in normal esophageal tissues and had statistically significant differences (P < 0.05).
The analysis results of the relationship between the expressions of RICTOR or p-AKT and clinicopathologic parameters show that the expressions of RICTOR or p-AKT in ESCC have no relevance with the age, gender, as well as histology classification and depth of infiltration (P > 0.05), while are positively correlated with lymph node metastasis and TNM phase (P < 0.05; Table 2), indicating that mTORC2/AKT signaling may involve in the tumorigenesis process of ESCC. The analysis of association between the expression of RICTOR and p-AKT (Ser473) is shown in Table 3. There are 68 tissues with positive expression of p-AKT (Ser473) in 92 tissues (68/92, 73.9%) with positive expression of RICTOR, while there are 28 tissues with negative expression of p-AKT (Ser473) in 58 tissues with negative expression of RICTOR (28/58, 48.3%), indicating a positive correlation between the expression of p-AKT (Ser473) and RICTOR in ESCC tissues (rs = 0.227, P < 0.05; Table 3).
Table 2.
Clinical significance of RICTOR and p-AKT (Ser473) expression.
| Variable | RICTOR |
p-AKT (Ser473) |
|||
|---|---|---|---|---|---|
| n | Positive (%) | P | Positive (%) | P | |
| Gender | |||||
| Male | 98 | 63 (64.3) | 0.308 | 59 (60.2) | 0.070 |
| Female | 52 | 29 (55.8) | 39 (75.0) | ||
| Age | |||||
| ≥60 | 88 | 59 (67.0) | 0.087 | 60 (68.2) | 0.382 |
| <60 | 62 | 33 (53.2) | 38 (61.3) | ||
| Histology classification | |||||
| I | 31 | 18 (58.1) | 0.907 | 19 (61.3) | 0.773 |
| II | 44 | 27 (61.4) | 28 (63.6) | ||
| III | 75 | 47 (62.7) | 51 (68.0) | ||
| Depth of infiltration | |||||
| Mucosa | 28 | 16 (57.1) | 0.089 | 20 (71.4) | 0.441 |
| Muscle layer | 59 | 31 (52.5) | 35 (59.3) | ||
| Fiber membrane | 63 | 45 (71.4) | 43 (68.3) | ||
| Lymph node metastasis | |||||
| No | 64 | 33 (51.6) | 0.034 | 36 (56.3) | 0.044 |
| Yes | 86 | 59 (68.6) | 62 (72.1) | ||
| TNM phase | |||||
| I, II | 69 | 35 (50.7) | 0.014 | 37 (53.6) | 0.005 |
| III, IV | 81 | 57 (70.4) | 61 (75.3) | ||
The relationships between the expressions of RICTOR or p-AKT protein and clinicopathologic parameters were analyzed. The expression of RICTOR or p-AKT protein in ESCC had no statistically significant differences with the age, gender, as well as histology classification, depth of infiltration and lymph node metastasis (P > 0.05), while was positively correlated with the TNM phase (P < 0.05).
Table 3.
Association between the expression level of RICTOR and p-AKT (Ser473) in ESCC tissues.
| RICTOR | n | p-AKT (Ser473) |
P | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 92 | 68 | 24 | 0.005 |
| Negative | 58 | 30 | 28 | |
The association between the expression level of RICTOR and p-AKT (Ser473) in ESCC tissues was explored. There was a positive correlation between the expression of p-AKT (Ser473) and RICTOR in ESCC tissues (rs = 0.227, P < 0.05).
Furthermore, the expressions of p-AKT (Ser473) and RICTOR were investigated in five human ESCC cell lines and a normal esophageal cell line Het-1A by Western blot. As shown in Fig. 1B, the expression levels of RICTOR and p-AKT (Ser473) are higher in the five ESCC cell lines than those in Het-1A cells, which is consistent with the above immunohistochemical results.
Taken together, the results in Fig. 1 and Table 1, Table 2, Table 3 highlight that mTORC2/AKT signaling is frequently over-activated in ESCC and may participate in metastasis and invasion of ESCC. Moreover, mTORC2 kinase may contribute to promote the activation of AKT in ESCC.
3.2. RAD001 or PP242 inhibited proliferation of ESCC cells through affecting AKT/mTOR/p70S6K pathway
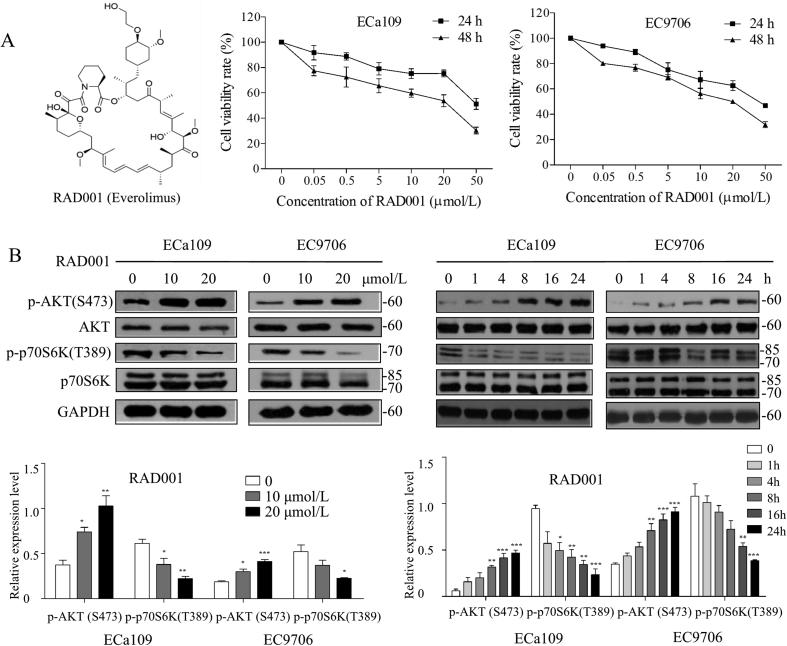
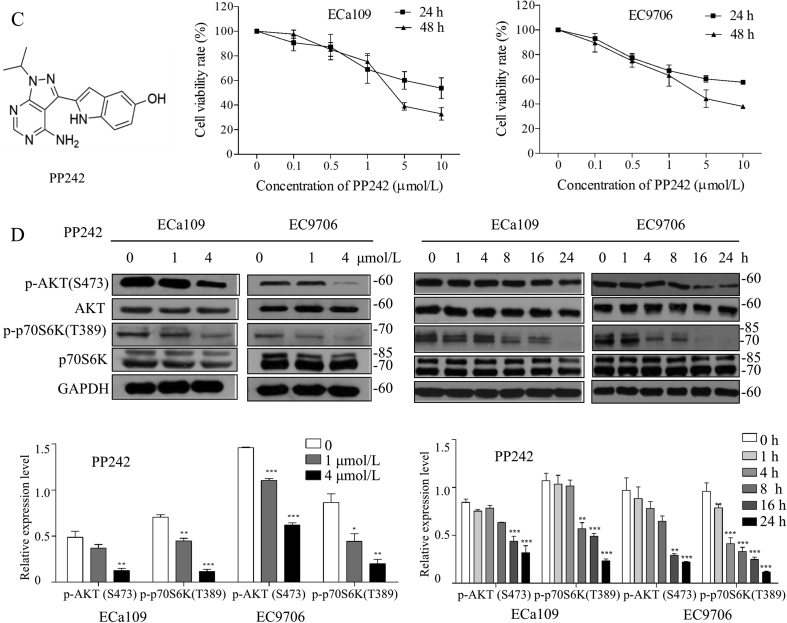
Our previous studies32,38 and above findings have confirmed the activation of mTORC1 and mTORC2 to explore the effects of mTORC1- and mTORC2-targeting inhibition on ESCC, and the in vitro anti-proliferative effects of RAD001 and PP242 were evaluated by CCK-8 assay. As shown in Fig. 2A and B, RAD001 or PP242 could inhibit proliferation of ESCC cells in a dose-dependent manner with the IC50 values (48 h) of 18.3 ± 5.6 and 17.1 ± 1.2 μmol/L for RAD001 on ECa109 and EC9706 cells, respectively. While PP242 had a better inhibitory effect on cell proliferation than RAD001 with IC50 value (48 h) of 3.7 ± 0.1 and 3.5 ± 0.5 μmol/L on ECa109 and EC9706 cells, respectively, suggesting that inhibition of both mTORC1 and mTORC2 by PP242 exhibited more powerful anti-proliferative effect than inhibition of mTORC1 by RAD001. Results from Western blot demonstrate that RAD001 inhibited the phosphorylation of p70S6K while promoted the phosphorylation of AKT in dose- and time-dependent manners (Fig. 2C). In contrast, PP242 decreased the expression of p-AKT (Ser473) and p-p70S6K (Thr389) in dose- and time-dependent manners (Fig. 2D). These findings suggest that the inhibition of mTORC1 by RAD001 triggered the feedback activation of AKT signaling, which may explain why PP242 exhibited relatively more powerful anti-proliferative effect on ESCC than that of RAD001.
