Abstract
Background
Coronavirus disease 2019 (COVID-19) has been widely spread and caused tens of thousands of deaths, especially in patients with severe COVID-19. This analysis aimed to explore risk factors for mortality of severe COVID-19, and establish a scoring system to predict in-hospital deaths.
Methods
Patients with COVID-19 were retrospectively analyzed and clinical characteristics were compared. LASSO regression as well as multivariable analysis were used to screen variables and establish prediction model.
Findings
A total of 2529 patients with COVID-19 was retrospectively analyzed, and 452 eligible severe COVID-19 were used for finally analysis. In training cohort, the median age was 66•0 years while it was 73•0 years in non-survivors. Patients aged 60–75 years accounted for the largest proportion of infected populations and mortality toll. Anti-SARS-CoV-2 antibodies were monitored up to 54 days, and IgG levels reached the highest during 20–30 days. No differences were observed of antibody levels between severe and non-severe patients. About 60.2% of severe patients had complications. Among acute myocardial injury (AMI), acute kidney injury (AKI) and acute liver injury (ALI), the heart was the earliest injured organ, whereas the time from AKI to death was the shortest. Age, diabetes, coronary heart disease (CHD), percentage of lymphocytes (LYM%), procalcitonin (PCT), serum urea, C reactive protein and D-dimer (DD), were identified associated with mortality by LASSO binary logistic regression. Then multivariable analysis was performed to conclude that old age, CHD, LYM%, PCT and DD remained independent risk factors for mortality. Based on the above variables, a scoring system of COVID-19 (CSS) was established to divide patients into low-risk and high-risk groups. This model displayed good discrimination (AUC=0·919) and calibration (P=0·264). Complications in low-risk and high-risk groups were significantly different (P<0·05). Use of corticosteroids in low-risk groups increased hospital stays by 4·5 days (P=0·036) and durations of disease by 7·5 days (P=0·012) compared with no corticosteroids.
Interpretation
Old age, CHD, LYM%, PCT and DD were independently related to mortality. CSS was useful for predicting in-hospital mortality and complications, and it could help clinicians to identify high-risk patients with poor prognosis.
Funding
This work was supported by the Key Project for Anti-2019 novel Coronavirus Pneumonia from the Ministry of Science and Technology, China (grant number 2020YFC0845500).
Keywords: COVID-19, Critical ill, Scoring system, Antibody, Mortality
Research in context.
Evidence before this study
We searched PubMed on March 11, 2020, for articles that documented the risk factors of mortality and scoring system to predict in-hospital death in patients with coronavirus disease 2019 (COVID-19), resulting from infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), using the search terms (“novel coronavirus” OR “SARS-CoV-2” OR “COVID-19”) AND (“death” OR “mortality”) AND (“model” OR “scoring system”) with no language or time restrictions. Older age, higher Sequential Organ Failure Assessment (SOFA) score, d-dimer levels, comorbidities, lymphocytopenia and elevated alanine aminotransferase, high-sensitive cardiac troponin I were reported to be associated with mortality. However, no published works were found to establish a scoring system to predict in-hospital deaths for patients with severe COVID-19.
Added value of this study
In this retrospective cohort study from three hospitals in Wuhan, China, we found that old age, coronary heart disease condition, percentage of lymphocytes <8%, procalcitonin>0·15 ng/ml and D-dimer >0·5 ug/ml were independent risk factors for mortality. Based on the above variables, a scoring system of COVID-19 (CSS) was established to divide patients into low-risk and high-risk groups. This model displayed good discrimination (AUC=0·919) and calibration (P=0·264). Complications in low-risk and high-risk groups were significantly different (P<0·05). Use of corticosteroids in low-risk groups increased hospital stays by 4·5 days (P=0·036) and durations of disease by 7·5 days (P=0·012) compared with no corticosteroids.
Implications of all the available evidence
Old age, coronary heart disease condition, lymphopenia, elevated procalcitonin and D-dimer were independently related to mortality. CSS was useful for predicting in-hospital mortality and complications, and it could help clinicians to identify high-risk patients with poor outcomes.
Alt-text: Unlabelled box
1. Introduction
In December 2019 a group of patients with pneumonia of unknown cause were later confirmed to be infected with a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in Wuhan, Hubei province, China [1,2]. The clinical spectrum of SARS-CoV-2 infection appeared to be wide, ranging from asymptomatic infection, mild upper respiratory tract illness to severe or even fatal in some cases. To accommodate the wide spectrum of clinical presentations and outcomes of infections caused by SARS-CoV-2 [3–5], the WHO later introduced a rather unspecific name COVID-19 (coronavirus disease 19) to denote this disease.
The rapid emergence of COVID-19 in Wuhan city, Hubei Province, China, has resulted in thousands of deaths [6]. Recently, the COVID-19 spread rapidly around the world and caused the death of hundreds of thousands of patients [7]. At present, there are no effective therapies or vaccines for COVID-19. It was reported that the death was mainly in severe patients, and many infected patients presented mild flu-like symptoms and quickly recover [5]. However, the differences in clinical characteristics between severe and non-severe cases were little reported [8,9]. In particular, the comparison of clinical characteristics between survivors and non-survivors of severe patients and risk factors for survival have not been reported.
In this study, we aimed to identify risk factors for mortality of severe patients and establish a risk model to predict mortality, so that clinicians can effectively consider medical resources of patients with different risks.
2. Methods
2.1. Patients
This retrospective cohort study included three institutions: Zhongnan Hospital of Wuhan University, No. 7 Hospital of Wuhan and Leishenshan Hospital (Wuhan, China). Zhongnan Hospital is responsible for the treatments for COVID-19 assigned by the government. No. 7 Hospital of Wuhan is one of the designated hospitals for the hospitalization of patients with COVID-19 and has been entrusted by Zhongnan Hospital of Wuhan University since January 2020. Leishenshan Hospital was a hospital specially built to treat patients with COVID-19 and was taken over by the Zhongnna hospital of Wuhan University. All consecutive patients with confirmed COVID-19 according to WHO interim guidance from January 1 to March 27, 2020 were enrolled in present study [10]. Patients absent of or with negative SARS-CoV-2 test results were excluded from this study. All patients involved in this study were living in Wuhan during the outbreak period of COVID-19. The clinical outcomes such as discharges or mortality were monitored up to March 31, 2020, the final date of follow-up, and all patients with COVID-19 have experienced a definite outcome. This study was conducted according to the principles of Helsinki and approved by the Ethics Committee of Zhongnan Hospital of Wuhan University (No. 2020074).
