Abstract
The advent of novel human coronavirus (SARS-CoV-2) and its potential transmission via fecal-oral and aerosols-borne routes are upcoming challenges to understand the fate of the virus in the environment. In this short communication, we specifically looked at the possibilities of these transmission routes based on the available literature directly related to the SARS-CoV-2 as well as on the closer phylogenetic relatives such as SARS-CoV-1. The available data suggest that, in addition to human-to-human contact, the virus may spread via fecal-oral and aerosols-borne routes. Existing knowledge states that coronaviruses have low stability in the environment due to the natural action of oxidants that disrupt the viral envelope. Previous recommended dosage of chlorination has been found to be not sufficient to inactivate SARS-CoV-2 in places where viral load is high such as hospitals and airports. Although there is no current evidence showing that coronaviruses can be transmitted through contaminated drinking water, there is a growing concern on the impact of the current pandemic wave on underprivileged societies because of their poor wastewater treatment infrastructures, overpopulation, and outbreak management strategies. More research is encouraged to trace the actual fate of SARS-CoV-2 in the environment and to develop/revise the disinfection strategies accordingly.
Keywords: Post-pandemic outbreaks, Sewage-borne transmission, SARS-CoV-2 inactivation, Virus-laden aerosols, Underprivileged societies
Graphical abstract
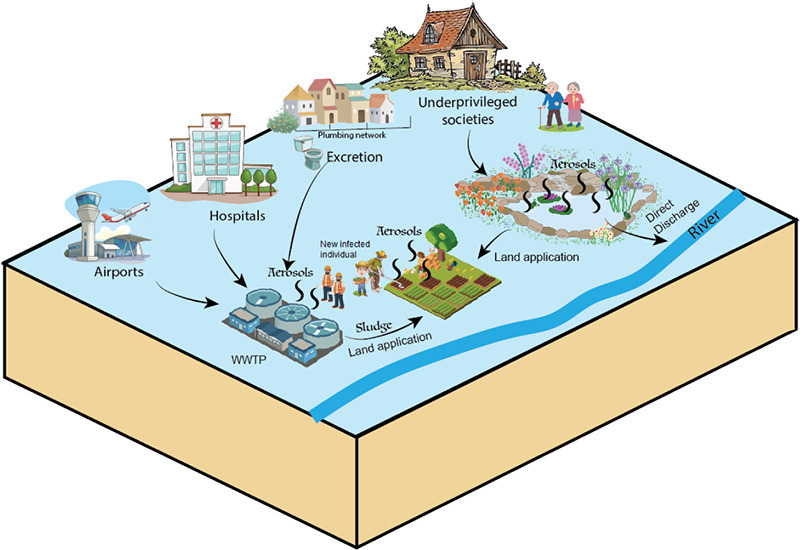
1. Introduction
The ongoing pandemic situation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is rapidly evolving with global distribution (WHO, 2020a). SARS CoV-2 belongs to the Coronaviridae family which is a large family of viruses known to cause diseases ranging from the common cold to severe acute respiratory diseases (Chan et al., 2020; Gorbalenya et al., 2020; Paules et al., 2020). The infection of SARS-CoV-2 has been largely associated with the coronavirus disease 2019 (COVID-19), which started in Wuhan, China in December 2019 (Huang et al., 2020). However, being highly pandemic, the virus has been transmitted to approximately all parts of the world in a very short time. The virus is known to spread by human-to-human contact via respiratory droplets or direct contact (Cascella et al., 2020). However, more recently, concerns on its spread via fecal-oral and virus-laden aerosols-borne routes are growing rapidly (Hindson, 2020; Wang and Du, 2020). This short communication gives a prospective outlook on how SARS-CoV-2 can be transmitted in the environment via these two routes. Furthermore, we discussed the impacts of these transmissions on underprivileged societies due to their poor wastewater management strategies and ineffective control measures.
2. Fecal–oral transmission
Recently, transmission via the fecal-oral route is getting serious attention after the viral RNA has been detected in the stool samples of infected patients (Ahmed et al., 2020; Grassia et al., 2020; Wen et al., 2020). To this end, Xiao et al. (2020) studied the possibility of gastrointestinal infection by SARS-CoV-2 through histological staining, as well as viral receptor angiotensin-converting enzyme 2 (ACE2), and viral nucleocapsid staining of gastrointestinal tissues. The authors found that receptor ACE2 was positively stained for gastrointestinal epithelial cells, and nucleocapsid protein was visualized in gastric, duodenal, and rectum glandular epithelial cells. Similar results have been reported by other authors (Wu et al., 2020; Zhang et al., 2020b). Briefly, Wu et al. (2020) found that viral shedding from the digestive tract might be greater compared to the respiratory tract, whereas Zhang et al. (2020a) stated that the digestive system is a potential route of SARS-CoV-2 transmission because viral RNA was identified from the samples obtained from pharynx, esophagus, gastric mucosa, rectal mucosa, duodenal mucosa, and stool.
It has been suggested that SARS-CoV-2 may persist longer in the digestive tract than in the respiratory tract (Grassia et al., 2020). At least two studies have reported that infected patients were capable of excreting viral RNA via feces even after several weeks from the day of developing symptoms. For example, Hu et al. (2020) reported two cases of positive viral nucleic acid in the anal swabs after 6 and 14 days of getting negative results from respiratory specimens. Likewise, Wu et al. (2020) found that the fecal samples of a patient in China were continuously positive for the viral RNA even after 33 days of seeing negative results for the respiratory samples. For another patient, the excretion period was further extended to 47 days from the first day of developing symptoms. This situation opens core questions related to the fate and distribution of the virus in the environment via fecal-oral routes because many patients discharged from the hospitals after being treated are most likely to excrete the virus in their feces.