Figure 2.
RAD001 or PP242 inhibited proliferation of ESCC cells through inhibiting AKT/mTOR/p70S6K pathway. (A) and (B) ECa109 and EC9706 cells were treated with RAD001 or PP242 for 24 or 48 h, respectively, and the cell viability was assessed by CCK-8 assay (n = 5). (C) and (D) After ECa109 and EC9706 cells were treated with RAD001 (0, 10 and 20 μmol/L) or PP242 (0, 1 and 4 μmol/L) for 24 h or at the same concentration (20 μmol/L of RAD001 or 4 μmol/L of PP242) for different time, total proteins were extracted to analyze the expression of p-AKT (Ser473), AKT, p-p70S6K and p70S6K by Western blot (n = 5). Values represent the mean ± SD. **P < 0.01 and ***P < 0.001 versus the control cells.
3.3. Knockdown of RICTOR enhanced sensitivity of ESCC cells to RAD001 and PP242
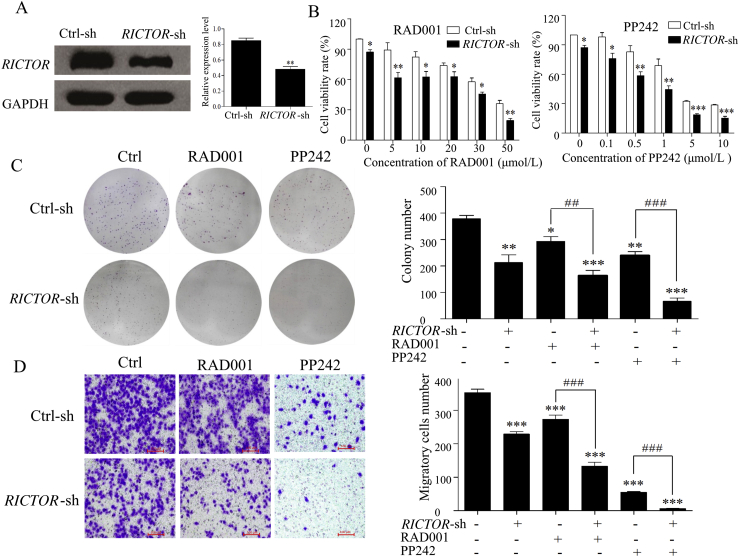
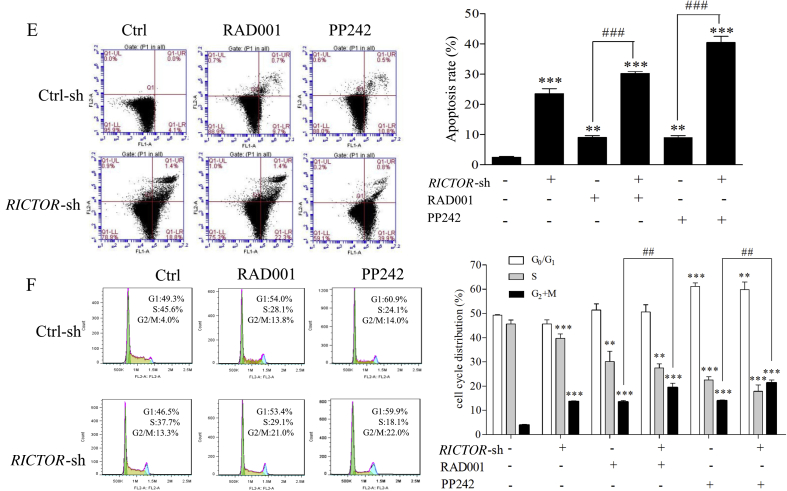
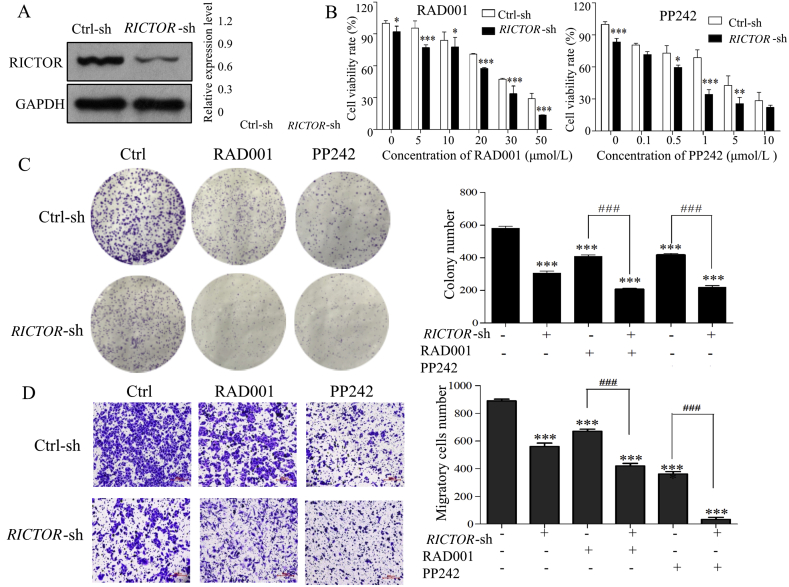
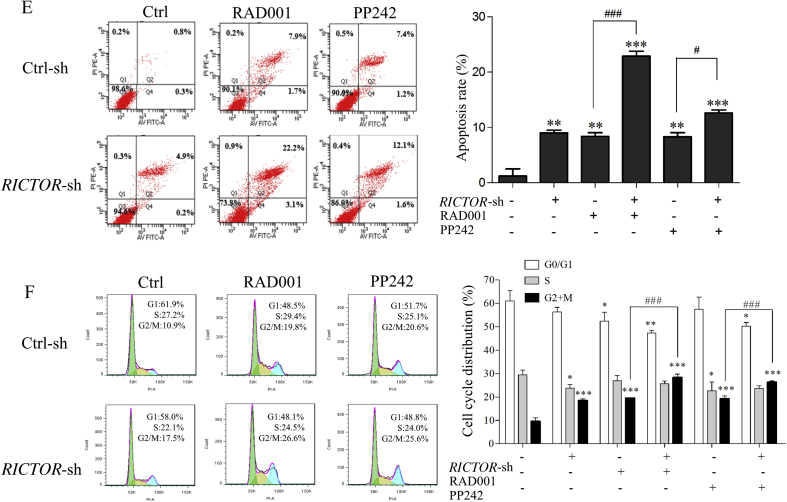
To explore the antitumor effect of RICTOR/mTORC2-targeting inhibition on ESCC cells, we respectively generated ECa109 and EC9706 cells with shRNA-mediated stable knockdown of RICTOR (decreased 43.2% in ECa109 and 68.0% in EC9706 compared to that in control cells, Figure 3, Figure 4A), and determined whether RICTOR-knockdown (RICTOR-KD) could enhance the anti-proliferative and anti-migratory effect of RAD001 and PP242. As shown in Figure 3, Figure 4B, at every concentration of RAD001 or PP242, the viability rate of RICTOR-KD cells significantly decreased compared to the control cells (P < 0.05). Furthermore, treatment of RAD001 or PP242 induced a lower viability rate in RICTOR-KD cells as compared with the control cells (P < 0.05). The colony number of RICTOR-KD cells decreased significantly compared with control cells (P < 0.05 or P < 0.001), and treatment of RAD001 and PP242 produced less colony number in RICTOR-KD cells than that in control cells (P < 0.01 or P < 0.001, Figure 3, Figure 4C). The above results indicated that RICTOR-KD could inhibit the proliferation of ESCC cells and enhance the growth-inhibitory effect of RAD001 and PP242 on ESCC cells. In the transwell migration assay (Figure 3, Figure 4D), the number of migratory cells decreased significantly in RICTOR-KD cells compared with control cells (P < 0.001), and RICTOR-KD cells migrated at significantly slower rates after treated by RAD001 or PP242 as compared to control cells (P < 0.001), suggesting that RICTOR-KD could inhibit the migration of ESCC cells and enhance the anti-migratory effect of RAD001 and PP242.