2.2. Data collection
Epidemiological, clinical, laboratory, radiological characteristics, treatment and outcomes data were obtained from electronic medical records with standardized data collection forms. The data were reviewed by a trained team of physicians. COVID-19 was defined as a positive result on high throughput sequencing or real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of nasal and pharyngeal swab specimens [3]. The targeted region of RT-PCR for viral RNA detection included open reading frame 1ab (ORF1ab) and nucleocapsid protein (N). Target 1 (ORF1ab): forward primer CCCTGTGGGTTTTACACTTAA; reverse primer ACGATTGTGCATCAGCTGA; and the probe 5’-VIC-CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1-3’. Target 2 (N): forward primer GGGGAACTTCTCCTGCTAGAAT; reverse primer CAGACATTTTGCTCTCAAGCTG; and the probe 5’-FAM- TTGCTGCTGCTTGACAGATT-TAMRA-3’. Radiologic assessments included chest radiography or computed tomography (CT). Laboratory examinations consisted of complete blood count, serum biochemical tests (including liver and renal function, creatine kinase MB isoenzyme and electrolytes), coagulation profile, C-reactive protein (CRP) and procalcitonin (PCT). Anti-SARS-CoV-2 antibodies (IgG and IgM) were also tracked. IgM and IgG antibodies were measured with COVID-19 IgM/IgG chemiluminescence test kit (Shenzhen Yahuilong Biotechnology Co., Ltd., China). Tests were performed on iFlash 3000 automated analyzer. The durations from illness onset to complications, hospital admission, discharged or death were recorded. The criteria for discharge were absence of fever for at least 3 days, substantial improvement in both lungs in chest CT, clinical remission of respiratory symptoms, and two throat-swab samples negative for SARS-CoV-2 RNA obtained at least 24 h apart.
2.3. Definitions
Fever was defined as axillary temperature of 37·3 °C or higher. Septic shock was defined according to the 2016 Third International Consensus Definition [3]. Acute kidney injury (AKI) was identified according to the Kidney Disease: Improving Global Outcomes definition [11,12]. Acute liver injury (ALI) was defined if liver enzymes or bilirubin were more than twice the upper limit of normal [13,14]. Acute myocardial injury (AMI) was defined if the serum levels of cardiac biomarkers were above the 99th percentile upper reference limit or new abnormalities were shown in electrocardiography and echocardiography [3]. Coagulopathy was defined as a 3-s extension of prothrombin time or a 5-s extension of activated partial thromboplastin time [8]. The illness severity of COVID-19 was defined according to the Chinese management guideline for COVID-19 (version 7·0) [15]. COVID-19 patients are stratified as follows: mild (i.e., mild clinical symptoms without imaging feature of pneumonia), ordinary (i.e., clinical symptoms such as fever, cough, with imaging feature of pneumonia), severe (i.e., dyspnea, respiratory frequency ≥30/min, blood oxygen saturation ≤93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300, and/or lung infiltrates > 50% within 24 to 48 h), and critical ill cases (i.e., respiratory failure, septic shock, and/or multiple organ dysfunction or failure). In this analysis, severe cases included the severe and critical ill classification in the 7th Edition. Survival time was defined as from illness onset to death.
2.4. Statistical analysis
Normality of distribution of continuous variables was tested by the Kolmogorov–Smirnov test. We used the Chi-square test or Fisher's exact test for categorical variables and t or Mann–Whitney U test for continuous variables to compare differences between survivors and non-survivors by IBM SPSS statistics software, version 25·0. The cut-off value of continuous variables was defined based on the receiver operating characteristic (ROC) curve with optimal sensitivity and specificity. In training cohort, there are few missing values for each variable, and the proportion of missing values does not exceed 10%, so multiple imputation methods were applied to supplement missing values to obtain complete data using “mice” package with R version 3·6·3 software. Then the least absolute shrinkage and selection operator (LASSO) regression was performed to identify variables with non-zero coefficients using package of “glmnet”. The coefficients and multivariable analyses were used to generate a prediction model. To quantify discrimination performance of the model, ROC curve was measured with “rms” package in R software. By comparing observed actual data with the predicted probability of the model, calibration curves were plotted, accompanied with the Hosmer–Lemeshow test. Cumulative risk events were estimated in training and independent validation cohort using the Kaplan–Meier method in high- and low-risk groups and the log-rank test to assess statistical significance of the difference. All reported P values were two-sided at significance-level of 0·05.
2.5. Role of the funding source
This work was supported by the Key Project for Anti-2019 novel Coronavirus Pneumonia from the Ministry of Science and Technology, China (grant number 2020YFC0845500).
3. Results
3.1. Demographics and clinical characteristics of total patients