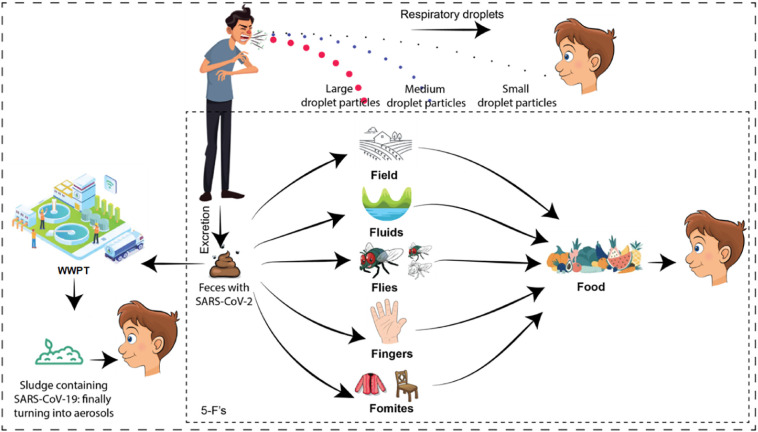
The fecal-oral route conservatively follows five-Fs paths such as fomites, fingers, flies, fluids, and fields (Fig. 1 ). In case of COVID-19, knowledge on disease spread via fields and fluids is limited; however, the contribution of fomites (e.g., clothes and surfaces), fingers (direct contact), and flies is being argued (Chen et al., 2020; Guo et al., 2020; Haas, 2020; Eslami and Jalili, 2020; Lai et al., 2020; Ong et al., 2020; WHO, 2020a). Further, the pathways can be linked to fecal-oral transmission by considering the examples of phylogenetic relatives of SARS-CoV-2 such as SARS coronavirus (SARS-CoV-1). Both viruses are similar in morphological features and chemical structures, as well as they share 82% genetic similarity (Yeo et al., 2020). In 2003, during the outbreak of SARS-CoV-1 in Hong Kong, inadequate plumbing system was identified as the main cause of fecal-oral transmission (Peiris et al., 2003; WHO, 2003a; WHO, 2003b). The virus entered the sewer systems and formed aerosols after settling on feces particles, i.e. virus-laden aerosols (Qu et al., 2020). The aerosols/droplets originating from virus-rich excreta were spread with non-functional water seals, inadequate traps, and strong upward air flows. The airborne transmission route was further aided by the bathroom extract ventilation system which ultimately drew air within the apartment thus mediating long-range human-to-human transmission via air movement. Resultantly, within a 50-storey building, 342 confirmed cases and 42 deaths were recorded. These observations were later supported by investigating the mechanisms of cross-contamination (Gormley et al., 2012; Gormley et al., 2014). Previously, other viruses were also found to be transmitted via fecal–oral route such as gastroenteritis viruses (Graham et al., 1994; Chan et al., 2006), adenoviruses (Reynolds, 2004), and enteroviruses (Klemola et al., 2008; Oliveira et al., 2014). Although no concrete evidence exists for the spread of SARS-CoV-2, these aspects are important to consider to understand the future of SARS-CoV-2 (Gormley et al., 2020).
Fig. 1.
Schematic representation of SARS-CoV-2 spread via the fecal-oral route.
3. Aerosols–borne transmission
Although SARS-CoV-2 is not an airborne virus, the adsorption on dust or particulate matter (PM) could allow its transport to long distances especially if these particles carry moisture. This phenomenon has also been coined as virus-laden-aerosols transmission. In a recent study conducted in Italy, high COVID-19 infection rate was linked to the air pollution for the cities exceeding the limits set for PM10 (Coccia, 2020). The cities with >100 days of air pollution had a high average number of infection rate which was further driven by low wind speed. It was suggested that air transmission dynamics of COVID-19 are not mere “human-to-human transmission” but could also be “pollution-to-human transmission” which is associated with the airborne viral infectivity. Similar observations were made in another study where the air quality of two regions in Italy (Lombardy and Emilia Romagna) was correlated with high SARS-CoV-2 transmission dynamics and infections (Conticini et al., 2020). The authors further stated that Lombardy and Emilia Romagna had the highest level of virus lethality in the world as well as they are among the most polluted areas in Europe. Another study suggested that airborne transmission route is highly virulent and dominant for the spread of COVID-19 as face covering reduced the infections to over 78,000 in Italy from April 6 to May 9, and over 66,000 in New York City from April 17 to May 9 (Zhang et al., 2020c).
Furthermore, Lu et al. (2020) reported a scenario where the spread of the virus was associated with air conditioning. In the very beginning phase of pandemic, three families that ate lunch at the same time in a restaurant in Guangzhou, China, developed the COVID-19 symptoms in a sequential manner. On the first day, only one person developed symptoms but 9 more persons got infected in later days. The authors argued that sitting distance among these families was sufficient, nevertheless, strong airflow from the air conditioner might have propagated the droplets. This might be the result of human atomization of virus-bearing particles which could occur from coughing, sneezing, or even normal breathing by an infected person (Zhang et al., 2020c). Generally, larger respiratory droplets with size >5 μm can stay in the air for a short time and settle at distances <1 m (Kutter et al., 2018; Pica and Bouvier, 2012). However, small aerosolized droplets (<5 μm) can remain in the air for a longer time and could travel long distances >1 m (Fernstrom and Goldblatt, 2013). The fine aerosols with PM2.5 may penetrate deeply into the respiratory tract finally reaching the other vital organs (Rychlik et al., 2019; Wu et al., 2019; Zhang et al., 2020c).These observations may correlate with the SARS outbreak in Hong Kong and the Middle East respiratory syndrome (MERS) coronavirus outbreak in South Korea (Kim et al., 2016). In South Korea, MERS-CoV was detected in the air samples of the hospitals which resulted in 186 infections and 36 deaths following the temporary shutdown of the hospitals.