Figure 3.
Stable knockdown of RICTOR enhanced the anti-tumor effects of RAD001 and PP242 on ECa109 cells. (A) Expression of RICTOR in ECa109 cells stably transfected with RICTOR-shRNA (RICTOR-KD) or control-shRNA (control), respectively (n = 5). (B) ECa109 RICTOR-KD cells or control cells were treated with RAD001 or PP242 for 48 h, and the cell viability was assessed by CCK-8 assay (n = 5). (C) ECa109 RICTOR-KD cells or control cells were treated with RAD001 (20 μmol/L) or PP242 (4 μmol/L) for 10 days, and the colonies were stained with crystal violet and counted using ImageJ software (n = 3). (D) ECa109 RICTOR-KD cells or control cells were treated with RAD001 (10 μmol/L) or PP242 (2 μmol/L) for 48 h, and then the cell migration was assessed by transwell migration assay (n = 3). Scale bar = 100 μm. (E) and (F) ECa109 RICTOR-KD cells or control cells were treated with RAD001 (10 μmol/L for cell cycle assay and 20 μmol/L for cell apoptosis) or PP242 (2 μmol/L for cell cycle assay and 4 μmol/L for cell apoptosis) for 48 h, and then the cell cycle and apoptosis were assessed by flow cytometer (n = 3). Values represent the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 significantly different from control group; ##P < 0.01, ###P < 0.001 versus single-factor treatment group.
Figure 4.
Stable knockdown of RICTOR enhanced the anti-tumor effects of RAD001 and PP242 on EC9706 cells. (A) Expression of RICTOR in and EC9706 cells stably transfected with RICTOR-shRNA (RICTOR-KD) or control-shRNA (control), respectively (n = 5). (B) EC9706 RICTOR-KD cells or control cells were treated with RAD001 or PP242 for 48 h, and the cell viability was assessed by CCK-8 assay (n = 5). (C) EC9706 RICTOR-KD cells or control cells were treated with RAD001 (20 μmol/L) or PP242 (4 μmol/L) for 10 days, and the colonies were stained with crystal violet and counted using ImageJ software (n = 3). (D) EC9706 RICTOR-KD cells or control cells were treated with RAD001 (10 μmol/L) or PP242 (2 μmol/L) for 48 h, and then the cell migration was assessed by transwell migration assay (n = 3). Scale bar = 100 μm. (E) and (F) EC9706 RICTOR-KD cells or control cells were treated with RAD001 (10 μmol/L for cell cycle assay and 20 μmol/L for cell apoptosis) or PP242 (2 μmol/L for cell cycle assay and 4 μmol/L for cell apoptosis) for 48 h, and then the cell cycle and apoptosis were assessed by flow cytometer (n = 3). Values represent the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 significantly different from control group; #P < 0.05, ###P < 0.001 versus single-factor treatment group.
To further detect whether RICTOR-KD affects the oncogenic properties of ESCC cells, the regulating effects of RICTOR-KD on cell apoptosis and cell cycle were then explored in ECa109 and EC9706 cells. As shown in Figure 3, Figure 4E, the apoptotic rates of RICTOR-KD cells increased significantly compared with control cells (P < 0.01 or P < 0.001). Moreover, RICTOR-KD cells were more sensitive to RAD001-or PP242-induced apoptosis as compared with control cells (P < 0.05 or P < 0.001), which indicates that RICTOR-KD could synergistically increase RAD001 or PP242-induced cell apoptosis. Next, the effects of RICTOR-KD on cell cycle progression were also assessed. As shown in Figure 3, Figure 4F, the percentage of cells in S-phase decreased significantly (P < 0.05) and the percentage of cells in G2/M-phase increased significantly (P < 0.001) in RICTOR-KD cells compared with control cells. Moreover, treatment of RAD001 or PP242 induced a higher percentage of G2/M-phase cells in RICTOR-KD cells than that in control cells (P < 0.01 or P < 0.001), indicating that RICTOR-KD could synergistically enhance the RAD001 or PP242-induced G2/M-phase arrest in ESCC cells.
Taken together, the results in Figure 3, Figure 4 demonstrate that knockdown of RICTOR could enhance the cell sensitivity to RAD001 or PP242 by suppressing proliferation and migration as well as inducing apoptosis and G2/M-phase cycle arrest in ESCC cells.