In total, 2529 patients were hospitalized in Zhongnan Hospital, No. 7 Hospital of Wuhan and Leishenshan Hospital with COVID-19. A total of 1830 cases with complete data and defined outcomes were included, among of which 452 cases (26.7%) were severe. The severe patients have relatively high mortality rates in Zhongnan Hospital of Wuhan University (39.1%) and No. 7 Hospital of Wuhan (46.3%), while the mortality rate in Leishenshan Hospital (8.8%) is lower. Therefore, 113 severe patients from Zhongnan Hospital and No. 7 Hospital of Wuhan were included as training cohort to compare the characteristics of survivors and non-survivors, while 339 from Leishenshan Hospital were assigned to independent validation cohort. The demographic and clinical characteristics were shown in Tables 1, 2 and S1.
Table 1.
Demographic and laboratory findings in patients with COVID-19 on admission in training cohort.
| Characteristics | Severe patients (n=113) No (%) or Median (IQR) | Presence of severe |
P | |
|---|---|---|---|---|
| Cut off value | Survivors (n=64) No (%) or Median (IQR) | Non-survivors (n=49) No (%) or Median (IQR) | ||
| General characteristics | ||||
| Sex | 0·085 | |||
| Male | 73(64·6) | 37(57·8) | 36(73·5) | |
| Female | 40(35·4) | 27(42·2) | 13(26·5) | |
| Age (years) | 66·0(57·5–73·5) | 62·0(53·0–71·0) | 73·0(64·5–79·0) | <0·001 |
| <60·0 | 31(27·4) | 26(40·6) | 5(10·2) | <0·001 |
| 60·0–75·0 | 58(51·3) | 33(51·6) | 25(51·0) | |
| >75·0 | 24(21·2) | 5(7·8) | 19(38·8) | |
| Routine peripheral blood | ||||
| WBC (× 109) | 5·2(3·7–8·5) | 4·5(3·2–7·6) | 7·0(4·7–10·5) | 0·001 |
| <3·5 | 23(20·9) | 18(28·6) | 5(10·6) | 0·004 |
| 3·5–9·5 | 64(58·2) | 38(60·3) | 26 (55·3) | |
| >9·5 | 23(20·9) | 7(11·1) | 16(34·0) | |
| Neu (× 109) | 4.0(2·5–7.4) | 3·2 (2·2–5·3) | 5·2(3·3–8·6) | 0·001 |
| >6·3 | 34(30·9) | 13(21·6) | 21(44·7) | 0·007 |
| Neu% (%) | 78·5(68·3–88·6) | 75·1(64·4–83·3) | 87·1(73·9–91·7) | 0·001 |
| >85·0 | 39(35·8) | 12(19·4) | 27(57·4) | <0·001 |
| Lym(× 109) | 0·7(0·5–1·0) | 0·7(0·6–1·0) | 0·6(0·4–1·0) | 0113 |
| <0·5 | 29(26·4) | 8(12·7) | 21(44·7) | <0·001 |
| Lym% (%) | 13·6(6·3–21·6) | 16·4(10·3–25·1) | 7·5(4·7–16·6) | <0·001 |
| <8·0 | 35(31·8) | 10(15·9) | 25(53·2) | <0·001 |
| Mon (× 109) | 0·4(0·2–0·5) | 0·4(0·2–0·5) | 0·4(0·3–0·5) | 0·929 |
| 0·1–0·6 | 88(80·7) | 52(83·9) | 36(76·6) | 0·340 |
| Mon% (%) | 6·2(4·3–9·7) | 7·5(5·0–10·7) | 5·0(3·5–6·9) | 0·001 |
| ≤6·0 | 52(47·7) | 21(33·9) | 31(66·0) | 0·001 |
| HGB (g/L) | 129·0(119·0–139·2) | 131·4(121·3–139·1) | 129·0(117·8–140·0) | 0·483 |
| Anemia | 42(38·5) | 21(33·9) | 21(44·7) | 0·251 |
| PLT (× 109) | 151·5(118·0–204·0) | 160·0(121·0–237·0) | 142·0(114·0–181·0) | 0·155 |
| <150·0 | 55(50·0) | 28(44·4) | 27(57·4) | 0·177 |
| Blood biochemical examination | ||||
| ALT (IU/L) | 34·0(20·0–47·0) | 28·0(18·5–41·5) | 35·5(25·0–48·8) | 0·078 |
| >40·0 | 32(29·9) | 15(24·6) | 17(37·0) | 0·167 |
| AST (IU/L) | 35·5(25·0–59·3) | 31·0(22·5–62·5) | 40·0(29·8–57·0) | 0·182 |
| >40·0 | 42(39·6) | 22(36·7) | 20(43·5) | 0·477 |
| TP (g/L) | 64·4(60·2–68·6) | 65·0(60·8–70·9) | 63·8(59·0–66·2) | 0·074 |
| <65·0 | 62(55·4) | 32(50·0) | 30(62·5) | 0·188 |
| Albumin (g/L) | 35·1(31·9–38·7) | 37·0(33·1–39·7) | 33·4(29·7–36·8) | 0·003 |
| <35·0 | 55(49·1) | 25(39·1) | 30(62·5) | 0·014 |
| Globulin (g/L) | 28·8(26·6–32·2) | 28·5(25·7–31·8) | 29·4(27·7–32·6) | 0·119 |
| >30·0 | 44(39·3) | 21(32·8) | 23(47·9) | 0·105 |
| UA (umol/L) | 251·3(212·4–371·2) | 245·0(202·6–319·2) | 288·0(230·0–415·6) | 0·052 |
| >428·0 | 16(14·5) | 7(11·1) | 9(19·1) | 0·237 |
| CREA (umol/L) | 70·0(57·7–89·4) | 70·0(55·0–81·7) | 73·0(64·5–106·4) | 0·046 |
| >133·0 | 7(6·4) | 2(3·2) | 5(10·6) | 0·233 |
| Urea (mmol/L) | 5·8(4·3–8·7) | 5·0(4·0–6·2) | 8·4(5·8–14·3) | <0·001 |
| >7·6 | 39(35·3) | 11(17·5) | 28(59·6) | <0·001 |
| Inflammatory indicators and coagulation function | ||||
| PCT (ng/ml) | 0·09(0·05–0·2) | 0·05(0·04–0·1) | 0·2(0·09–0·7) | <0·001 |
| >0·15 | 33(31·7) | 5(8·5) | 28(62·2) | <0·001 |
| CRP (mg/L) | 57·4(25·1–101·6) | 42·9(21·3–68·3) | 83·5(38·8–165·5) | <0·001 |
| >55·0 | 54(52·4) | 22(37·9) | 32(71·1) | 0·001 |
| CKMB (ng/ml) | 13·2(5·5–21·3) | 9·6(3·1–15·2) | 17·0(12·2–26·9) | <0·001 |
| >15·0 | 41(40·2) | 14(24·6) | 27(60·0) | <0·001 |
| D-dimer (ug/ml) | 0·4(0·2–2·8) | 0·23(0·15–0·40) | 3·4(0·5–16·7) | <0·001 |
| >0·5 | 42(44·2) | 12(22·2) | 30(73·2) | <0·001 |
| TT (s) | 14·7(13·8–15·8) | 14·6(13·7–15·3) | 14·7(14·1–17·1) | 0·057 |
| >16·8 | 12(11·9) | 2(3·4) | 10(23·3) | 0·002 |
| PT (s) | 13·1(12·3–13·8) | 13·1(12·3–13·6) | 13·3(12·2–14·9) | 0·339 |
| >12·5 | 60(59·4) | 35(60·3) | 25(58·1) | 0·823 |
ALT, Alanine aminotransferase; AST, Aspertate Aminotransferase; CKMB, MB isoenzyme of creatine kinase; CREA, Creatinine; CRP, C reactive protein; HGB, hemoglobin; IQR, interquartile range; Lym, lymphocyte count, LYM%; percentage of lymphocytes; Mon, monocyte count; Mon%, percentage of monocytes· Neu, neutrophil count; Neu%, percentage of neutrophils; PCT, procalcitonin; PLT, platelet; PT, prothrombin time; TP, total protein; TT, thrombin time; UA, Uric acid; WBC, white blood cell.
Table 2.
Clinical, radiographic findings and treatment in severe COVID-19 on admission in training cohort.
| Characteristics | Severe patients No (%) | Presence of severe |
P | |
|---|---|---|---|---|
| Survivors (n=64) | Non-survivors (n=49) | |||
| Coexisting conditions | ||||
| Any | 72(63·7) | 34(53·1) | 38(77·6) | 0·007 |
| Hypertension | 50(44·2) | 24(37·5) | 26 (53·1) | 0·099 |
| Diabetes | 20(17·7) | 5(7·8) | 15(30·6) | 0·002 |
| Coronary heart disease | 28(24·8) | 8(12·5) | 20(40·8) | 0·001 |
| Chronic obstructive pulmonary disease | 5(4·4) | 1(1·6) | 4(8·2) | 0·219 |
| Tumor | 8(7·1) | 3(4·7) | 5(10·2) | 0·445 |
| Renal diseases | 9(8·0) | 2(3·1) | 7(14·3) | 0·069 |