To assess the transmission of SARS-CoV-2 via aerosols, van Doremalen et al. (2020) assessed the stability of both SARS-CoV-2 and SARS-CoV-1 on laboratory-generated aerosols. The authors reported that SARS-CoV-2 could stay in the aerosols up to 3 h. Although a reduction in infectious titer was observed over time, i.e. TCID50 reduced from 103.5 to 102.7/L of air, half-lives of both SARS-CoV-2 and SARS-CoV-1 were comparable. Recently, Zhang et al. (2020c) detected 285–1130 copies/m3 of SARS-CoV-2 in the aerosols of a hospital environment. This finding suggests a significant viral spill-over that was possibly via respiratory droplets from patients or airborne aerosols. Such transmission of SARS-CoV-2 might lead to the nosocomial spread and super-spreading events as reported previously for SARS-CoV-1 (Chen et al., 2004). Previously, during the outbreak of SARS-CoV-1 in Hong Kong, the virus was able to enter the individual homes and buildings.
It is being debated that an increase in ambient temperature might impact the survival of SARS-CoV-2 in the air. To this end, Zhu and Xie (2020) conducted a study on COVID-19 infections for 122 cities in China between January and February 2020. The authors did not find a decrease in infection rate with warming weather. This observation is in contrast to the findings reported previously by other authors regarding poor survival of SARS-CoV-1 at high temperature (Tan et al., 2005) and lower temperatures, facilitating the high transmission and infections (Prata et al., 2020; Tobías and Molina, 2020; Wang et al., 2020).
4. Presence of SARS-CoV-2 in the wastewater
SARS-CoV-2 could also become part of wastewater treatment plants (WWTPs) (Heller et al., 2020). This is because the virus could embed within the feces particles as seen for coronaviruses and ultimately then settle its fate in the WWTPs. The presence of SARS-CoV-2 in sewage samples is already confirmed by studies from different countries (Ahmed et al., 2020; Mallapaty, 2020; Medema et al., 2020; Wu et al., 2020). For example, in the Netherlands, sewage samples of seven cities and the Schiphol airport (Amsterdam) were tested for SARS-CoV-2 between February to March 2020 (Medema et al., 2020). The samples of February were negative for the viral RNA but in March, the viral load started increasing gradually. Similar results have been seen in France where a peak in the viral concentrations was reported earlier in the wastewater of Paris before the hospitals noticed a surge in cases (Leste-Lasserre, 2020). Viral loads as high as 1.9 × 104 copies/L have been detected in the wastewaters from locations close to departments receiving COVID-19 patients in Wuhan, China (Zhang et al., 2020a). On one hand, this detection of SARS-CoV-2 in the wastewater is beneficial in terms of early warning (i.e. wastewater-based epidemiology); whereas on the other hand, it is also risk-oriented as it may cause recurrent outbreaks if the virus could survive longer in the environment (i.e., post-pandemic outbreaks) following the incomplete removal of the virus by the WWTPs (Mallapaty, 2020; Orive et al., 2020).
Survival/persistence of the coronaviruses in WWTPs is driven by several parameters such as the presence of organic matter and oxidants; fluctuations in temperature and pH; and abundance of antagonistic bacteria (Gundy et al., 2008; WHO, 2020a). Firstly, organic matter and suspended solids in the wastewater can provide protection for viruses that adsorb to these particles, reducing the inactivation efficiency (Gundy et al., 2008). This is the reason that coronaviruses in pasteurized settled sewage and pure water were found to survive up to several days (Casanova et al., 2009). Secondly, the presence of oxidants could affect the integrity of the viral envelope, which is a very fragile structure (La Rosa et al., 2020). For example, SARS-CoV-1 was found to be inactivated in the wastewater when strong oxidants (e.g., free chlorine) were abundant (Gundy et al., 2008). Zhang et al. (2020a) reported that the concentration of free chlorine towards chlorination (≥0.5 mg/L after at least 30 min of contact time at pH < 8.0) recommended by WHO might not be sufficient to treat SARS-CoV-2 when the viral load is high. These authors were able to detect the viral RNA in a septic tank containing hospital wastewater, which was disinfected with sodium hypochlorite at 800 g/m3 dose (free chlorine >6.5 mg/L and contact time was 1.5 h). Thirdly, the temperature is found to have an effect on the survival/persistence of coronaviruses, in general. For example, at 20 °C, SARS-CoV-1 was found to survive up to 2 days in the domestic sewage, hospital wastewater, and dechlorinated tap water. However, this period was prolonged to 17 days when present in the urine and 3 days when it embeds in the feces (organic matter). At lower temperatures (4 °C), SARS-CoV-1 was further able to persist up to 14 days in the wastewater and >17 days in feces or urine (Wang et al., 2005). Lastly, the presence of antagonistic microorganisms could increase the extent of inactivation. This has been well-studied at WWTPs where membrane bioreactors have been used. In membrane bioreactors, viruses are inactivated via predation and enzymatic breakdown (Bosch et al., 2006; Hao et al., 2010) and the extent of inactivation further increases in the solid phase (e.g. sludge) as compared to the liquid phase. This is because of a high density of enzymes and predators in the solid phase (Chaudhry et al., 2015). Most of these results are tested for enveloped viruses including members of the Coronaviridae family (Bodzek et al., 2019; Chaudhry et al., 2015). As such, further investigations on SARS-CoV-2 are required to generate more relevant conclusions.