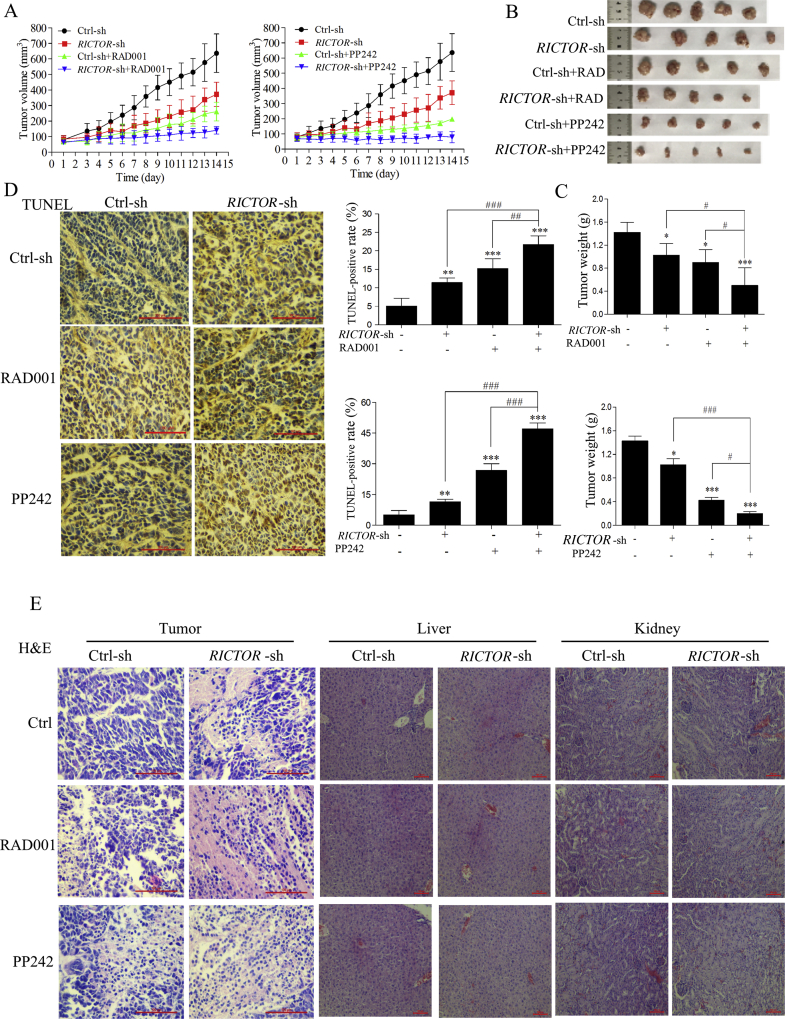
3.4. Stable knockdown of RICTOR inhibited tumor growth and potentiated the antitumor effect of RAD001 or PP242 in nude mice
To explore whether RICTOR-KD could potentiate the in vivo antitumor effect of RAD001 and PP242, nude mice bearing tumors derived from ECa109 RICTOR-KD cells or control cells were treated by RAD001 or PP242. As shown in Fig. 5A, when mice were treated with placebo, tumors derived from RICTOR-KD cells grew at significantly slower rates compared to tumors derived from control cells. When mice were treated with RAD001 or PP242, tumors derived from RICTOR-KD cells were more sensitive to RAD001 or PP242 than tumors derived from control cells. As shown in Fig. 5B and C, the weights of tumors derived from RICTOR-KD cells decreased significantly compared with that tumors derived from control cells (P < 0.05). After treated with RAD001 or PP242, tumors derived from RICTOR-KD cells were significantly smaller than tumors derived from control cells (P < 0.05). The above results indicate RICTOR-KD could inhibit tumor growth and enhance the antitumor effect of RAD001 and PP242 in vivo.
Figure 5.
Stable knockdown of RICTOR inhibited tumor growth and potentiated the antitumor effect of RAD001 or PP242 in nude mice. Nude mice bearing tumors derived from ECa109 RICTOR-KD cells or control cells were treated by RAD001 (3 mg/kg every other day, intragastric administration) or PP242 (5 mg/kg every other day, intraperitoneal injection) for 14 days (n = 5). (A) Tumor growth curves were graphed with the tumor volume of each mouse measured and recorded every day (n = 5). (B) and (C) Tumor and its weight from each group at treatment termination were shown (n = 5). (D) The tumor from each mouse was used to analyze the cell apoptosis by in situ TUNEL assay, the number of TUNEL-positive cells (brown-stained) was counted based on an examination of 1500 tumor cells of each section (400 × , scale bar = 100 μm) (n = 5). (E) Paraffin-embedded tumor was used to analyze the cell apoptosis as well as livers and kidneys of mice were used to evaluate the potential hepatorenal toxicity by H&E staining (n = 5). Scale bar = 100 μm. Values represent the mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group; #P < 0.05; ##P < 0.01; ###P < 0.001 versus single-factor treatment group.
Next, the cell apoptosis in tumors derived from RICTOR-KD cells or control cells was evaluated by in situ TUNEL assay and H&E staining. The results of in situ TUNEL assay (Fig. 5D) show the increased cell apoptotic rates in RICTOR-KD group compared with control group (P < 0.01), and treatment of RAD001 or PP242 induced higher apoptotic rates in RICTOR-KD group compared with that in control group (P < 0.01). The results of H&E staining show increased necrosis in the tumors derived from RICTOR-KD cells compared to tumors from control cells (Fig. 5E). The above results indicate that knockdown of RICTOR could promote cell apoptosis in vivo, and synergistically increase RAD001 or PP242-induced cell apoptosis, which is consistent with our in vitro results.
Moreover, the potential adverse effect of this combinatorial strategy was evaluated preliminarily. During necropsy, no obvious macroscopic pathological changes were observed in any organs of each mouse, including liver and kidney according to the H&E staining results (Fig. 5E). Compared to the control group, no statistically significant difference (P > 0.05) was observed in haematological parameters (Table 4) and relative organ weights (Table 5). These results indicate that no obvious adverse effect was observed in xenograft mice during treatment.
Table 4.
Effect of RAD001 or PP242 on haematological parameters in xenografts.
| Parameter | Ctrl-sh | RICTOR-sh | C-sh + RAD | R-sh + RAD | C-sh + PP242 | R-sh + PP242 |
|---|---|---|---|---|---|---|
| WBC (× 109/L) | 5.34 ± 0.35 | 5.76 ± 0.58 | 5.60 ± 0.54 | 5.19 ± 0.49 | 5.04 ± 0.72 | 5.09 ± 0.49 |
| RBC (× 1012/L) | 11.34 ± 0.27 | 10.85 ± 0.52 | 11.41 ± 0.65 | 11.19 ± 0.73 | 11.06 ± 0.70 | 10.89 ± 0.41 |
| HGB (g/L) | 154.01 ± 4.38 | 153.86 ± 3.06 | 152.57 ± 5.88 | 154.05 ± 6.48 | 165.65 ± 4.60 | 161.28 ± 7.43 |
| HCT (%) | 0.50 ± 0.02 | 0.49 ± 0.03 | 0.47 ± 0.02 | 0.46 ± 0.01 | 0.51 ± 0.01 | 0.47 ± 0.05 |
| MCV (fL) | 49.34 ± 3.95 | 45.07 ± 4.81 | 45.78 ± 3.10 | 45.03 ± 4.52 | 46.00 ± 1.49 | 45.02 ± 4.99 |
| RDW (%) | 15.25 ± 0.47 | 14.62 ± 0.15 | 14.99 ± 0.50 | 14.35 ± 0.99 | 14.26 ± 0.32 | 14.66 ± 0.49 |
| MCH (pg) | 316.85 ± 9.35 | 317.01 ± 9.60 | 315.04 ± 3.74 | 323.16 ± 14.97 | 338.18 ± 7.66 | 344.63 ± 7.29 |
| MCHC (g/L) | 27.19 ± 0.80 | 27.66 ± 0.79 | 27.36 ± 0.97 | 27.37 ± 0.52 | 28.01 ± 0.61 | 28.48 ± 0.61 |
| PLT (× 109/L) | 991.74 ± 105.04 | 968.31 ± 115.71 | 997.24 ± 101.69 | 1039.72 ± 138.94 | 1002.75 ± 158.78 | 979.38 ± 118.11 |
After mice were treated with RAD001 or PP242 for 2 weeks, blood samples were collected from mice orbit to measure the routine haematological parameters. No statistically significant differences were found. Note: C-sh, Ctrl-sh; R-sh, RICTOR-sh; RAD, RAD001.
Table 5.