| Liver disease | 8(7·1) | 3(4·7) | 5(10·2) | 0·445 |
| Fever | 88(77·9) | 53(82·8) | 35(71·4) | 0·149 |
| <37·3 °C | 25(22·1) | 11(17·2) | 14(28·6) | 0·345 |
| 37·3–38·0 °C | 34(30·1) | 18(28·1) | 16(32·7) | |
| 38·01–39·0 °C | 46(40·7) | 30(46·9) | 16(32·7) | |
| >39·0 °C | 8(7·1) | 5(7·8) | 3(6·1) | |
| Symptoms | ||||
| Chills | 12(10·6) | 7(10·9) | 5(10·2) | 0·900 |
| Cough | 74(65·5) | 43(67·2) | 31(63·3) | 0·664 |
| Expectoration | 39(34·5) | 23(35·9) | 16(32·7) | 0·716 |
| Sore throat | 6(5·3) | 4(6·3) | 2(4·1) | 0·931 |
| Fatigue | 48(42·5) | 23(35·9) | 25(51·0) | 0·108 |
| Myalgia or arthralgia | 8(7·1) | 8(12·5) | 0 | 0·028 |
| Diarrhoea | 9(8·0) | 5(7·8) | 4(8·2) | 1·0 |
| Nausea or vomiting | 7(6·2) | 5(7·8) | 2(4·1) | 0·673 |
| Shortness of breath | 45(39·8) | 21(32·8) | 24(49·0) | 0·082 |
| Stuffiness | 31(27·4) | 15(23·4) | 16(32·7) | 0·277 |
| Gasping | 24(21·2) | 10(15·6) | 14(28·6) | 0·095 |
| Coinfection status at admission | ||||
| Virus infections | 19(18·3) | 6(10·2) | 13(28·9) | 0·014 |
| Mycoplasma | 6(5·7) | 1(1·7) | 5(10·9) | 0·113 |
| Chlamydia | 2(1·9) | 0 | 2(4·3) | – |
| Influenza A | 5(4·8) | 1(1·7) | 4(8·7) | 0·226 |
| Influenza B | 8(7·6) | 3(5·1) | 5(10·9) | 0·461 |
| Respiratory syncytial virus | 4(3·8) | 3(5·1) | 1(2·2) | 0·795 |
| Adenovirus | 2(1·9) | 0 | 2(4·3) | – |
| Klebsiella pneumoniae | 3(2·9) | 0 | 3(6·5) | 0·162 |
| Radiologic findings on chest | – | |||
| Normal | 0 | 0 | 0 | |
| Abnormal | 107(100·0) | 64(59·8) | 43(40·2) | |
| Treatments | ||||
| Antibiotics | 109(96·5) | 62(96·9) | 47(95·9) | 1·0 |
| Antiviral drugs | 97(85·8) | 57(89·1) | 40(81·6) | 0·262 |
| Systemic glucocorticoids | 75(66·4) | 35(54·7) | 40(81·6) | 0·003 |
| Intravenous immunoglobin | 28(24·8) | 14(21·9) | 14(28·6) | 0·414 |
| Mechanical ventilation | 38(33·6) | 7(10·9) | 31(63·3) | <0·001 |
| Invasive | 14(12·4) | 1(1·6) | 13(26·5) | <0·001 |
In training cohort, there were 49 non-survivors during hospitalization. The median age of severe patients was 66 years (Range: 24–96 years; interquartile ranges: 57·5–73·5 years), while it was 73·0 years for the non-survivors. The number of men was more than women (Table 1). Patients aged 60–75 years accounted for the largest proportion, with a proportion of 51·3%. Among the population in training cohort, 72 (63·7%) patients had at least one underlying comorbidity, the most common of which were hypertension (44·2%) followed by coronary heart disease (24·8%) and diabetes (17·7%) (Table 2). The coronary heart disease (CHD) or diabetes had statistical differences between survivors and non-survivors. All severe COVID-19 had abnormal radiologic findings on chest in present study. Nineteen patients (18·3%) had co-infection with the virus on admission, of which Influenza B co-infection (7.6%) was the most common (Table 2).
3.2. The treatment and outcomes of severe COVID-19
For treatment, 109 (96·5%) patients received antibiotics and 97 (85·8%) received antiviral drugs (Table 2). Intravenous immunoglobulin use was in 24·8%. Systematic corticosteroid application accounted for 54.7% in survivors, while it was 81.6% in non-survivors (P=0·003). The comparison of treatment, and outcomes in training cohort were shown in Tables 2 and 3. Respiratory failure (41·6%) was the most frequently observed complication, followed by AMI (38·9%), ALI (25·7%) and AKI (23·0%) (Table 3). The secondary infection occurred in 16·8% patients. Sepsis and acidosis all occurred in non-survivors. The frequency of complications was higher in non-survivors than survivors (all P<0·05, Table 3).
Table 3.
Comparisons of outcomes and durations between survivors and non-survivors or different risk group in patients with severe COVID-19.
| Characteristics | Patients (n=113) No. (%) or Median (rang) | Presence of severe |
P | Hazard subgroup |
P | ||
|---|---|---|---|---|---|---|---|
| Survivors (n=64) No. (%) or Median (rang) | Non-survivors (n=49) No. (%) or Median (rang) | Low risk (n=60) No. (%) or Median (rang) | High risk (n=53) No. (%) or Median (rang) | ||||
| Complications-n (%) | |||||||
| Sepsis | 9(8·0) | 0 | 9(18·4) | 0·001 | 3(5·0) | 6(11·3) | 0·373 |
| Respiratory failure | 47(41·6) | 7(10·9) | 40(81·6) | <0·001 | 6(10·0) | 41(77·4) | <0·001 |
| Acute myocardial injury | 44(38·9) | 7(10·9) | 37(75·5) | <0·001 | 8(13·3) | 36(67·9) | <0·001 |
| Acute kidney injury | 26(23·0) | 6(9·4) | 20(40·8) | <0·001 | 6(10·0) | 20(37·7) | <0·001 |
| Acute liver injury | 29(25·7) | 8(12·5) | 21(42·9) | <0·001 | 9(15·0) | 20(37·7) | 0·006 |
| Acidosis | 21(18·6) | 0 | 21(42·9) | <0·001 | 3(5·0) | 18(34·0) | <0·001 |
| Secondary infection | 19(16·8) | 6(9·4) | 13(26·5) | 0·016 | 5(8·3) | 14(26·4) | 0·010 |
| Coagulopathy | 21(18·6) | 4(6·3) | 17(34·7) | <0·001 | 7(11·7) | 14(26·4) | 0·044 |
| Durations-median (rang) | |||||||
| Time from illness onset to admission | 7·0(0–41·0) | 7·0(1·0–41·0) | 8·0(0–30·0) | 0·958 | 7·0(1·0–41·0) | 8·0(0–30·0) | 0·411 |
| Time from illness onset to death or discharge | 26·0(2·0–67·0) | 30·0(11·0–67·0) | 21·0(2·0–54·0) | <0·001 | 30·0(11·0–67·0) | 22·0(2·0–52·0) | 0·005 |
| Time from illness onset to sepsis | 22·0(8·0–31·0) | NA | 22·0(8·0–31·0) | – | 25·0(22·0–31·0) | 21·5(8·0–28·0) | 0·262 |
| Time from illness onset to respiratory failure | 11·0(2·0–35·0) | 12·0(8·0–33·0) | 10·0(2·0–35·0) | 0·256 | 14·0(11·0–33·0) | 10·0(2·0–35·0) | 0·056 |
| Time from illness onset to acute myocardial injury | 14·5(2·0–34·0) | 14·0(7·0–25·0) | 15·0(2·0–34·0) | 0·950 | 17·0(8·0–26·0) | 13·5(2·0–34·0) | 0·247 |