5. Potential risks for underprivileged societies
In underprivileged societies, SARS-CoV-2 may bring major challenges if environmental conditions could favor its persistence in the environment. At first, their health care system is not very strong which results in an outbreak in a short time, ultimately increasing the viral load in their wastewater (WHO, 2020b). This puts all pressures on the WWTPs to treat the virus-contaminated wastewater. Many underprivileged societies have basic wastewater treatment systems that are not equipped with the necessary technology to remove viruses effectively. For example, in Pakistan, not even a single WWTP is operational in the whole country, rather only stabilization ponds are present in one city (Faisalabad) to treat city's wastewater. Similarly, in Nigeria, many states have non-functional wastewater treatment facilities (Benue, Kogi, Kwara, Niger, and Plateau); whereas other states do not have any treatment facility at all (Abia, Akwalbom, Anambra, Bayelsa, Imo, and Ondo) (Adesogan, 2013; Adewumi and Oguntuase, 2016; Omole et al., 2019). As such, the wastewater is discharged directly into local rivers and streams with limited or no treatment at all (Afzal et al., 2019; Azizullah et al., 2011).
Wastewater treatment ponds are used worldwide for wastewater management in small cities and towns as well as in developing countries. These ponds have been reported to achieve, on average, one log reduction of viruses for every 14.5 to 20.9 days (Verbyla and Mihelcic, 2015). However, their performance in terms of SARS-CoV-2 reduction has not being reported so far. Therefore, if wastewaters of these small communities or underdeveloped societies are not appropriately handled, the situation may result into frequent, recurrent, or periodic post-pandemic outbreaks as seen previously for other viral diseases (Rose, 1999).
WWTPs produce a large amounts of solids sludge. It is a well-established fact that sludge can carry variety of viruses including SARS-CoV-2 (Xagoraraki et al., 2014; Xiao et al., 2019). These viruses are concentrated onto solid particles during viral shedding. The concentrations of the viral RNA in the primary municipal sewage sludge could be up to several orders of magnitude higher than their respective concentrations in the raw wastewater (Ahmed et al., 2020; Xiao et al., 2020). These results are consistent to the previous findings of other coronavirus strains such as HKU1 which were found to concentrate on the sewage sludge (Bibby and Peccia, 2013).
Under appropriate conditions, the viruses could persist for several months in the sludge particles (Schlindwein et al., 2010). For example, human enteric viruses were detected in the sediments obtained from sewage sludge disposal sites in the Atlantic Ocean after 17 months of dumping (Goyal et al., 1984). Although, some communities treat their sewage sludge to reduce the presence of microorganisms via thickening, dewatering, digesting or composting processes, it is still unknown if these practices can sufficiently remove/inactivate the SARS-CoV-2. The current knowledge on adsorption capabilities of the virus onto sludge particles recommends appropriate treatment before disposing/discharging into the environment. Taking these issues into consideration, the French Agency for Food, Environmental and Occupational Health & Safety has already recommended that sewage sludge produced during COVID-19 outbreak should not be spread without being disinfected (Anses, 2020). On the other hand, in many developing countries sludge is directly applied on land as a fertilizer (Jiménez et al., 2009). For example, in Benin, Ghana, and Mali, farmers used to bribe septic truck drivers to dispose the sludge on their agricultural lands (Asare et al., 2003; Cofie et al., 2005). The recurrent disease outbreaks in the past have been linked to the direct application of sludge in many societies (Hellmér et al., 2014; Okoh et al., 2010).
Recently, Zaneti et al. (2020) used quantitative microbial risk assessment (QMRA) approach to quantify the health risk to the workers at WWTPs for three COVID-19 scenarios (three-tiered approach: moderate, aggressive and extreme). The viral load was determined in the range of 1.03 × 102 to 1.31 × 104 copies/mL with estimated risks for infection to be 6.5 × 10−3 and 3.1 × 10−2, respectively, for the aggressive and extreme scenarios. The calculated risk was above the WHO benchmark of tolerable risk used for virus infection of 10−3 and also for the risk of infection of E. coli, which is used as common pathogen indicator. From here, it can be suggested that the virus particles associated with sludge can potentially complete all of the transmission routes, finally reaching back to humans (Hurst and Gerba, 1989). Such transmission in underprivileged societies is likely where workers or people living in the close vicinity are not provided with personal and collective protective equipment (PPEs and CPEs).
Another aspect of SARS-CoV-2 transmission is the improper solid waste management practices. In developing countries, the use of facemasks and other PPEs has become compulsory at public places. Nzediegwu and Chang (2020) estimated that the total daily use of facemasks in fifteen different African countries is approximately 586,833,053. On one hand, governments of these countries are taking strict measures to contain and reduce the spread of COVID-19; whereas on the other hand, the appropriate disposal of PPEs is being overlooked. As observed recently, SARS-CoV-2 could stay on different surfaces such as plastic and stainless steel up to 72 h (van Doremalen et al., 2020); an improper disposal or recurrent use of the contaminated facemasks could lead to the spread of the virus both locally and globally.