Effect of RAD001 or PP242 on relative organ weighs (%) of xenograft mice.
| Organ | Relative organ weigh (%) |
|||||
|---|---|---|---|---|---|---|
| Ctrl-sh | RICTOR-sh | C-sh + RAD | R-sh + RAD | C-sh + PP242 | R-sh + PP242 | |
| Heart | 0.55 ± 0.03 | 0.62 ± 0.06 | 0.53 ± 0.04 | 0.59 ± 0.06 | 0.55 ± 0.09 | 0.58 ± 0.06 |
| Liver | 7.37 ± 0.38 | 7.08 ± 0.44 | 6.58 ± 0.19 | 6.36 ± 0.07 | 6.2 ± 0.27 | 6.30 ± 0.23 |
| Spleen | 0.51 ± 0.10 | 0.55 ± 0.07 | 0.52 ± 0.06 | 0.44 ± 0.09 | 0.45 ± 0.03 | 0.48 ± 0.09 |
| Lung | 0.73 ± 0.09 | 0.73 ± 0.26 | 0.75 ± 0.07 | 0.64 ± 0.08 | 0.64 ± 0.02 | 0.70 ± 0.05 |
| Kidney-L | 0.97 ± 0.05 | 0.96 ± 0.05 | 0.96 ± 0.06 | 0.89 ± 0.04 | 0.88 ± 0.06 | 0.95 ± 0.06 |
| Kidney-R | 0.99 ± 0.06 | 0.96 ± 0.08 | 0.99 ± 0.03 | 0.87 ± 0.08 | 0.92 ± 0.01 | 0.92 ± 0.07 |
During necropsy, the heart, liver, spleen, lung and kidney from each mouse were collected and weighed separately to calculated the relative organ weight [Relative organ weight (%) = organ weight/body weight × 100]. No statistically significant differences were found. Note: C-sh, Ctrl-sh; R-sh, RICTOR-sh; RAD, RAD001.
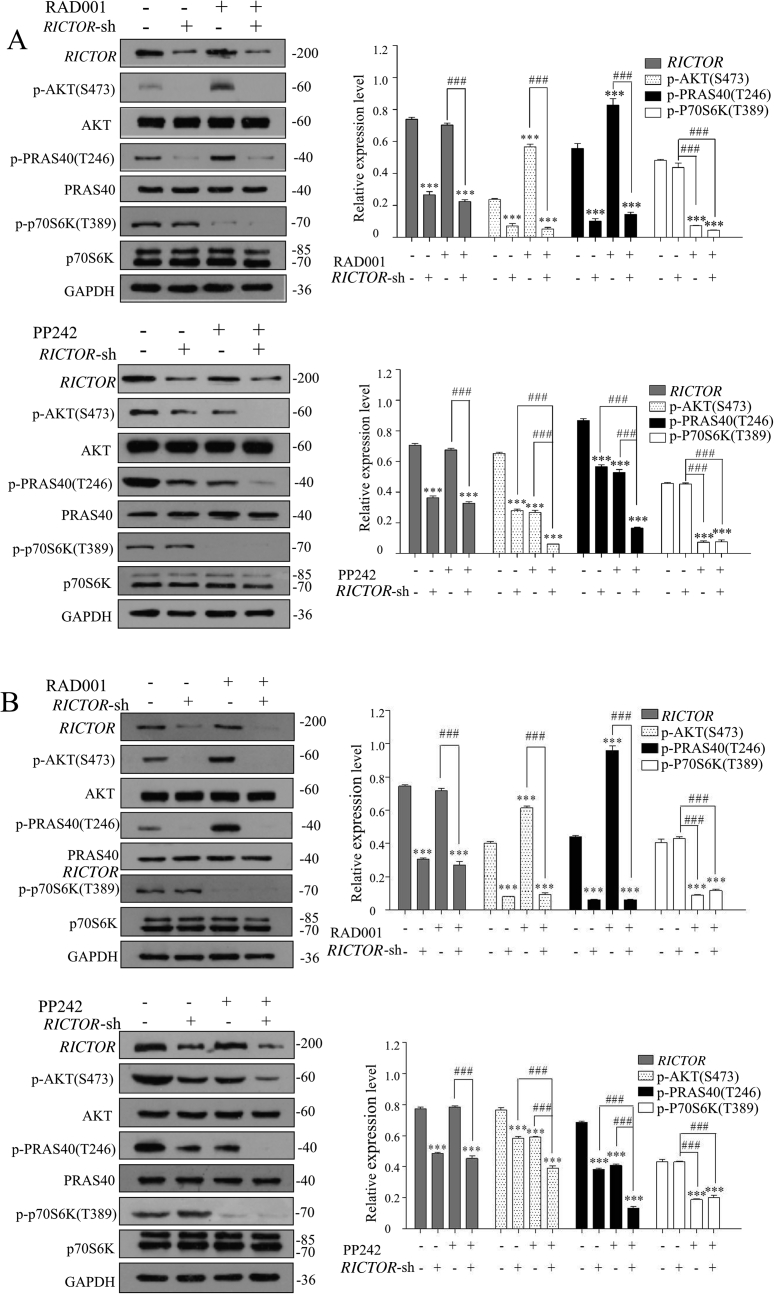
3.5. Knockdown of RICTOR inhibited RAD001-induced feedback activation of AKT/PRAS40 signaling in vitro and in vivo
To investigate the molecular mechanism underlying the increased cell sensitivity to RAD001 and PP242 produced by RICTOR-KD, the expressions of p70S6K, AKT and PRAS40, a proline-rich AKT substrate that regulates mTORC1 kinase activity34, in RICTOR-KD or control cells treated with RAD001 and PP242 were explored. As shown in Fig. 6A, the expression of p-AKT (Ser473) and p-PRAS40 (Thr246) increased significantly after control cells were treated with RAD001 (P < 0.001), while the expressions of p-AKT (Ser473) and p-PRAS40 (Thr246) were decreased significantly after RICTOR was knocked down (P < 0.001), compared with the untreated control cells. Most interestingly, the RAD001-induced phosphorylation of AKT and PRAS40 could be significantly abrogated by RICTOR-KD (P < 0.001), which may explain why RICTOR-KD could enhance the growth-inhibitory effect of RAD001. In contrast, PP242 significantly inhibited the phosphorylation of AKT and PRAS40 (P < 0.001), and synergistically acted with RICTOR-knockdown to inhibit the activation of AKT/PRAS40 signaling (P < 0.001), which may be the mechanism that RICTOR-knockdown cells were sensitized to PP242-induced growth inhibition compared to control cells. In addition, the phosphorylation of p70S6K was inhibited significantly by treatment of RAD001 or PP242, while knockdown of RICTOR did not affect the expression and phosphorylated status of p70S6K.
Figure 6.
Stable knockdown of RICTOR abrogated the activation of RAD001 and enhanced the inhibition of PP242 to AKT/PRAS40 signaling in vitro and in vivo. (A) ECa109 cells stably transfected with control shRNA or RICTOR shRNA were treated with RAD001 (10 μmol/L) or PP242 (2 μmol/L) for 48 h, and total proteins were extracted to analysis the expression of RICTOR, p-AKT (Ser473), AKT, p-PRAS40 (Thr246), PRAS40, p-p70S6K and p70S6K by Western blot (n = 5). (B) Total proteins in tumor tissues from nude mice were extracted and the expressions of RICTOR, p-AKT (Ser473), AKT, p-PRAS40 (Thr246), PRAS40, p-p70S6K (Thr389) and p70S6K were evaluated by Western blot (n = 5). Values represent the mean ± SD. ***P < 0.001 versus control group; ###P < 0.001 versus single-factor treatment group.
The molecular mechanism underlying the in vivo antitumor effect of RICTOR-KD was also explored using the xenograft from nude mice by Western blot. As shown in Fig. 6B, in tumors derived from control cells, RAD001 promoted the phosphorylation of AKT and PRAS40 (P < 0.001), while PP242 inhibited significantly the phosphorylation of AKT and PRAS40 (P < 0.001). In contrast, in tumors derived from RICTOR-KD cells, the RAD001-induced phosphorylation of AKT and PRAS40 was significantly inhibited (P < 0.001), and PP242 synergistically enhanced the inhibition effect of RICTOR-KD on phosphorylation of AKT and PRAS40 (P < 0.001). The findings above are consistent with our in vitro results, which may be the reason that knockdown of RICTOR could enhance the in vivo antitumor effect of RAD001 and PP242.
