Highlights
-
•
Early diagnosis and prevention of aggravation are important in COVID-19 medical care.
-
•
Lymphocyte counts can be used to identify patients who may develop severe COVID-19.
-
•
The ciclesonide treatment group had a lower incidence of severe pneumonia.
-
•
The lymphocyte count after ciclesonide therapy was significantly higher compared to before treatment.
-
•
Treatment of the asthma drug ciclesonide may prevent severe pneumonia.
Keywords: Coronavirus, COVID-19, Ciclesonide, Pneumonia
Abstract
We investigated whether reduced lymphocyte count, could predict the development of severe COVID-19. We also examined whether ciclesonide could prevent the development of severe COVID-19 among patients with the predictors. This was a retrospective cohort study. Of the 30 included patients, 12, 14, and 4 were allocated to severe pneumonia, non-severe pneumonia, and non-pneumonia groups, respectively. The group of the low level of lymphocyte counts of the sixth day after onset was significantly intubated approximately three days later. The incidence of the severe pneumoniae requiring intubation are significantly lower in the patients treated with ciclesonide than without it (11.18 % vs 83.33 %, p = 0.0033). The lymphocyte count after ciclesonide treatment in the non-severe pneumonia group was significantly higher (p = 0. 0156) than before. The lymphocyte count could be used to identify patients that may develop severe COVID-19. Treatment with ciclesonide may prevent the development of severe COVID-19.
1. Introduction
Since first appearing in Wuhan, Hubei Province, China, in December 2019, a novel coronavirus (SARS-CoV-2) has spread rapidly around the world. Many patients with coronavirus infection disease 2019(COVID-19) are subclinical, and it has been reported that people are contagious even when asymptomatic (Li et al., 2020; Wei et al., 2020), which means preventing the spread of SARS-CoV-2 is challenging (Liu et al., 2020a). In addition, some patients have been reported to deteriorate rapidly in the early stages (Wang et al., 2020). Therefore, early detection and preventing cases from progressing to a severe stage is essential.
About 20 % of COVID-19 cases progress to a severe stage, of which about 3% die. Risk factors of severe pneumonia include age, comorbidities, smoking, reduced lymphocyte count, elevated ferritin levels, and elevated C-reactive protein (CRP) levels (Wang et al., 2020; Henderson et al., 2020; Henry et al., 2020; Lagunas-Rangel, 2020; Liu et al., 2020b; Liu et al., 2020c). However, it is unclear which of these risk factors are predictors of progression to severe COVID-19.
As yet, no effective treatment has been found for COVID-19. There have been many medications suggested, including remdesivir (Agostini et al., 2018; Ko et al., 2020a; Sheahan et al., 2017), lopinavir and ritonavir (Cao et al., 2020), and chloroquine (Cortegiani et al., 2020), but their efficaciousness have yet to be verified. Ciclesonide is an inhaled corticosteroid that is approved to treat asthma. It has demonstrated antiviral effects in vitro (Matsuyama et al., 2020) and has been reported to be effective in treating COVID-19 (Iwabuchi et al., 2020). According to a report by Meehyun Koa et al., the infection inhibitory effect of ciclesonide was confirmed in the MERS-CoV strain isolated in South Korea (Ko et al., 2020b). Furthermore, because Ciclesonide is a local administration, there are few side effects, and administration is possible for a pregnant woman relatively safely.
We believe that preventing the development of severe COVID-19 will help to reduce the mortality rate. We investigated whether any of the factors that have been reported to correlate with severe pneumonia could predict the development of severe COVID-19. In addition, we examined whether ciclesonide could prevent the development of severe COVID-19 among patients with these predictors.
2. Materials and methods
This was a retrospective cohort study. All the patients were hospitalized at our institution between February 16 and April 14, 2020, and had tested positive for SARS-CoV-2 using polymerase chain reaction testing of pharyngeal or nasopharyngeal swabs taken. For all patients, the date of onset was the day clinical symptoms appeared, such as fever, cough, runny nose, and dysgeusia. The presence of pneumonia was confirmed by chest computed tomography (CT). Patients who underwent intubation and respiratory management were defined as severe pneumonia group. Written informed consent for this study was obtained. The study was conducted with the approval of our hospital’s institutional review board (approval number: 4712).
