Abstract
Background and aims
Data show that vitamin D deficiency may play a role in patients with diabetes mellitus and COVID-19 infection. In this article, we review evidence of vitamin D deficiency and COVID-19 infection in context of diabetes mellitus.
Methods
A literature search was carried out by using the key term ‘COVID 19’ combined with ‘Diabetes’, ‘Vitamin D’, ‘Extra skeletal effects’, ‘immunity’, ‘infection’, ‘India’ from Pub Med (National Library of Medicine, Bethesda, MD and Google Scholar from December 2019 to May 2020. A manual search of the references was also carried out.
Results
Vitamin D deficiency has been linked to increased morbidity and mortality in COVID -19 infections but convincing data on diabetic subgroup of patients in particular is still awaited.
Conclusion
Robust studies are required to ascertain if Vitamin D supplementation could be beneficial in patients with diabetes and COVID-19.
Keywords: COVID-19, Diabetes mellitus, Vitamin D
1. Introduction
The world is in the midst of a global pandemic due to COVID-19, a disease caused by a novel beta corona virus namely Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) [1]. The outbreak has engulfed whole world in a period of four months keeping everyone home locked. Knowledge about the natural history of this disease, its pathophysiology and treatment is unfolding day by day but mystery remains as to how this pandemic will eventually fade out. Patients infected with COVID -19 remain asymptomatic in 80% of the cases that become a concern for spreading the disease, symptomatic in 15% and require hospitalization in 5% with high mortality [2]. Fatality rates are higher in elderly population, patients with various co morbidities like hypertension, diabetes mellitus, cardiovascular disease, chronic lung, renal disease and malignancy [3].
Diabetes mellitus has been recognised as a risk factor for hospitalization and increased mortality in COVID-19 infection. It was co morbidity in 22% of 32 non-survivors in a study of 52 intensive care patients [4]. In another study of 173 patients with severe disease, 16.2% had diabetes [2], and in further study of 140 hospitalised patients, 12% had diabetes [5]. When comparing intensive care and non-intensive care patients of COVID-19, there was twofold increase in the incidence of diabetes in intensive care patients [6]. Mortality was about threefold higher in people with diabetes compared with the general mortality of COVID-19 in China [6]. Diabetes was also a risk factor for severe disease and mortality in the previous SARS, MERS (Middle East respiratory syndrome) corona virus infections and the severe influenza A H1N1 pandemic in 2009 [7]. Interestingly, we all have fear that diabetics have more COVID 19 infection than non diabetics but reported data by Yang et al. 2020 [6] did not support basis of this fear.
Many observational studies have linked low vitamin D status to major human diseases, diabetes being one of them. Vitamin D is recognised to have a host of antioxidant and immunomodulatory properties [8]. In a deadly disease of COVID -19 SARS-CoV-2 infection, vitamin D attracted us to offer a ray of hope in treating diabetic patients with COVID -19 infection, particularly in the presence of its deficiency. Deficiency of vitamin D is global and more so in a country like India due to lack of provision of supplementation in food stuffs [9].
We reviewed the plausible mechanisms linking vitamin D deficiency to increased susceptibility to severe COVID -19 infection in patients with diabetes.
2. Search methodology
A literature search was carried out by using the key term ‘COVID 19’ combined with ‘Diabetes’, ‘Vitamin D’, ‘Extra skeletal effects’, ‘immunity’, ‘infection’, ‘India’ from PubMed (National Library of Medicine, Bethesda, MD and Google Scholar from December 2019 to May 2020. A manual search of the references was also carried out. Articles from several non – academic sources (i.e news, websites) were also accessed.
3. Diabetes, increased tendency of infection and COVID -19
Diabetic patients have impaired immune-response to infection both in relation to cytokine profile and changes in immune-responses including T-cell and macrophage activation. Poor glycaemic control impairs several aspects of the immune response to viral infection and also to the potential secondary bacterial infection in the lungs [10]. Various bacterial, viral, parasitic and mycotic infections with increased severity as compared to non diabetics are common in diabetic patients [10]. In one of the study [11], cytokine response was heightened in diabetic foot infection and the cytokine response to infection was linked to vitamin D deficiency particularly below 10 ng/ml. All these perturbations may dispose diabetic patients to have increased release of cytokines (“cytokine storm”) in response to infection be it bacterial or viral.