4. Discussion
ESCC is a subtype of esophageal carcinoma that occurs at a high frequency in many areas including China, South America, Western Europe, Southern Africa and Japan3,39, and this disease always accompanies with the insensitivity to traditional chemotherapy and the poor prognosis, which urge the researchers to explore the etiology and pathogenesis of ESCC and develop novel treatment strategies.
In earlier studies, mTORC1 was considered to be a promising target for the treatment of squamous cell carcinoma in head and neck40. Consistently, our earlier studies also confirmed that mTORC1/p70S6K signaling is hyperactivated in ESCC, and targeted inhibition of mTORC1 by rapamycin could effectively suppress proliferation of ESCC cells and enhance the antitumor effect of cisplatin both in vitro and in vivo33,38,41. Because of the seemingly clear rationale for the utilization of mTORC1 inhibitors in cancer treatment, rapalogs such as RAD001 and CCI-779 have been used clinically in many tumor types for the past decade years5,13, 14, 15,42. However, recent clinical trials have revealed that the antitumor effect of rapalogs is probably not as promising as we initially expected5,43, the existence of several negative feedback loops emanating from p70S6K to AKT is considered to be the main reason that leads to the relatively modest efficacy of rapalogs in clinical treatment5,13,44. Mechanistically, inhibition of p-p70S6K by rapalog activates insulin receptor substrate 1 (IRS-1) and phosphatidylinositol 3 kinase (PI3K), resulting in the phosphorylated activation of AKT at Thr308 site44, 45, 46. Meanwhile, inhibition of p-p70S6K can phosphorylate RICTOR at Thr1135 site and results in the dissociation of RICTOR from mTORC2, thus promoting phosphorylation of AKT at Ser473 site, the direct downstream targets of RICTOR17,18. To sum up, inhibition of mTORC1 by rapalogs would reactivate AKT signaling through the p70S6K/IRS-1/PI3K/AKT (Thr308) and p70S6K/mTORC2/AKT (Ser473) feedback loops, and ultimately attenuate the antitumor effect of rapalogs.
For these reasons above, the newly-developed pan-mTOR inhibitors seem to have broader impacts than the “old” rapalogs. PP242, the first reported pan-mTOR inhibitor, was reported to inhibit both mTORC1 and mTORC2 activities, and thus has more complete inhibition on the output of mTOR than rapalogs22. A previous preclinical study demonstrated that PP242, but not rapamycin, could effectively suppress cell proliferation, induce apoptosis, and arrest cell cycle of ESCC cells by attenuating the activities of both mTORC1 and mTORC2 signaling and abrogating mTORC1-dependent PI3K/AKT feedback activation23. In this study, we confirmed that PP242 exhibited more powerful anti-proliferative effect against ESCC cells than RAD001, and found that inhibition of mTORC1 by RAD001 increased the phosphorylated levels of AKT at Ser473, whereas PP242 inhibited phosphorylation of both AKT and p70S6K (Fig. 2).
However, some studies thought that PP242 could not completely inhibit the activation of AKT because PP242 could relieve feedback inhibition of RTKs47 and induce inhibition of p-4EBP-1 in cancers with KRAS mutation48, which will lead to the resistance of cancer cells to PP242, thus. Thus, the hypothesis that using the pan-mTOR inhibitor to suppress these feedback loops seems infeasible and impractical47,48. Considering the above studies, selectively blocking mTORC2 activity could avoid the mTORC1-mediated feedback loops, and should be undoubtedly effective for cancer therapy. Several recent studies have confirmed that targeted inhibition of mTORC2 inhibits tumorigenesis in ovarian and pancreatic cancer49,50, which provide a rationale for developing inhibitors specifically targeting mTORC2. Unfortunately, the inhibitors specifically targeting mTORC2 are still unavailable currently. RICTOR, as the key component of mTORC2, has critical roles for mTORC2 function by regulation of the activation of AKT26. Some recent studies have demonstrated the overexpression of RICTOR and its association with tumor progression and poor prognosis in many cancers such as lung cancer, pancreatic cancer, and gastric cancer29,30,51,52, and knockdown of RICTOR by RNA interference has inhibitory effects on tumor growth in vitro and in vivo25,29,30,50. These studies above highlight the role of RICTOR in tumorigenesis and RICTOR is therefore becoming an important actor in cancer diagnosis, prognosis and treatment as a therapeutic target26,27. Therefore, exploring the unique impacts of RICTOR/mTORC2 pathway on oncogenic properties will facilitate the research and development of mTORC2-specific inhibitors21. Although Jiang et al.31 have determined the overexpression of RICTOR and its relationship with tumor metastasis and prognosis in ESCC, the functional effects of RICTOR/mTORC2 on tumorigenesis of ESCC are still unknown, this study therefore explored the potential role of mTORC2 as a therapeutic target in ESCC.
In this study, we first explored the expression of p-AKT (Ser473) and RICTOR as well as the clinical significance in 150 tissues from ESCC patients, and demonstrated that p-AKT (Ser473) and RICTOR were more frequently activated in ESCC tissues. Moreover, the overactivation of RICTOR is positively correlated with elevated p-AKT (Ser473) level in ESCC tissues, and their overexpression is related to lymph node metastasis and tumor-node-metastasis (TNM) phase of ESCC patients, suggesting that mTORC2/AKT pathway may participate in metastasis and invasion of ESCC, which might contribute to diagnose the progress of ESCC. Second, we confirmed that inhibition of mTORC1 by RAD001 increased the phosphorylated levels of AKT at Ser473 via the p70S6K-mediated negative feedback loops, whereas inhibition of both mTORC1 and mTORC2 by PP242 inhibited phosphorylation of both AKT and p70S6K, which might explain, at least partly, the reason that PP242 exhibited more powerful anti-proliferative effect against ESCC cells than RAD001. Third, we found that stable knockdown of RICTOR could inhibit proliferation and migration as well as induce cell cycle arrest and apoptosis of ESCC cells. Noteworthy, an important finding in our study was that inhibition of mTORC2 by knocking-down RICTOR significantly suppressed the RAD001-induced feedback activation of AKT/PRAS40 signaling, and also enhanced the inhibition efficacy of PP242 on the phosphorylation of AKT and PRAS40, and therefore potentiated the antitumor effect of RAD001 and PP242 both in vitro and in vivo, which provide a rationale for developing inhibitors specifically targeting mTORC2 as well as its combination with mTOR inhibitors in clinical therapy of ESCC.
Recent studies conducted by Sakre et al.29 and Kim et al.53 revealed that the amplification of RICTOR increased sensitivity of cancer cells to mTORC1/mTORC2 inhibitors, and silencing or knocking-down RICTOR counteracted the inhibitory effects of mTORC1/mTORC2 inhibitor AZD2014 in lung cancer and gastric cancer. However, based on our data in this study, knocking-down RICTOR obviously improved the antitumor effect of mTORC1 inhibitor RAD001 and mTORC1/mTORC2 inhibitor PP242 on ESCC cells. We speculated that knocking-down RICTOR combined with PP242 might inhibit activation of AKT at a larger extent, which was demonstrated in the exploration of molecular mechanism in vitro and in vivo by Western blot (Fig. 6). From our results, we could speculate that the pan-mTOR inhibitors targeting the mTOR–ATP binding domain will be more efficacious than rapalogs in the clinical treatment of ESCC. Furthermore, since mTORC2 regulates a wider range of targets of downstream mTOR and does not perturb mTORC1-dependent negative feedback loops, it will be a more promising therapeutic target in ESCC treatment than mTORC1. In addition, although the mTORC2-specific inhibitors are still unavailable currently, this study supports the combined use of mTORC2-specific inhibition and rapalogs/pan-mTOR inhibitors as an effective approach to treat ESCC in the future. Our recent study reported a novel diterpenoid compound that targeting PI3K and mTORC2 signaling pathway significantly potentiates the antitumor effect of rapamycin in ESCC36, which further supported the opinion above.