Additionally, underprivileged societies cannot afford to purchase or produce their own disinfection chemicals that could help them inactivate the virus. For instance, in many countries such as Egypt, India, and Tunisia, wastewater is not treated beyond secondary stage (Adewumi and Oguntuase, 2016). Hence, their treatment systems mostly rely on natural systems such as stabilization ponds and/or lagoons. This raises the question whether natural conditions are sufficient to inactivate the virus in the environment, which is presumed effective for other coronaviruses. The risk associated with the presence of SARS-CoV-2 in the surface water appears to be negligible, nevertheless, the situation for developing countries might be different due to improper sanitation and poor waste management practices.
Another concern in developing countries is the cross-contamination of the drinking water systems by the improper management of the wastewater. Although no current evidence shows that human coronaviruses are present in surface or groundwater or are transmitted through contaminated drinking-water (La Rosa et al., 2020), previous waterborne outbreaks due to fecal-oral pathogens from developing countries have been linked to cross-contamination due to poorly maintained distribution systems, failure to disinfect water or maintain a proper disinfection residual, intermittent service, excessive network leakages, and inadequate sewage disposal, among others (Lee and Schwab, 2005; Anakhasyan et al., 2012).
As temperature effect is found notable in inactivating coronaviruses, colder regions may allow the longer survival/persistence of SARS-CoV-2 in the environment. This situation could cause post-pandemic outbreaks (Kissler et al., 2020) as seen previously for other viral diseases such as influenza (Sakoda et al., 2012). Moreover, the intensity and timing of control measures should be handled carefully in underprivileged societies because the fatality rate might rise for these regions due to poor health care, wastewater management, and overpopulation. A similar situation has already seen in Iran recently where a surge in number of COVID-19 infections was seen after people did not follow the health guidelines during Persian new year holidays, i.e. Nowruz festival (Hafezi, 2020). Likewise, Wuhan (China) is fearing a second wave of COVID-19 outbreak because a cluster of cases are seen after businesses and schools are reopened on 8th of April 2020 (Jiang and Goh, 2020). In the 20th century, during the Spanish flu outbreak, up to 30% of the fatality was recorded from India alone due to the recurrent outbreaks (Barro et al., 2020). For SARS-CoV-2, Kissler et al. (2020) projected the post-pandemic transmission dynamics and forecasted its future accordingly. The factors such as seasonal variations, duration of immunity, and degree of cross-immunity between SARS-CoV-2 and other coronaviruses, and timing of the control measures were highlighted as key components that may contribute to post-pandemic transmission. Furthermore, modeled outcomes suggested that SARS-CoV-2 is capable of producing a substantial outbreak regardless of establishment time.
Last but not least, rigorous monitoring of the wastewaters in underprivileged societies should be given top priority unless most of the population is immunized. This could help in early detection of the outbreak following appropriate remedial solutions to keep the virus out of the water cycle. A major challenge in these societies is to develop cost-effective screening systems because advanced methods are less feasible. To this end, paper-based detection methods are being developed for COVID-19 which would help assess wastewater-based epidemiology for these societies (Mao et al., 2020).
6. Conclusions
Based on current literature, it can be concluded that:
-
•
SARS-CoV-2 may transmit via fecal-oral and virus-laden aerosols-borne routes. Nevertheless, the intensity of unwanted consequences may vary in different societies depending on the level of control measures, environmental conditions, and treatment facilities.
-
•
Currently established disinfection strategies such as chlorination at WHO recommended doses might not be sufficient to inactivate the SARS-CoV-2 in places where viral load is high; therefore, more research is encouraged to decipher the fate of the virus in different compartments of the environment.
-
•
Because underprivileged societies lack basic infrastructure to remove SARS-CoV-2 from the water cycle, the situation could lead to frequent outbreaks as observed in the past for other viruses.
-
•
More research is encouraged to trace the actual fate of SARS-CoV-2 in the environment and to develop/revise the disinfection strategies accordingly.
-
•
The information presented in this short review might be useful for the risk analysis of SARS-CoV-2 in the water cycle.
CRediT authorship contribution statement
Muhammad Arslan: Conceptualization, Writing - original draft. Bin Xu: Writing - review & editing. Mohamed Gamal El-Din: Conceptualization, Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The financial support provided by the Canada First Research Excellence Fund as a part of the University of Alberta's Future Energy Systems Research Initiative is greatly appreciated.