| Time from illness onset to acute kidney injury | 16·5(2·0–52·0) | 11·5(2·0–52·0) | 17·0(2·0–37·0) | 0·355 | 16·5(2·0–52·0) | 16·5(2·0–37·0) | 0·929 |
| Time from illness onset to acute liver injury | 16·0(2·0–39·0) | 13·0(4·0–31·0) | 17·0(2·0–39·0) | 0·301 | 17·0(4·0–31·0) | 12·0(2·0–39·0) | 0·871 |
| Time from illness onset to acidosis | 19·0(5·0–35·0) | NA | 19·0(5·0–35·0) | – | 18·0(17·0–20·0) | 19·0(5·0–35·0) | 0·814 |
| Time from illness onset to secondary infection | 13·0(4·0–25·0) | 12·0(4·0–25·0) | 13·0(4·0–25·0) | 0·701 | 10·0(4·0–25·0) | 14·5(4·0–25·0) | 0·500 |
| Time from illness onset to coagulopathy | 19·0(4·0–41·0) | 14·5(11·0–22·0) | 20·0(4·0–41·0) | 0·144 | 17·0(11·0–27·0) | 20·5(4·0–41·0) | 0·322 |
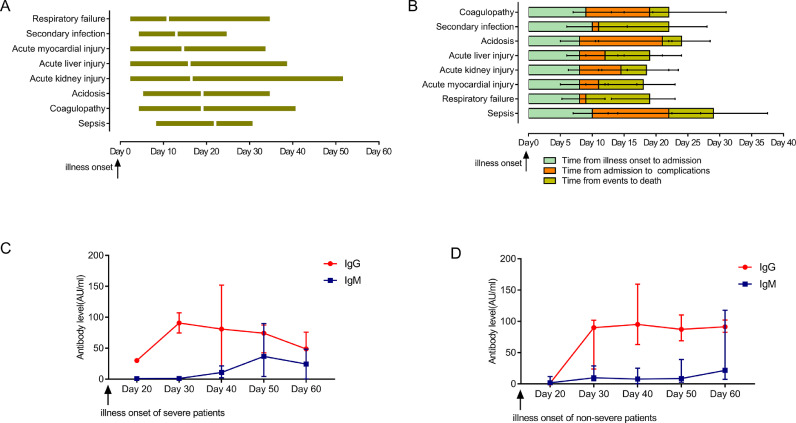
Median time from illness onset to admission was similar in survivors and non-survivors (P=0·958) with a median duration of 7·0 days (1·0–41·0) for survivors and 8·0 days (0–30·0) for non-survivors (Table 3). The median time from illness onset to discharge was 30·0 days (Range: 11·0–67·0; interquartile ranges: 23·0–38·0) for survivors, whereas the median time to death was 21·0 days (Range: 2·0–54·0; interquartile ranges: 15·0–31·5) for non-survivors (P<0·01). The median time from illness onset to complication had no statistical differences between survivors and non-survivors (all P>0·05, Table 3). Median time from illness onset to progression of respiratory failure was 11·0 days, followed by secondary infection (13·0 days), AMI (14.5 days), ALI (16·0 days), AKI (16·5 days), acidosis (19·0 days), coagulopathy (19·0 days) and sepsis (22·0 days) (Table 3, Fig. 1(A)). Among non-survivors, the median time from illness onset to admission, from admission to progression of complication, from complication occurred to death was shown in Fig. 1(B). The median time from coagulopathy or acidosis to death was the shortest (3 days), followed by AKI, ALI, AMI, sepsis, respiratory failure and secondary infection.
Fig. 1.
Dynamic profile of complication, clinical course, and anti-SARS-CoV-2 antibody. Dynamic profile of complication from illness onset in patients with severe COVID-19 (A). Timeline of non-survivors from illness onset to death (B). Dynamic profile of Anti-SARS-CoV-2 antibody in severe COVID-19 (C). Dynamic profile of Anti-SARS-CoV-2 antibody in non-severe COVID-19 (D). The bars represent median with interquartile range.
3.3. Temporal profiles of serum antibody responses
Of cases from Zhongnan Hospital, 56 non-severe serums and 15 severe serums were tested for anti-SARS-CoV-2 antibodies from patients with COVID-19. The IgG and IgM levels of severe and non-severe patients were shown in Fig. 1(C) and (D). The maximum time for antibody monitoring was up to 54 days from illness onset. Antibody levels between severe and non-severe patients had no statistical differences (P=0.179 for IgG, P=0.926 for IgM). IgG levels reached the highest during 20–30 days both in severe and non-severe COVID-19 patients. In severe patients, the level of IgG gradually declined after 30 days, while in non-severe patients it remained stable. The IgM levels stabilized at low levels in non-severe patients, whereas it gradually increased after 30 days from the onset of symptoms in severe patients, and then declined after 50 days.
3.4. Risk factors and prediction model for death in severe cases
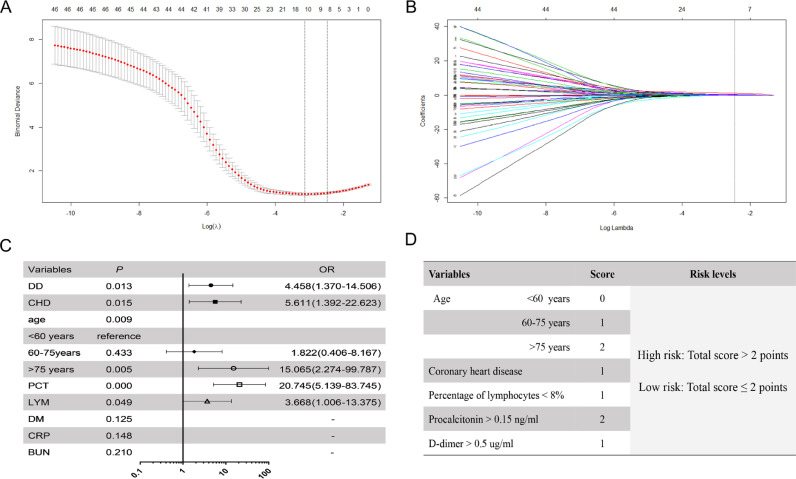
In total, 52 characteristics were analyzed by LASSO binary logistic regression in training cohort, and 8 candidate variables that were reliably associated with survival were selected (Fig. 2). The weight for each factor associated with infection were obtained by calculating coefficients when log(λ)=−2·475 and λ=0·0841 from LASSO binary logistic regression model (Fig. 2(A) and (B)). The coefficients for each parameter were as follows: 0·4565 for Age, 0·0997 for diabetes, 0·2487 for CHD, 0·1760 for percentage of lymphocytes (LYM%),<8%, 1·371 for PCT>0·15 ng/ml, 0·2150 for blood urea nitrogen (BUN)>7·6 mmol/L, 0·0815 for C reactive protein (CRP) >55 mg/L and 0·5865 for D-dimer (DD) >0·5 ug/ml. Then, by multivariable analysis, Age, CHD, LYM%, PCT and DD remained independent risk factors for mortality, and hazard ratios (HRs) of the five variables were shown in Fig. 2(C).
Fig. 2.
Clinical feature selection using the least absolute shrinkage and selection operator (LASSO) binary logistic regression model and multivariable analysis in the training cohort. Eight variables selected by LASSO binary logistic regression analysis. Two dotted vertical lines mark the optimal values by minimum criteria and 1-s.e. criteria (A). LASSO coefficient profiles of the 52 variables. A vertical line indicates the optimal value based on the 1-s.e. criterion giving five non-zero coefficients (B). The hazard ratio (HR) for each of 8 variables by the multivariable analysis (C). The score of each variable (D).
According to multivariable analysis and coefficients of Lasso binary logistic regression, a scoring system of COVID-19 (CSS) was generated as shown in Fig. 2(D). The score distributions were as follows: age between 60 and 75 points was 1 point, and those older than 75 was 2 points, CHD condition was 1 point, LYM% <8% was 1 point, PCT>0·15 ng/ml was 2 points and DD>0·5 ug/ml was 1 point. We then defined a total of 0 to 2 points as low risk, and total points more than 2 as high risk. The cut-off value between these groups was selected based on the best sensitivity/specificity ratio.