5. Conclusions
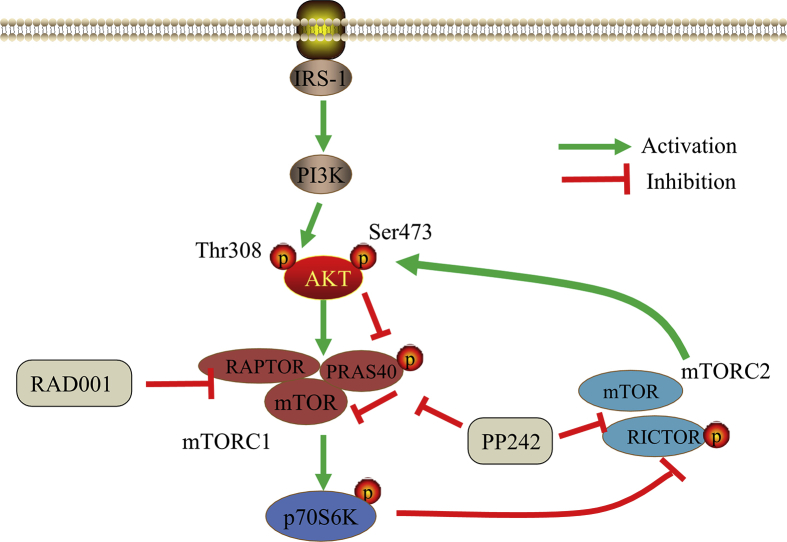
Our findings highlight the crucial role of mTORC2 in tumorigenesis of ESCC, and provide preclinical rationale for selectively targeting mTORC2 as a feasible and promising therapeutic strategy to enhance the antitumor efficacy of mTOR inhibitors in future treatment of ESCC (Fig. 7).
Figure 7.
Schematic diagram that the potential effect of RICTOR KD on sensitivity of ESCC cells to RAD001 and PP242.
Acknowledgments
This work was supported by the Open Foundation Project of Pharmacy in Zhejiang Province, China (Grant No.YKFJ2-010), the National Natural Science Foundation of Henan Province, China (Grant No.182300410312), Henan Provincial University Science and Technology Innovation Team, Department of Education of Henan Province (Grant No. 19IRTSTHN001, China), Key Research Project of University, Department of Education of Henan Province (Grant No. 20A350019, China) and the National Science and Technology Major Project of China (Grant No. 2018ZX10302205). The authors would like to thank all members of the study team, the patients involved in this study and Dr. Xuejian Feng from School of Pharmaceutical Sciences of Zhengzhou University (Zhengzhou, China).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.01.010.
Author contributions
Guiqin Hou and Zhaoming Lu conceived the project. Guiqin Hou, Fanghua Gong, Wen Zhao, and Jianying Zhang designed the experiments and secured funding. Zhaoming Lu, Xiaojing Shi, Shenglei Li, Yang Wang, Yandan Ren, Mengying Zhang, and Yan Li performed the experiments. Zhaoming Lu, Xiaojing Shi, Shenglei Li and Bin Yu analyzed the data. Zhaoming Lu wrote the manuscript. Guiqin Hou and Zhaoming Lu provided critical discussion, editing and final approval of the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Network CGAR Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Pickens A., Orringer M.B. Geographical distribution and racial disparity in esophageal cancer. Ann Thorac Surg. 2003;76:S1367–S1369. doi: 10.1016/s0003-4975(03)01202-5. [DOI] [PubMed] [Google Scholar]
- 4.De Angelis R., Sant M., Coleman M.P., Francisci S., Baili P., Pierannunzio D. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE—5-a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 5.Chiarini F., Evangelisti C., McCubrey J.A., Martelli A.M. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci. 2015;36:124–135. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G., Boyle J.W., Ko C.N., Zeng W., Wong V.K.W., Wan J.B. Aurone derivatives as Vps34 inhibitors that modulate autophagy. Acta Pharm Sin B. 2019;9:537–544. doi: 10.1016/j.apsb.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sengupta S., Peterson T.R., Sabatini D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarbassov D.D., Guertin D.A., Ali S.M., Sabatini D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 10.Wander S.A., Hennessy B.T., Slingerland J.M. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao W., Qiu Y., Kon g. D. Class I phosphatidylinositol 3-kinase inhibitors for cancer therapy. Acta Pharm Sin B. 2017;7:27–37. doi: 10.1016/j.apsb.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basnet R., Gong G.Q., Li C., Wang M.W. Serum and glucocorticoid inducible protein kinases (SGKs): a potential target for cancer intervention. Acta Pharm Sin B. 2018;8:767–771. doi: 10.1016/j.apsb.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baselga J., Campone M., Piccart M., Burris H.A., 3rd, Rugo H.S., Sahmoud T. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapoor A., Figlin R.A. Targeted inhibition of mammalian target of rapamycin for the treatment of advanced renal cell carcinoma. Cancer. 2009;115:3618–3630. doi: 10.1002/cncr.24409. [DOI] [PubMed] [Google Scholar]
- 15.Yao J.C., Shah M.H., Ito T., Bohas C.L., Wolin E.M., Van Cutsem E. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Efeyan A., Sabatini D.M. mTOR and cancer: many loops in one pathway. Curr Opin Cell Biol. 2010;22:169–176. doi: 10.1016/j.ceb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breuleux M., Klopfenstein M., Stephan C., Doughty C.A., Barys L., Maira S.M. Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol Canc Therapeut. 2009;8:742–753. doi: 10.1158/1535-7163.MCT-08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julien L.A., Carriere A., Moreau J., Roux P.P. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol Cell Biol. 2010;30:908–921. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson V., Altman J.K., Platanias L.C. Next generation of mammalian target of rapamycin inhibitors for the treatment of cancer. Expet Opin Invest Drugs. 2013;22:715–722. doi: 10.1517/13543784.2013.787066. [DOI] [PubMed] [Google Scholar]
- 20.Yori J.L., Lozada K.L., Seachrist D.D., Mosley J.D., Abdul-Karim F.W., Booth C.N. Combined SFK/mTOR inhibition prevents rapamycin-induced feedback activation of AKT and elicits efficient tumor regression. Cancer Res. 2014;74:4762–4771. doi: 10.1158/0008-5472.CAN-13-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou Z., Chen J., Yang J., Bai X. Targeted inhibition of Rictor/mTORC2 in cancer treatment: a new era after rapamycin. Curr Cancer Drug Targets. 2016;16:288–304. doi: 10.2174/1568009616666151113120830. [DOI] [PubMed] [Google Scholar]
- 22.Feldman M.E., Apsel B., Uotila A., Loewith R., Knight Z.A., Ruggero D. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y., Xi Q., Chen Y., Wang J., Peng P., Xia S. A dual mTORC1 and mTORC2 inhibitor shows antitumor activity in esophageal squamous cell carcinoma cells and sensitizes them to cisplatin. Anti Cancer Drugs. 2013;24:889–898. doi: 10.1097/CAD.0b013e328363c64e. [DOI] [PubMed] [Google Scholar]
- 24.Zeng Z., Shi Y., Tsao T., Qiu Y., Kornblau S.M., Baggerly K.A. Targeting of mTORC1/2 by the mTOR kinase inhibitor PP242 induces apoptosis in AML cells under conditions mimicking the bone marrow microenvironment. Blood. 2012;120:2679–2689. doi: 10.1182/blood-2011-11-393934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bian Y.H., Xu J., Zhao W.Y., Zhang Z.Z., Tu L., Cao H. Targeting mTORC2 component rictor inhibits cell proliferation and promotes apoptosis in gastric cancer. Am J Transl Res. 2017;9:4317–4330. [PMC free article] [PubMed] [Google Scholar]
- 26.Gkountakos A., Pilotto S., Mafficini A., Vicentini C., Simbolo M., Milella M. Unmasking the impact of Rictor in cancer: novel insights of mTORC2 complex. Carcinogenesis. 2018;39:971–980. doi: 10.1093/carcin/bgy086. [DOI] [PubMed] [Google Scholar]