Editor: Damia Barcelo
References
- Adesogan S. Sewage technology in Nigeria: a pragmatic approach. Science Journal of Environmental Engineering Research. 2013;2013:1–9. doi: 10.7237/sjeer/266. [DOI] [Google Scholar]
- Adewumi J.R., Oguntuase A.M. Planning of wastewater reuse programme in Nigeria. Consilience. 2016;15(2016):1–33. [Google Scholar]
- Afzal M., Arslan M., Müller J.A., Shabir G., Islam E., Tahseen R., Anwar-ul-Haq M., Hashmat A.J., Iqbal S., Khan Q.M. Floating treatment wetlands as a suitable option for large-scale wastewater treatment. Nature Sustainability. 2019;2(9):863–871. [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anakhasyan E., Hoeser C., Stenström T.A., Kistemann T. Cross-contamination of distributed drinking water as the cause of waterborne outbreaks in Armenia 1992–2010. Journal of Water, Sanitation and Hygiene for Development. 2012;2(3):146–156. [Google Scholar]
- Anses, A. 2020. de l'Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail: https://www.anses.fr/en/system/files/MFSC2020SA0043.pdf (Accessed 14th May 2020)
- Asare I., Kranjac-Berisavljevic G., Cofie O. Vol. 10. Urban Agriculture Magazine; 2003. Faecal Sludge Application for Agriculture in Tamale; pp. 31–33. [Google Scholar]
- Azizullah A., Khattak M.N.K., Richter P., Häder D.-P. Water pollution in Pakistan and its impact on public health—a review. Environ. Int. 2011;37(2):479–497. doi: 10.1016/j.envint.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Barro R.J., Ursúa J.F., Weng J. The coronavirus and the great influenza pandemic: lessons from the “Spanish flu” for the coronavirus’s potential effects on mortality and economic activity. National Bureau of Economic Research. 2020:0898–2937. [Google Scholar]
- Bibby K., Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environmental science & technology. 2013;47(4):1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodzek M., Konieczny K., Rajca M. Membranes in water and wastewater disinfection. Archives of Environmental Protection. 2019;(1):45. [Google Scholar]
- Bosch A., Pintó R.M., Abad F.X. Viruses in Foods. Springer; 2006. Survival and Transport of Enteric Viruses in the Environment; pp. 151–187. [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. Statpearls [Internet] StatPearls Publishing; 2020. Features, evaluation and treatment coronavirus (COVID-19) [PubMed] [Google Scholar]
- Chan J.F.-W., Kok K.-H., Zhu Z., Chu H., To K.K.-W., Yuan S., Yuen K.-Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging microbes & infections. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M.C., Sung J.J., Lam R.K., Chan P.K., Lee N.L., Lai R.W., Leung W.K. Fecal viral load and norovirus-associated gastroenteritis. Emerg. Infect. Dis. 2006;12(8):1278. doi: 10.3201/eid1208.060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry R.M., Nelson K.L., Drewes J.r.E. Mechanisms of pathogenic virus removal in a full-scale membrane bioreactor. Environmental science & technology. 2015;49(5):2815–2822. doi: 10.1021/es505332n. [DOI] [PubMed] [Google Scholar]
- Chen D., Xu W., Lei Z., Huang Z., Liu J., Gao Z., Peng L. Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report. Int. J. Infect. Dis. 2020;93:297–299. doi: 10.1016/j.ijid.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-C., Huang L.-M., Chan C.-C., Su C.-P., Chang S.-C., Chang Y.-Y., Chen M.-L., Hung C.-C., Chen W.-J., Lin F.-Y. SARS in hospital emergency room. Emerg. Infect. Dis. 2004;10(5):782. doi: 10.3201/eid1005.030579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cofie O.O., Kranjac-Berisavljevic G., Drechsel P. The use of human waste for peri-urban agriculture in northern Ghana. Renewable Agriculture and Food Systems. 2005;20(2):73–80. [Google Scholar]
- Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in northern Italy? Environ. Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami H., Jalili M. The role of environmental factors to transmission of SARS-CoV-2 (COVID-19) AMB Express. 2020;10(1):1–8. doi: 10.1186/s13568-020-01028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom A., Goldblatt M. Aerobiology and its role in the transmission of infectious diseases. Journal of Pathogens. 2013;2013 doi: 10.1155/2013/493960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., et al. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Swaffield J., Sleigh P., Noakes C. An assessment of, and response to, potential cross-contamination routes due to defective appliance water trap seals in building drainage systems. Build. Serv. Eng. Res. Technol. 2012;33(2):203–222. [Google Scholar]
- Gormley M., Templeton K., Kelly D., Hardie A. Environmental conditions and the prevalence of norovirus in hospital building drainage system wastewater and airflows. Build. Serv. Eng. Res. Technol. 2014;35(3):244–253. [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A. COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob. Health. 2020;8(5):e643. doi: 10.1016/S2214-109X(20)30112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S.M., Adams W., O’Malley M., Lear D. Human pathogenic viruses at sewage sludge disposal sites in the middle Atlantic region. Appl. Environ. Microbiol. 1984;48(4):758–763. doi: 10.1128/aem.48.4.758-763.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D.Y., Jiang X., Tanaka T., Opekun A.R., Madore H.P., Estes M.K. Norwalk virus infection of volunteers: new insights based on improved assays. J. Infect. Dis. 1994;170(1):34–43. doi: 10.1093/infdis/170.1.34. [DOI] [PubMed] [Google Scholar]
- Grassia R., Testa S., Pan A., Conti C.B. SARS-CoV-2 and gastrointestinal tract: the dark side of the pandemic. Dig. Liver Dis. 2020;52(7):700–701. doi: 10.1016/j.dld.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food and Environmental Virology. 2008;1(1):10. [Google Scholar]
- Guo Z.-D., Wang Z.-Y., Zhang S.-F., Li X., Li L., Li C., Cui Y., Fu R.-B., Dong Y.-Z., Chi X.-Y. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerging Infectous Diseases. 2020;26(7) doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C.N. Coronavirus and environmental engineering science. Environ. Eng. Sci. 2020;37:233–234. (https://doi.org/0.1089/ees.2020.0096) [Google Scholar]
- Hafezi, P. 2020. Iran fears second wave of coronavirus as death toll rises to 2,077. in: The Guardian: https://www.theguardian.pe.ca/news/world/iran-coronavirus-toll-rises-to-2077-after-143-new-deaths-429209/ (Accessed 12th May 2020).