3.5. Performance of the prediction model in the training and independent validation cohort
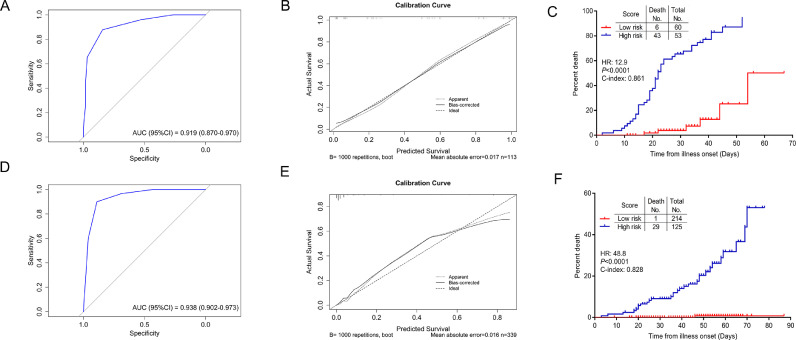
The model showed great prognostic accuracy in both training cohort and independent validation cohort by using ROC analysis (Fig. 3(A) and (D)). Performance of this model was validated by calibration plots for the probability of death, which demonstrated good agreement between prediction and actual observation in training cohort (Fig. 3(B)). The Hosmer–Lemeshow test yielded a nonsignificant statistic (P=0·264), indicating that there was no departure from perfect fit. For illustration, the cumulative risk events were performed in training and independent validation cohorts, which showed significantly increased deaths in high-risk group compared with the low-risk group (Fig. 3(C) and (F)).
Fig. 3.
The performance of the scoring system to predict mortality of severe COVID-19 patients. ROC curves and AUCs to assess the prediction accuracy in the training cohort (A) and the independent validation cohort (D). Calibration curves of the model in the training cohort (B) and the independent validation cohort (E). Cumulative risk events for high- and low-risk groups in the training cohort (C) and the independent validation cohort (F). AUC, area under the curve, ROC, receiver operating characteristic.
3.6. Predictive value of COVID-19 scoring system
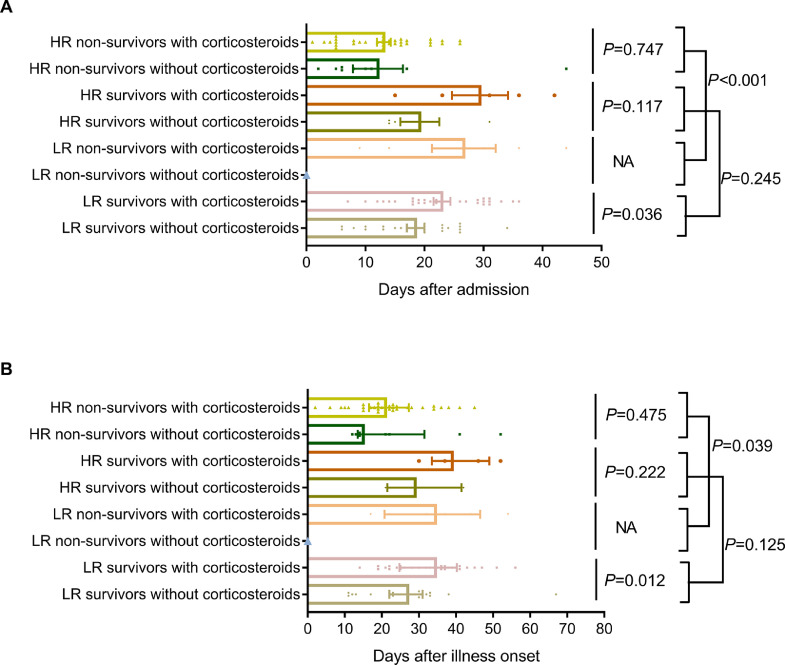
In low-risk group, the number of patients was 60 with 6 non-survivors, and in high-risk group, the number of patients was 53 with 43 non-survivors. The mortality rates were 10·0% in low-risk group and 81·1% in high-risk group (P<0·01). There were also statistical differences in complications in low-risk and high-risk groups except for sepsis. In low-risk group, the median time from illness to death was 34·5 days, whereas it was 21·0 days in high-risk group (P=0·039, Fig. 4(B)), indicating that low-risk patients had a longer survival time. For survivors, the median time from illness to discharge was 20·0 days for low-risk group, and 22·5 for high-risk group (P=0·125, Fig. 4(B)). In low-risk group, 30 survivors received systemic corticosteroids treatment and 24 survivors did not, whose mean hospital durations were 23·0 days and 18·5 days respectively (P=0·036, Fig. 4(A)), and the median time from illness onset to discharge was 34·5 days and 27·0 days (P=0·012, Fig. 4(B)). In high-risk group, 34 non-survivors received systemic corticosteroids treatment and 9 non-survivors did not, and their median survival time were 21·0 days and 15·0 days respectively (P=0·475, Fig. 4(B)). In conclusion, in low-risk group, the absence of systemic corticosteroids could shorten duration of disease by 7·5 days and hospital duration by 4·5 days, meanwhile, whether to use of systemic corticosteroids in high-risk group had no effect on survival.
Fig. 4.
Comparison of durations in different risk groups between groups with corticosteroids and without corticosteroids. Comparison of hospitalization days in different risk groups between groups with corticosteroids and without corticosteroids (A). The bars represent mean with Standard Error of Mean (SEM). Comparison of survival time in different risk groups between groups with corticosteroids and without corticosteroids (B). The bars represent median with interquartile range.
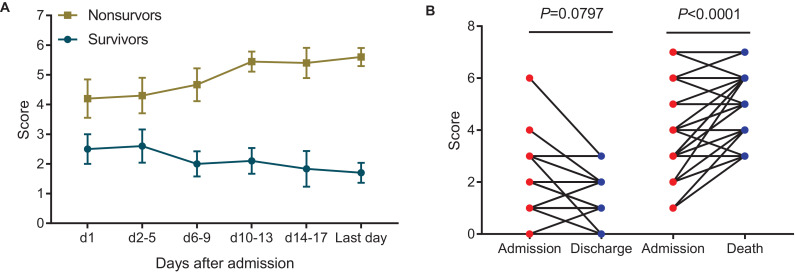
3.7. Dynamic profile of points and comparison of scores between admission and outcomes in severe COVID-19 patients
Data from 20 patients with complete clinical course (including 10 severe survivors, 10 severe non-survivors) were analyzed to track scores from admission to discharge or death (Fig. 5(A)). Among survivors, scores gradually decreased during hospitalization until discharge, whereas scores gradually increased until death in non-survivors. Meanwhile, the last laboratory findings with LYM%, DD, PCT were recorded to obtain discharge or death scores. Finally, 36 non-survivors and 36 survivors were included to compare scores between admission and discharge or death (Fig. 5(B)). For non-survivors, scores at death was higher than that at admission (P<0.0001). For survivors, scores at discharge seemed lower than that at admission, but the difference was not statistically significant (P=0.080).
Fig. 5.