2.1. Initial testing for predictors of severe COVID-19
Thirteen patients with COVID-19, hospitalized between February 16 and March 18, 2020, before treatment with ciclesonide starts, were enrolled in this study. Blood tests performed less than 14 days from the date of onset and before intubation were examined. If multiple blood tests were performed during the evaluation period, the minimum and maximum values were examined. The leukocyte count, lymphocyte count, platelet count, CRP, ferritin, d-dimer, and KL-6 were examined. Patients were divided into three groups: severe pneumonia, non-severe pneumonia, and non-pneumonia.
2.2. Investigation of the therapeutic effect of ciclesonide
For the lymphocyte count, the mean+1SD was used as the cutoff value of severe COVID-19 pneumonia. The cases at or below this cutoff value were evaluated, and patients who started ciclesonide after intubation were excluded. The treatment group received 2 inhalations of 400 μg ciclesonide once a day, for a daily total of 800 μg. The relationship between ciclesonide use and severe pneumonia were examined. In addition, the lymphocyte count prior to and approximately 7 days after starting treatment were compared.
2.3. Statistical analysis
Data were analyzed with the Mann-Whitney U, Fisher's exact and Wilcoxon matched-pairs signed rank tests using GraphPad Prism ver.6.00 for Windows, GraphPad Software, San Diego California USA.
3. Results
3.1. Patients
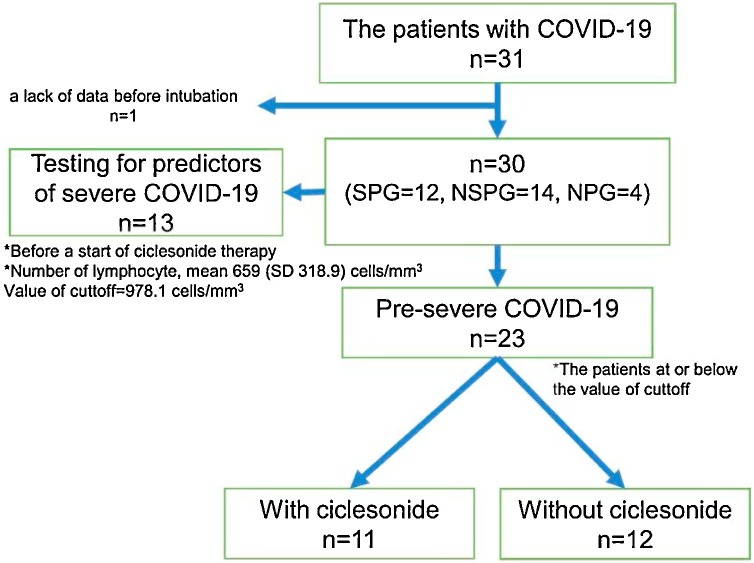
Of the 31 patients who were hospitalized during the observation period, 1 was excluded due to a lack of data before intubation. Of the 30 included patients, 12 were allocated to the severe pneumonia group, 14 to the non-severe pneumonia group, and 4 to the non-pneumonia group. The study design of this study was shown in Fig. 1 .
Fig. 1.
The study design of this COVID-19 study.
The value of cutoff by testing for predictors of severe COVID-19 is 978.1 cells/mm3.
The patients of pre-severe COVID-19 is at or below the value of cutoff.
SPG: severe pneumonia group (n = 12); NSPG: non-severe pneumonia group (n = 14); NPG: non-pneumonia group (n = 4).
3.2. Baseline characteristics
Table 1 details the patients’ demographic information. The mean age was 54.5 years, and 83.3 % were male. Of the total and those with pneumonia, 53.3 % and 57.7 % had comorbidities, respectively.
Table 1.
Baseline characteristics of patients with COVID-19 (n = 30).
| Variable | Value |
|---|---|
| age, mean(SD), years | 54.5(13.97) |
| female, n(%) | 5(16.7) |
| Associated disease, n(%) | 16(53.3) |
| Sample collection from nasopharynx, n(%) | 17(56.7) |
| a period to first blood test, mean(SD), days | 5.8(2.72) |
| Pneumonia, n(%) | 26(86.7) |
| intubation, n(%) | 12(40.0) |
| a period to intubation, mean(SD), days | 9.0(2.43) |
n: number, SD: standard deviation.