- Hao X.D., Wang Q.L., Zhu J.Y., Van Loosdrecht M.C. Microbiological endogenous processes in biological wastewater treatment systems. Crit. Rev. Environ. Sci. Technol. 2010;40(3):239–265. [Google Scholar]
- Heller L., Mota C.R., Greco D.B. COVID-19 faecal-oral transmission: Are we asking the right questions? Sci. Total Environ. 2020:138919. doi: 10.1016/j.scitotenv.2020.138919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H. Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Appl. Environ. Microbiol. 2014;80(21):6771–6781. doi: 10.1128/AEM.01981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindson J. COVID-19: faecal–oral transmission? Nat. Rev. Gastroenterol. Hepatol. 2020;17 doi: 10.1038/s41575-020-0295-7. 259–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Shen L., Xu Z., Zhou J., Zhou H. Preprints; 2020. SARS-CoV-2 May Persist in Digestive Tract Longer than Respiratory Tract. 2020020354. [DOI] [Google Scholar]
- Huang Y., Tu M., Wang S., Chen S., Zhou W., Chen D., Zhou L., Wang M., Zhao Y., Zeng W. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis. Travel Med. Infect. Dis. 2020 doi: 10.1016/j.tmaid.2020.101606. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst C.J., Gerba C.P. Fate of viruses during wastewater sludge treatment processes. Crit. Rev. Environ. Sci. Technol. 1989;18(4):317–343. [Google Scholar]
- Jiang, X., Goh, B., (2020). Fearing second wave, China's Wuhan ramps up coronavirus tests. Thompson Reuters: https://www.reuters.com/article/us-coronavirus-health-china-toll/fearing-second-wave-chinas-wuhan-ramps-up-coronavirus-tests-idUSKBN22R035. (Accessed 15th May 2020).
- Jiménez B., Drechsel P., Koné D., Bahri A. Wastewater Irrigation and Health. Routledge; 2009. Wastewater, sludge and excreta use in developing countries: An overview; pp. 29–54. [Google Scholar]
- Kim S.-H., Chang S.Y., Sung M., Park J.H., Bin Kim H., Lee H., Choi J.-P., Choi W.S., Min J.-Y. Extensive viable Middle East Respiratory Syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin. Infect. Dis. 2016;63(3):363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020:eabb5793. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemola P., Kaijalainen S., Ylipaasto P., Roivainen M. Diabetogenic effects of the most prevalent enteroviruses in Finnish sewage. Ann. N. Y. Acad. Sci. 2008;1150(1):210–212. doi: 10.1196/annals.1447.012. [DOI] [PubMed] [Google Scholar]
- Kutter J.S., Spronken M.I., Fraaij P.L., Fouchier R.A.M., Herfst S. Transmission routes of respiratory viruses among humans. Current Opinion in Virology. 2018;28:142–151. doi: 10.1016/j.coviro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods-a scoping review. Water Res. 2020;179:115899. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.J., Schwab K.J. Deficiencies in drinking water distribution systems in developing countries. J. Water Health. 2005;3(2):109–127. [PubMed] [Google Scholar]
- Leste-Lasserre C. Coronavirus found in Paris sewage points to early warning system. Science. 2020;368:6489. [Google Scholar]
- Lu J., Gu J., Li K., Xu C., Su W., Lai Z., Zhou D., Yu C., Xu B., Yang Z. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China. Emerg. Infect. Dis. 2020;26(7) doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty S. How sewage could reveal true scale of coronavirus outbreak. Nature. 2020;580(7802):176–177. doi: 10.1038/d41586-020-00973-x. [DOI] [PubMed] [Google Scholar]
- Mao K., Zhang H., Yang Z. Can a paper-based device trace COVID-19 sources with wastewater-based epidemiology? Environmental Science & Technology. 2020;54(7):3733–3735. doi: 10.1021/acs.est.0c01174. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and 3 correlation with reported COVID-19 prevalence in the early 4 stage of the epidemic in the Netherlands. Environmental Science & Technology Letters. 2020 doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Nzediegwu C., Chang S.X. Improper solid waste management increases potential for COVID-19 spread in developing countries. Resour. Conserv. Recycl. 2020;161:104947. doi: 10.1016/j.resconrec.2020.104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoh A.I., Sibanda T., Gusha S.S. Inadequately treated wastewater as a source of human enteric viruses in the environment. Int. J. Environ. Res. Public Health. 2010;7(6):2620–2637. doi: 10.3390/ijerph7062620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira D.B., Campos R.K., Soares M.S., Barros R.B., Batista T.C.A., Ferreira P.C.P., Bonjardim C.A., Trindade G.S., Abrahão J.S., Kroon E.G. Outbreak of herpangina in the Brazilian Amazon in 2009 caused by Enterovirus B. Arch. Virol. 2014;159(5):1155–1157. doi: 10.1007/s00705-013-1858-5. [DOI] [PubMed] [Google Scholar]
- Omole D.O., Jim-George T., Akpan V.E. Economic analysis of wastewater reuse in Covenant University. J. Phys. Conf. Ser. 2019;1299(1):12125. August. (IOP Publishing) [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orive G., Lertxundi U., Barcelo D. Early SARS-CoV-2 outbreak detection by sewage-based epidemiology. Sci. Total Environ. 2020:139298. doi: 10.1016/j.scitotenv.2020.139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. Jama. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- Peiris J., Lai S., Poon L., Guan Y., Yam L., Lim W., Nicholls J., Yee W., Yan W., Cheung M. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica N., Bouvier N.M. Environmental factors affecting the transmission of respiratory viruses. Current Opinion in Virology. 2012;2(1):90–95. doi: 10.1016/j.coviro.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata D.N., Rodrigues W., Bermejo P.H. Temperature significantly changes COVID-19 transmission in (sub) tropical cities of Brazil. Sci. Total Environ. 2020;729:138862. doi: 10.1016/j.scitotenv.2020.138862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G., Li X., Hu L., Jiang G. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19) Environmental Science & Technology. 2020;54(7):3730–3732. doi: 10.1021/acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- Reynolds K.A. Water Conditioning Purification. 2004. Adenovirus: balancing water treatment challenges; pp. 42–43. [Google Scholar]