Dynamic profile of points and comparison of scores between admission and outcomes in severe COVID-19. Dynamic profile of points in non-survivors and survivors (A). The bars represent mean with Standard Error of Mean (SEM). Comparison of scores between admission and discharge or death in severe COVID-19 patients (B).
4. Discussion
In this retrospective cohort study, the median time from illness onset to death was 21·0 days for severe COVID-19 non-survivors. A total of 60·2% patients had complications, and the proportion in non-survivors was higher than that of survivors (P<0·05). It was reported that organ damage during hospitalization in patients with COVID-19 was associated with in-hospital mortality [16], [17], [18]. Respiratory failure (41·6%) was the most common complication followed by AMI (38·9%), ALI (25·7%) and AKI (23·0%). For non-survivors, respiratory failure and secondary infection occurred firstly, followed by AMI, AKI, ALI. AMI occurred earlier than AKI and ALI, and that heart becomes the first organ involved might be explained by the fact that the cardiac highly expressed angiotensin-converting enzyme 2 (ACE2) which was a human cell receptor with a strong binding affinity to the Spike protein of SARS-CoV-2, leading to directly attacked by the virus [17,19,20]. COVID-19-induced kidney and liver injury might be related to drug hepatotoxicity or subsequent immune-mediated inflammation, such as cytokine storm, pneumonia-associated hypoxia and shock [16,18,21]. It was worth noting that time from AKI to death was shorter than that of AMI or ALI. Cui and co-workers indicated that cytopathic effects of SARS-CoV-2 on podocytes and proximal straight tubule cells may cause AKI in patients with COVID-19 [22]. The early monitoring of renal function should be paid more attention.
The study identified several factors associated with mortality in severe patients with COVID-19. The aged, CHD condition, decreased LYM%, elevated levels of PCT and DD on admission were associated with high odds of in-hospital death. All non-survivors scored no less than 3 points before death, and dynamic profile of points showed that a high score indicated progression of disease and a higher risk of death (Fig. 5). CSS was established to divide mortality risk of severe cases into high-risk and low-risk groups. The risk of death in high-risk group increased significantly (P<0·01), and there were also statistical differences in complications between low-risk and high-risk group. The CSS demonstrated good predictive performance with ROC curve (AUC=0·919 in training cohort and 0·938 in independent validation cohort) and Calibration curve (P=0·264 in training cohort) analysis. In validation cohort, CSS seemed to underestimate mortality of low-risk patients but overestimate mortality of high-risk patients in the calibration curve (Fig. 3(D)). It could be explained by the low mortality rate in Wuhan Leishenshan Hospital since some critically ill patients were transferred to other hospitals to reduce mortality, which lead to the predicted death inconsistent with the actual observed death. The data from Wuhan Leishenshan Hospital might not reflect the actual mortality rate of severe COVID-19 in Wuhan or China.
The elderly was considered an important independent predictor of mortality in SARS and MERS, and also has been confirmed to be associated with mortality in patients with COVID-19 [8,[23], [24], [25]], which was also confirmed in current study. The mortality rate was the highest in patients with severe COVID-19 older than 75 years (79·2%), followed by patients aged 60–75 years old (43·1%) and younger than 60 years (16·1%). It was worth noting that the number of patients aged 60–75 years was the largest (Table 1). The elderly patients with COVID-19 deserved special attention.
Myocardial injury was reported to be a common complication and significantly associated with fatal outcome of COVID-19 [17,26,27]. Lu et al. demonstrated that patients with underlying cardiovascular disease including CHD was more prone to experience myocardial injury during the course of COVID-19 and higher risk of death [27]. CHD has also been found to be associated with acute cardiac events and poor outcomes in influenza and other respiratory viral infections [28,29]. In present study, CHD was one of independent risk factors to patients with COVID-19. The mechanism might be that the release of inflammatory cytokines after infection probably caused reduction in coronary blood flow, decreased in oxygen supply, destabilization of coronary plaque, and microthrombogenesis, leading to myocardial injury and heart failure [17,19,20].
The majority of patients died of multiple organ failure. Overexpression of proinflammatory cytokines and chemokines released by activated immune cells and infected cells was involved in the development of organ dysfunction in SARS patients and the COVID-19 mortality [30]. In this analysis, PCT, one of the inflammatory indicators, had a strong correlation with mortality of severe COVID-19 patients, and D-dimer greater than 0·5 μg/mL was also associated with fatal outcome. Elevated D-dimer levels indicating the coagulation activation was considered to be related to sustained inflammatory response. Consequently, COVID-19 mortality might be due to virus-activated cytokine storm. It was valuable to incorporate inflammation indicators into the scoring system to predict the risk of mortality.
Lymphopenia was believed to be related to increased disease severity and decease in COVID-19 patients [31]. Lymphocytopenia was also common in the critically ill patients with MERS infection, which was considered the result of lymphocytes apoptosis [32,33]. Therefore, it could be postulated that necrosis or apoptosis of lymphocytes also induced lymphocytopenia in severe patients with SARS-CoV-2 infection [31]. The severity of lymphocytopenia reflected the severity of COVID-19 and possessed the ability to predict mortality as a powerful predictor [4]. Besides, the decreased LYM% partly reflected an increase in the proportion of neutrophils, which may be related to cytokine storm induced by virus invasion.
This analysis also tracked antibodies response in serum of patients with COVID-19, which was considered the longest tracking to our best knowledge. It can be seen from Fig. 1 that IgG reached its peak at about 20–30 days both in severe and non-severe COVID-19 patients. In severe patients, the level of IgG declined after 30 days, which might be due to lymphocyte depletion [32]. That IgM levels were still high after 30 days from illness onset in severe patients might be explained that viruses existed in severe patients for a relatively long time [34]. It was reported that the serum antibody in COVID-19 had potential diagnostic value, and a higher titer of antibody was independently associated with a worse clinical classification [35,36]. But there was no statistically significant difference in antibody levels between severe and non-severe patients in this analysis. The number of severe cases was small in present study, which might result in less accurate results. More cases and time period tests are needed to verify.
No antiviral treatment for coronavirus infection has been proven to be effective. Given the high levels of cytokines induced by SARS-CoV, MERS-CoV and SARS-CoV-2 infections, systemic corticosteroids were often used to treat severe patients, for possible benefit by reducing inflammatory-induced lung injury [37], [38], [39], [40]. However, it was controversial to use corticosteroids for treating influenza-associated pneumonia or ARDS [41]. Several studies demonstrated the association of corticosteroid use with mortality, bacterial and fungal infection and the emergence of antiviral resistance [24,42]. Some evidence show that receiving corticosteroids in patients with SARS and MERS did not have an effect on mortality, but rather delayed viral clearance [43], [44], [45], [46]. Further evidence was urgently needed to assess whether systematic corticosteroid treatment was beneficial or harmful for patients with COVID-193. In present cohort, 16·8% of severe COVID-19 patients had complications of secondary infection, but it was not associated with the use of corticosteroids, and the difference was not statistically significant (P=0·203). The secondary infection may be due to the immune deficiency caused by virus [47]. In addition, whether to use corticosteroids had no effect on mortality, but the use of corticosteroids in low-risk group prolonged hospital stay by 4·5 days and durations by 7·5 days. The CSS provided some guidance for the rational application of corticosteroids and the applicable populations.