Blood tests were on average performed 5.8 days after onset (SD 2.72) and 12 days after treatment (SD 3.58). On average, patients developed severe COVID-19 and underwent intubation and respiratory management 9 days after onset (SD 2.43).
3.3. Investigation of the predictors of severe COVID-19
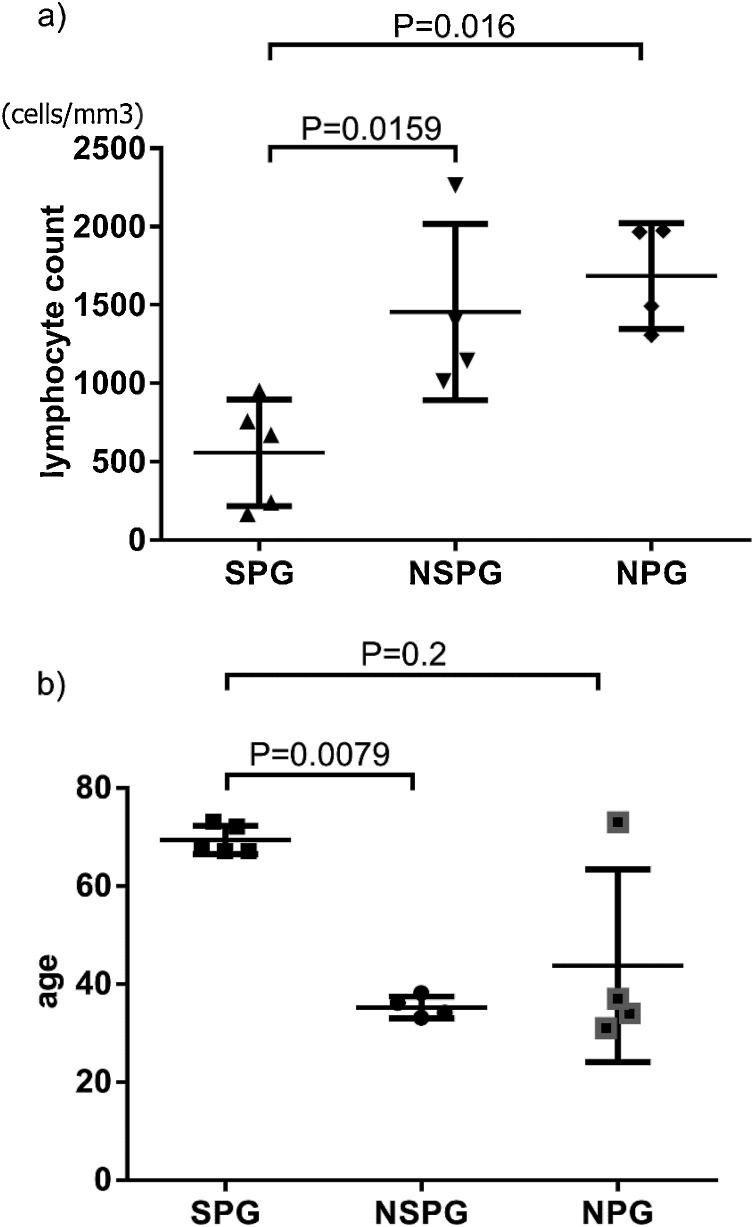
Of the 13 patients with COVID-19 hospitalized between February 16 and March 18, 2020, before the start of ciclesonide therapy, there were 5 in the severe pneumonia group, 4 in the non-severe pneumonia group, and 4 in the non-pneumonia group (Fig. 2 ).
Fig. 2.
Predictors of the severity of COVID-19 pneumonia.
a) Lymphocyte count b) Age.
SPG: severe pneumonia group (n = 5); NSPG: non-severe pneumonia group (n = 4); NPG: non-pneumonia group (n = 4). The significant difference used by Mann-Whitney U test.