- Rose G.D. 1999. Community-Based Technologies for Domestic Wastewater Treatment and Reuse: Options for Urban Agriculture. (Cities Feeding People Series; Rept. 27). [Google Scholar]
- Rychlik K.A., Secrest J.R., Lau C., Pulczinski J., Zamora M.L., Leal J., Langley R., Myatt L.G., Raju M., Chang R.C.A., Li Y. In utero ultrafine particulate matter exposure causes offspring pulmonary immunosuppression. Proc. Natl. Acad. Sci. 2019;116(9):3443–3448. doi: 10.1073/pnas.1816103116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoda Y., Ito H., Uchida Y., Okamatsu M., Yamamoto N., Soda K., Nomura N., Kuribayashi S., Shichinohe S., Sunden Y. Reintroduction of H5N1 highly pathogenic avian influenza virus by migratory water birds, causing poultry outbreaks in the 2010–2011 winter season in Japan. J. Gen. Virol. 2012;93(3):541–550. doi: 10.1099/vir.0.037572-0. [DOI] [PubMed] [Google Scholar]
- Schlindwein A., Rigotto C., Simões C., Barardi C. Detection of enteric viruses in sewage sludge and treated wastewater effluent. Water Sci. Technol. 2010;61(2):537–544. doi: 10.2166/wst.2010.845. [DOI] [PubMed] [Google Scholar]
- Tan J., Mu L., Huang J., Yu S., Chen B., Yin J. An initial investigation of the association between the SARS outbreak and weather: with the view of the environmental temperature and its variation. J. Epidemiol. Community Health. 2005;59(3):186–192. doi: 10.1136/jech.2004.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobías A., Molina T. Is temperature reducing the transmission of COVID-19? Environ. Res. 2020;186:109553. doi: 10.1016/j.envres.2020.109553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbyla M.E., Mihelcic J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015;71:107–124. doi: 10.1016/j.watres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Wang J., Du G. COVID-19 may transmit through aerosol. Ir. J. Med. Sci. (1971-) 2020:1–2. doi: 10.1007/s11845-020-02218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Tang K., Feng K., Lv W. 2020. High Temperature and High Humidity Reduce the Transmission of COVID-19. (Available at SSRN 3551767) [Google Scholar]
- Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N., Xiao W.-J., Yin J., Wei W., Wang G.-J., Si B.-y., Guo B.-Z., Liu C., Ou G.-R., Wang M.-N., Fang T.-Y., Chao F.-H., Li J.-W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126(1):171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q., Yang J., Luo T. First case of Covid-19 in the United States. N. Engl. J. Med. 2020;382:e53. doi: 10.1056/NEJMc2004794. [DOI] [PubMed] [Google Scholar]
- WHO. 2003a. Consensus Document on the Epidemiology of Severe Acute Respiratory Syndrome (SARS). World Health Organization: https://apps.who.int/iris/bitstream/handle/10665/70863/WHO_CDS_CSR_GAR_2003.11_eng.pdf?sequence=1&isAllowed=y (Accessed 26th April 2020).
- WHO. 2003b. Inadequate plumbing systems likely contributed to SARS transmission. in: Inadequate Plumbing Systems Likely Contributed to SARS Transmission: https://www.who.int/mediacentre/news/releases/2003/pr70/en/ (Accessed 29th April, 2020). [PubMed]
- WHO. 2020a. Water, Sanitation, Hygiene, and Waste Management for the COVID-19 Virus: Interim Guidance, 23 April 2020. World Health Organization: https://apps.who.int/iris/handle/10665/331846 (Accessed 25th April 2020).
- WHO. 2020b. WHO releases guidelines to help countries maintain essential health services during the COVID-19 pandemic: https://www.who.int/news-room/detail/30-03-2020-who-releases-guidelines-to-help-countries-maintain-essential-health-services-during-the-covid-19-pandemic (Accessed 10th May 2020).
- Wu G., Brown J., Zamora M.L., Miller A., Satterfield M.C., Meininger C.J., Steinhauser C.B., Johnson G.A., Burghardt R.C., Bazer F.W., Li Y. Adverse organogenesis and predisposed long-term metabolic syndrome from prenatal exposure to fine particulate matter. Proc. Natl. Acad. Sci. 2019;116(24):11590–11595. doi: 10.1073/pnas.1902925116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. The lancet Gastroenterology & hepatology. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xagoraraki I., Yin Z., Svambayev Z. Fate of viruses in water systems. J. Environ. Eng. 2014;140 [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Liang S., Wang X., Chen C., Huang X. Current state and challenges of full-scale membrane bioreactor applications: a critical review. Bioresour. Technol. 2019;271:473–481. doi: 10.1016/j.biortech.2018.09.061. [DOI] [PubMed] [Google Scholar]
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? The Lancet Gastroenterology & Hepatology. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaneti R.N., Girardi V., Spilki F.R., Mena K., Westphalen A.P.C., da Costa Colares E.R., Pozzebon A.G., Etchepare R.G. medRxiv; 2020. QMRA of SARS-CoV-2 for Workers in Wastewater Treatment Plants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S., Zhang X. Potential spreading risks and disinfection challenges of medical wastewater by the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kang Z., Gong H., Xu D., Wang J., Li Z., Li Z., Cui X., Xiao J., Zhan J., Meng T., Zhou W., Liu J., Xu H. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69(6):1010–1018. [Google Scholar]
- Zhang R., Li Y., Zhang A.L., Wang Y., Molina M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. 2020:202009637. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020:138201. doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]