Our study had several limitations. Firstly, only 113 severe patients with COVID-19 were included in the training cohort, and in independent validation group, the number of deaths was small. Larger cohort studies are needed to validate the scoring system we have established. Secondly, as a retrospective study, some possible risk factors reported by other studies such as lactate dehydrogenase, and interleukin 6 were not presented in the study because not all laboratory tests were done in all patients resulting in the incomplete data [48]. Their role might be underestimated in predicting in-hospital death. It was reasonable to assume that other possible risk factors not included to analyses might be independently associated with in-hospital death, but the current model based on the five variables has shown good performance to predict clinical outcomes. Thirdly, since the vast majority of chest imaging findings were bilateral lung lesions [3], we only used the presence of lung lesions on admission as a positive indicator of chest imaging, without considering the severity of lungs, which may underestimate the role of lung lesions. A predictive model of combined lung lesions also needs to be evaluated. Finally, the data for score development and validation are entirely from Wuhan, China. Due to differences in medical resources, the data in this study may not be fully representative of the population in China or even the world, which could potentially limit the generalizability of the risk score in other areas of the world. Additional validation studies from areas outside China should be completed. However, in the absence of a severe COVID-19 death risk scoring system, this scoring system can help clinicians worldwide to judge in-hospital deaths.
In conclusion, complications were associated with mortality of COVID-19. The time from AKI to death was the shorter than that of ALI and AMI. Early monitoring of renal function should be paid more attention. The dynamic changes of antibodies were worth tracking, since a higher titer of antibody was considered to be independently associated with a worse clinical classification. We found that the aged, CHD condition, decreased percentage of lymphocyte, elevated D-dimer and PCT at admission were related to mortality of severe patients with COVID-19. The CSS well classified severe patients into low-risk and high-risk groups, which was helpful for predicting in-hospital mortality and complications. CSS could help clinicians to identify high-risk patients and be alert to the occurrence of complications.
Declaration of Competing Interest
All authors declare no competing interests.
Acknowledgments
Acknowledgments
We acknowledge all health-care workers participated in the diagnosis and treatment of COVID-19 patients in Zhongnan Hospital of Wuhan University.
Funding
This work was supported by the Key Project for Anti-2019 novel Coronavirus Pneumonia from the Ministry of Science and Technology, China (grant number 2020YFC0845500).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100426.
Contributor Information
Fuling Zhou, Email: zhoufuling@whu.edu.cn.
Xinghuan Wang, Email: wangxinghuan@whu.edu.cn.
Appendix. Supplementary materials
References
- 1.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323(8):709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of V The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahase E. Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. doi: 10.1136/bmj.m641. [DOI] [PubMed] [Google Scholar]
- 7.Fanidi A., Jouven X., Gaye B. Strategies to control COVID-19 and future pandemics in Africa and around the globe. Eur Heart J. 2020:ehaa278. doi: 10.1093/eurheartj/ehaa278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Xu S., Yu M. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. S0091-6749(20):30495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization; 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. [Google Scholar]
- 11.Ad-hoc working group of ERBP. Fliser D, Laville M. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transpl. 2012;27(12):4263–4272. doi: 10.1093/ndt/gfs375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey A.S., Levin A., Kellum J.A. Definition and classification of kidney diseases. Am J Kidney Dis. 2013;61(5):686–688. doi: 10.1053/j.ajkd.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11(2):272–276. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 14.Medina-Caliz I., Robles-Diaz M., Garcia-Munoz B. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J Hepatol. 2016;65(3):532–542. doi: 10.1016/j.jhep.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Health Commission of the People's Republic of China; 2020. Chinese management guideline for COVID-19 (version 7.0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020:e200950. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie H., Zhao J., Lian N., Lin S., Xie Q., Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40(6):1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan X.W., Xu D., Zhang H., Zhou W., Wang L.H., Cui X.G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intens Care Med. 2020;46(6):1114–1116. doi: 10.1007/s00134-020-06026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong K.H., Choi J.P., Hong S.H. Predictors of mortality in Middle East respiratory syndrome (MERS) Thorax. 2018;73(3):286–289. doi: 10.1136/thoraxjnl-2016-209313. [DOI] [PubMed] [Google Scholar]
- 24.Alfaraj S.H., Al-Tawfiq J.A., Assiri A.Y., Alzahrani N.A., Alanazi A.A., Memish Z.A. Clinical predictors of mortality of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: a cohort study. Travel Med Infect Dis. 2019;29:48–50. doi: 10.1016/j.tmaid.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi K.W., Chau T.N., Tsang O. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139(9):715–723. doi: 10.7326/0003-4819-139-9-200311040-00005. [DOI] [PubMed] [Google Scholar]
- 26.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corrales-Medina V.F., Musher D.M., Shachkina S., Chirinos J.A. Acute pneumonia and the cardiovascular system. Lancet. 2013;381(9865):496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 29.Blackburn R., Zhao H., Pebody R., Hayward A., Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis. 2018;67(1):8–17. doi: 10.1093/cid/cix1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang K.J., Su I.J., Theron M. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(8):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu H., Zhou J., Wong B.H. Middle east respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis. 2016;213(6):904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W.J., Zhao M., Liu K. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antiviral Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.To K.K., Tsang O.T., Leung W.S. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin Y., Wang M., Zuo Z. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int J Infect Dis. 2020;94:49–52. doi: 10.1016/j.ijid.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J., Yuan Q., Wang H. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong C.K., Lam C.W., Wu A.K. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136(1):95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahallawi W.H., Khabour O.F., Zhang Q., Makhdoum H.M., Suliman B.A. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8–13. doi: 10.1016/j.cyto.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He L., Ding Y., Zhang Q. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210(3):288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Josset L., Menachery V.D., Gralinski L.E. Cell host response to infection with novel human coronavirus EMC predicts potential antivirals and important differences with SARS coronavirus. mBio. 2013;4(3):e00165–e00213. doi: 10.1128/mBio.00165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arabi Y.M., Fowler R., Hayden F.G. Critical care management of adults with community-acquired severe respiratory viral infection. Intens Care Med. 2020;46(2):315–328. doi: 10.1007/s00134-020-05943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han K., Ma H., An X. Early use of glucocorticoids was a risk factor for critical disease and death from pH1N1 infection. Clin Infect Dis. 2011;53(4):326–333. doi: 10.1093/cid/cir398. [DOI] [PubMed] [Google Scholar]
- 43.Hui D.S. Systemic corticosteroid therapy may delay viral clearance in patients with middle east respiratory syndrome coronavirus infection. Am J Respir Crit Care Med. 2018;197(6):700–701. doi: 10.1164/rccm.201712-2371ED. [DOI] [PubMed] [Google Scholar]
- 44.Arabi Y.M., Mandourah Y., Al-Hameed F. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 45.Lee N., Allen Chan K.C., Hui D.S. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delaney J.W., Pinto R., Long J. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care. 2016;20:75. doi: 10.1186/s13054-016-1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen G., Wu D., Guo W. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Investig. 2020;30(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGonagle D., Sharif K., O'Regan A., Bridgewood C. Interleukin-6 use in COVID-19 pneumonia related macrophage activation syndrome. Autoimmun Rev. 2020 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.