Lymphocyte counts of approximately sixth days after onset were significantly lower in the severe pneumonia group compared to both the non-severe pneumonia group and the non-pneumonia group (p = 0.0159, 0.0016, respectively) (Fig. 2a). The severe pneumonia group had a low mean lymphocyte count at 659 cells/mm3 (SD 318.9). Patients in the severe pneumonia group were significantly older than those in the non-severe pneumonia group (p = 0.0079), but not significantly different from those in the non-pneumonia group (Fig. 2b). Significant differences were not observed between the severe and non-severe pneumonia groups in relation to ferritin, CRP, and D-dimer. Regarding sex differences, there tended to be more males in the severe and non-severe pneumonia groups. However, there was no significant difference in sex for the pneumonia cases. While 57.7 % of patients in the pneumonia groups had a comorbidity, the difference between the severe and non-severe pneumonia groups was not significant.
3.4. Investigation of the therapeutic effect of ciclesonide
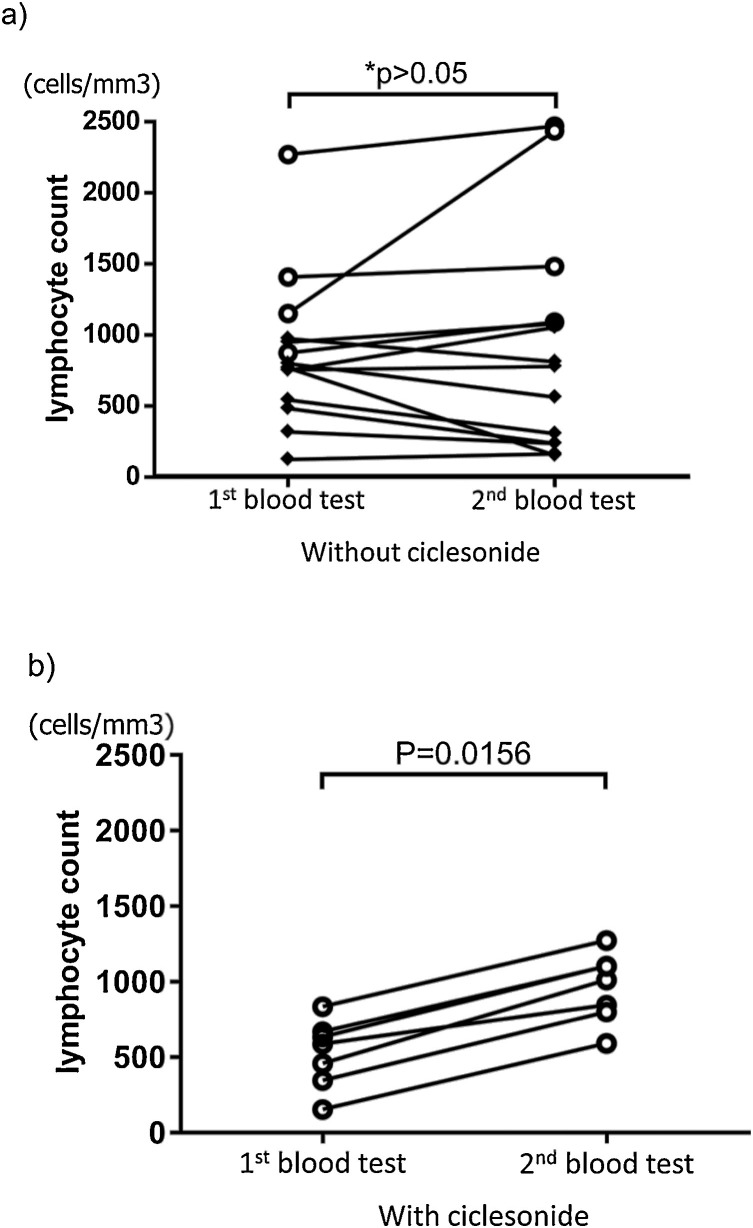
To evaluate the ability of ciclesonide to suppress the development of severe COVID-19, we examined patients with lymphocyte counts at or below the cuttoff value (978,1 cells/mm3) of severe pneumonia. Overall, this included 23 patients (Table 2 ), 12 patients had severe pneumonia, and 11 had non-severe pneumonia. Eleven patients from 23 with a lymphocyte count at or below the cutoff value could be treated with ciclesonide. Of these, 2 had severe COVID-19 pneumonia, and the incidence of the severe pneumoniae requiring intubation are significantly lower in the patients treated with ciclesonide than without it (11.18 % vs 83.33 %, p = 0.0033). Thus ciclesonide therapy is suspected to exhibit a significant correlation with the non-severe pneumonia group. Moreover, the lymphocyte count after ciclesonide therapy in the non-severe pneumonia group was significantly higher (p = 0.0156) compared to before treatment (mean 6.14 days, SD 2.17) (Fig. 3 b). Five patients with pneumonia were subsequently transferred to other hospitals, so their lymphocyte counts after treatment are unknown.