- 27.Jebali A., Dumaz N. The role of RICTOR downstream of receptor tyrosine kinase in cancers. Mol Canc. 2018;17:39. doi: 10.1186/s12943-018-0794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Lin J., Wang X., Yao G., Wang L., Zheng H. Targeting of mTORC2 prevents cell migration and promotes apoptosis in breast cancer. Breast Canc Res Treat. 2012;134:1057–1066. doi: 10.1007/s10549-012-2036-2. [DOI] [PubMed] [Google Scholar]
- 29.Sakre N., Wildey G., Behtaj M., Kresak A., Yang M., Fu P. RICTOR amplification identifies a subgroup in small cell lung cancer and predicts response to drugs targeting mTOR. Oncotarget. 2017;8:5992–6002. doi: 10.18632/oncotarget.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt K.M., Hellerbrand C., Ruemmele P., Michalski C.W., Kong B., Kroemer A. Inhibition of mTORC2 component RICTOR impairs tumor growth in pancreatic cancer models. Oncotarget. 2017;8:24491–24505. doi: 10.18632/oncotarget.15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang W.J., Feng R.X., Liu J.T., Fan L.L., Wang H., Sun G.P. RICTOR expression in esophageal squamous cell carcinoma and its clinical significance. Med Oncol. 2017;34:32. doi: 10.1007/s12032-017-0894-5. [DOI] [PubMed] [Google Scholar]
- 32.Hou G., Zhang Q., Wang L., Liu M., Wang J., Xue L. mTOR inhibitor rapamycin alone or combined with cisplatin inhibits growth of esophageal squamous cell carcinoma in nude mice. Cancer Lett. 2010;290:248–254. doi: 10.1016/j.canlet.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Hou G., Xue L., Lu Z., Fan T., Tian F., Xue Y. An activated mTOR/p70S6K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett. 2007;253:236–248. doi: 10.1016/j.canlet.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 34.Hou G., Zhao Q., Zhang M., Fan T., Liu M., Shi X.J. Down-regulation of Rictor enhances cell sensitivity to PI3K inhibitor LY294002 by blocking mTORC2-medicated phosphorylation of Akt/PRAS40 in esophageal squamous cell carcinoma. Biomed Pharmacother. 2018;106:1348–1356. doi: 10.1016/j.biopha.2018.07.075. [DOI] [PubMed] [Google Scholar]
- 35.Dong M., Nio Y., Sato Y., Tamura K., Song M., Tian Y. Comparative study of p53 expression in primary invasive ductal carcinoma of the pancreas between Chinese and Japanese. Pancreas. 1998;17:229–237. doi: 10.1097/00006676-199810000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Peng K.Z., Ke Y., Zhao Q., Tian F., Liu H.M., Hou G. OP16, a novel ent-kaurene diterpenoid, potentiates the antitumor effect of rapamycin by inhibiting rapamycin-induced feedback activation of Akt signaling in esophageal squamous cell carcinoma. Biochem Pharmacol. 2017;140:16–27. doi: 10.1016/j.bcp.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y.J., Ko B.S., Liang S.M., Lu Y.J., Jan Y.J., Jiang S.S. ZNF479 downregulates metallothionein-1 expression by regulating ASH2L and DNMT1 in hepatocellular carcinoma. Cell Death Dis. 2019;10:408. doi: 10.1038/s41419-019-1651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou G., Yang S., Zhou Y., Wang C., Zhao W., Lu Z. Targeted inhibition of mTOR signaling improves sensitivity of esophageal squamous cell carcinoma cells to cisplatin. J Immunol Res. 2014;2014:845763. doi: 10.1155/2014/845763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 40.Amornphimoltham P., Patel V., Sodhi A., Nikitakis N.G., Sauk J.J., Sausville E.A. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65:9953–9961. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 41.Lu Z., Peng K., Wang N., Liu H.M., Hou G. Downregulation of p70S6K enhances cell sensitivity to rapamycin in esophageal squamous cell carcinoma. J Immunol Res. 2016;2016:7828916. doi: 10.1155/2016/7828916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atkins M.B., Hidalgo M., Stadler W.M., Logan T.F., Dutcher J.P., Hudes G.R. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 43.Evangelisti C., Ricci F., Tazzari P., Tabellini G., Battistelli M., Falcieri E. Targeted inhibition of mTORC1 and mTORC2 by active-site mTOR inhibitors has cytotoxic effects in T-cell acute lymphoblastic leukemia. Leukemia. 2011;25:781–791. doi: 10.1038/leu.2011.20. [DOI] [PubMed] [Google Scholar]
- 44.Wan X., Harkavy B., Shen N., Grohar P., Helman L.J. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 45.Carracedo A., Ma L., Teruya-Feldstein J., Rojo F., Salmena L., Alimonti A. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gual P., Le Marchand-Brustel Y., Tanti J.F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005;87:99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 47.Rodrik-Outmezguine V.S., Chandarlapaty S., Pagano N.C., Poulikakos P.I., Scaltriti M., Moskatel E. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1:248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ducker G.S., Atreya C.E., Simko J.P., Hom Y.K., Matli M.R., Benes C.H. Incomplete inhibition of phosphorylation of 4E-BP1 as a mechanism of primary resistance to ATP-competitive mTOR inhibitors. Oncogene. 2014;33:1590–1600. doi: 10.1038/onc.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hisamatsu T., Mabuchi S., Matsumoto Y., Kawano M., Sasano T., Takahashi R. Potential role of mTORC2 as a therapeutic target in clear cell carcinoma of the ovary. Mol Canc Therapeut. 2013;12:1367–1377. doi: 10.1158/1535-7163.MCT-12-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Driscoll D.R., Karim S.A., Sano M., Gay D.M., Jacob W., Yu J. mTORC2 signaling drives the development and progression of pancreatic cancer. Canc Res. 2016;76:6911–6923. doi: 10.1158/0008-5472.CAN-16-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng H., Zou Y., Ross J.S., Wang K., Liu X., Halmos B. RICTOR amplification defines a novel subset of patients with lung cancer who may benefit from treatment with mTORC1/2 inhibitors. Cancer Discov. 2015;5:1262–1270. doi: 10.1158/2159-8290.CD-14-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gulhati P., Bowen K.A., Liu J., Stevens P.D., Rychahou P.G., Chen M. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011;71:3246–3256. doi: 10.1158/0008-5472.CAN-10-4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S.T., Kim S.Y., Klempner S.J., Yoon J., Kim N., Ahn S. Rapamycin-insensitive companion of mTOR (RICTOR) amplification defines a subset of advanced gastric cancer and is sensitive to AZD2014-mediated mTORC1/2 inhibition. Ann Oncol. 2017;28:547–554. doi: 10.1093/annonc/mdw669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.