Table 2.
Characteristics of the patients with low lymphocyte cout (n = 23).
| Variable | Ciclesonide |
P value | |
|---|---|---|---|
| with(n = 11) | without(n = 12) | ||
| age, mean(SD), years | 54.7(11.17) | 62.6(8.76) | n.s.** |
| female, n(%) | 1(9.1) | 4(33.3) | n.s.* |
| Associated disease, n(%) | 6.2(54.6) | 5(75.0) | n.s.* |
| a period to first blood test, mean(SD), days | 6.2(1.85) | 6.8(2.80) | n.s.** |
| number of lymphocytes, mean(SD), /mm3 | 561(203.88) | 680(256.83) | n.s.** |
| consequence with intubation, n(%) | 2(18.2) | 10(83.3) | p = 0.0033* |
Mann-Whitney U test.
Fisher's exact test.
Fig. 3.
Changes in the lymphocyte count in pneumonia group.
The patients without second blood test data did not include it in this figure. A average period to blood test was 6.1(SD = 2.58) (1st), 12.0 (SD = 3.58) (2nd) days from the onset. The significant difference test used Wilcoxon matched-pairs signed rank test (Without ciclesonide: SPG n = 10, NSPG n = 4, With ciclesonide: n = 7) 〇: NSPG, ◆ :SPG.
a) Pneumonia group without ciclesonide.
*No significant increase in lymphocyte count was observed. (only NSPG (p = 0.125)、only SPG (p = 0.275)、Both pneumonia group (p > 0.999)).
b) Pneumonia group with ciclesonide.
Significant increase in lymphocyte count was observed after treatment.
1st blood test:Before treatment, 2nd blood test:After treatment.
4. Discussion
There is currently no therapy that has been proven to be efficacious in treating COVID-19. As many COVID-19 cases are subclinical, it is challenging to track infected individuals, making it hard to prevent infections from occurring (Li et al., 2020; Wei et al., 2020; Liu et al., 2020a). However, some patients with COVID-19 progress to a severe stage, some of whom die. Therefore, we believe it is vital to find an approach that prevents patients with COVID-19 progressing to a severe stage. In the current study, we first examined the factors that predict severe disease, which suggested the importance of the lymphocyte count. Moreover, we showed that treatment with ciclesonide was significantly associated with the non-severe pneumonia group. This finding indicates that ciclesonide could be used for preventing the development of severe COVID-19 in patients who are highly likely to become critical. No one in the non-pneumonia group had a lymphocyte count below the cutoff value.
In the present study, 2 patients who received ciclesonide developed severe pneumonia. Patients with COVID-19 are known to deteriorate rapidly. Both of these cases began treatment with ciclesonide 2 days before intubation, which suggests that the drug may have been introduced too late. Previous research has found that age, comorbidities, lymphocyte count, ferritin, CRP, and D-dimer are associated with severe pneumonia. Of these, only the pre-intubation lymphocyte count appeared to be a possible predictor with counts in the non-pneumonia and non-severe pneumonia groups being significantly different from the severe pneumonia group. Regarding age, there was a significant difference between the severe and non-severe pneumonia groups, but not with the non-pneumonia group. The 73 year old case in the non-pneumonia group accounts for the lack of significant difference. We believe that this case did not cause pneumonia because there is no underlying disease and there are no risk factors other than age. Significant differences were not observed for ferritin or D-dimer. There were cases with high ferritin and D-dimer levels in both the severe and non-severe pneumonia groups. In addition, there was a lot of missing data, making it difficult to accurately assess whether ferritin or D-dimer could be a predictor of severe COVID-19.
While the presence of subclinical COVID-19 cases makes controlling infections difficult, death occurs suddenly in some cases (Wang et al., 2020). COVID-19 is known to be contagious 2 days before onset, with increased viral loads in the respiratory tract. It has been suggested that cytokine storms are associated with the development of severe COVID-19. Therefore, the early administration of antivirals could be efficacious, similar to influenza. It is important to identify patients with COVID-19 as soon as possible and prevent it from progressing to a severe stage. The lymphocyte count could be used as an indicator for identifying patients that may develop severe COVID-19. Our results suggest that treatment with ciclesonide may prevent the development of severe COVID-19 in these circumstances. It is best to introduce the drug as soon as possible in patients with reduced lymphocyte counts and other predictors of severe COVID-19 (Cockrell et al., 2016).
This study had several limitations. This was a retrospective study with a small sample size. Therefore, the results need to be confirmed in a larger, prospective study. The viral load determination could not be mentioned because it was not measured in all cases.
We showed treatment with ciclesonide as the candidate of the factor which inhibited severe COVID-19 in this study. We urgently need to establish a testing system that includes the antigen-antibody method, develop a vaccine, and find treatments that can prevent the development of and treat severe COVID-19.
CRediT authorship contribution statement
Yukitaka Yamasaki: Writing - original draft. Seido Ooka: Writing - review & editing, Conceptualization. Tomoya Tsuchida: Writing - review & editing, Validation. Yuta Nakamura: Writing - review & editing, Methodology. Yuta Hagiwara: Writing - review & editing. Yoshiyuki Naitou: Writing - review & editing. Yuki Ishibashi: Writing - review & editing. Hiroki Ikeda: Writing - review & editing. Tsutomu Sakurada: Writing - review & editing. Hiroshi Handa: Writing - review & editing. Hiroki Nishine: Writing - review & editing. Mumon Takita: Resources. Daiki Morikawa: Resources. Hideki Yoshida: Resources. Shuichi Fujii: Resources. Kenichiro Morisawa: Resources. Hiromu Takemura: Supervision, Visualization. Shigeki Fujitani: Supervision. Hiroyuki Kunishima: Project administration.
Acknowledgments
We thank all medical staff who treated COVID19 with us.
The authors have no conflicts of interest directly relevant to the content of this article.
Biography
Dr. Yamasaki is a physician in the Department of Infectious Diseases, St. Marianna University School of Medicine, Kanagawa, Japan. His research interests include Infection Control.
References
- Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell A.S., Yount B.L., Scobey T., Jensen K., Douglas M., Beall A. A mouse model for MERS coronavirus-induced acute respiratory distress syndrome. Nat. Microbiol. 2016;2:16226. doi: 10.1038/nmicrobiol.2016.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.A., Canna S.W., Schulert G.S., Volpi S., Lee P.Y., Kernan K.F. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. 2020 doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- Iwabuchi K., Yoshie K., Kurakami Y., Takahashi K., Kato Y., Morishima T. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: report of three cases. J. Infect. Chemother. 2020 doi: 10.1016/j.jiac.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W.C., Rolain J.M., Lee N.Y., Chen P.L., Huang C.T., Lee P.I. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M., Chang S.Y., Byun S.Y., Choi I. Screening of FDA-approved drugs using a MERS-CoV clinical isolate from South Korea identifies potential therapeutic options for COVID-19. bioRxiv. 2020 doi: 10.3390/v13040651. 02.25.965582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunas-Rangel F.A. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Li W., He X., Cao Y. Asymptomatic and presymptomatic infectors: hidden sources of COVID-19 disease. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gayle A.A., Wilder-Smith A., Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020:27. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liao W., Wan L., Xiang T., Zhang W. Correlation between relative nasopharyngeal virus RNA load and lymphocyte count disease severity in patients with COVID-19. Viral Immunol. 2020 doi: 10.1089/vim.2020.0062. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani Wataru. The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. BioRxiv. 2020 doi: 10.1128/JVI.01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lu X., Chen H., Chen T., Su N., Huang F. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W.E., Li Z., Chiew C.J., Yong S.E., Toh M.P., Lee V.J. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23–March 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:411–415. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]