Consistent with the immune and glycaemic response to infection, various studies have shown dysregulated cellular and metabolic profile in Covid-19 infection in diabetics. There is decrease in the peripheral counts of CD4+ and CD8+ T cells with a concomitant increase in highly proinflammatory Th17 CD4+ T cells and various cytokines. Acute phase reactants like serum ferritin, ESR, CRP, IL-6 are much higher in diabetics with Covid-19 infection as compared to patients without diabetes. The “stress response” to infection in the body worsens hyperglycaemia by antagonising the insulin action or by inhibiting insulin secretion by the beta cells [12]. Such observations support the proposition that the patients with diabetes are susceptible to enhanced inflammatory response, which could lead to rapid deterioration of COVID-19 infection with high mortality [13].
4. Vitamin D deficiency– linked to both diabetes and infection
Vitamin D has a host of extra skeletal effects. Studies have shown that vitamin D plays a pivotal role in preserving the function of islet cells. The endocrine pancreas is a recognised vitamin D receptor (VDR) target. It improves both insulin sensitivity and insulin synthesis. Low vitamin D levels have repeatedly been shown to be associated with increased risk of type 2 diabetes mellitus [14].
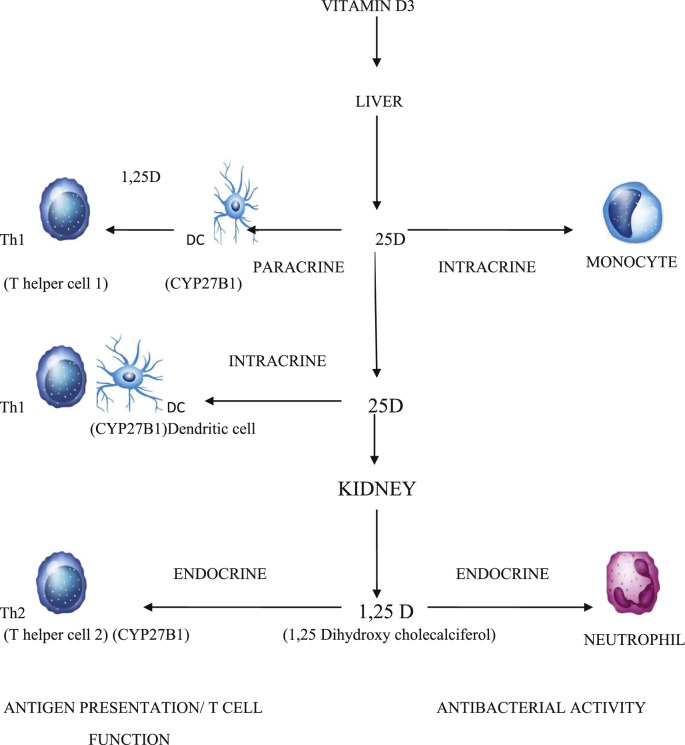
Almost all the cells of the immune system express vitamin D receptor (VDR). Cells of monocyte lineage can even produce local vitamin D. Vitamin D has a role in innate immune response to eliminating pathogens. Upon activation of VDR by pathogens, there is activation of several downstream antimicrobial factors like cathelicidin, CD 14, nucleotide oligomerization domain protein 2 (NOD 2) and many other signalling molecules [15]. VDR is also present in T lymphocytes where it acts as inhibitor of T lymphocyte activation. Vitamin D suppresses T cell mediated inflammation and enhances the effects of anti-inflammatory Treg cells [16]. Hence 1,25(OH) 2 D has a stimulatory effect on the native immune system (comprising mainly of monocytes/macrophages), whereas it has down regulatory effect on adaptive immune system (comprising mainly dependent of T-helper cells) [16]. These mechanisms explain how vitamin D deficiency is associated with increased risk of infections. [Fig. 1 ].
Fig. 1.
Role of vitamin D on immune regulation:Vitamin D is converted into 25-hydroxy cholecalciferol in liver and into 1,25 dihydroxy cholecalciferol in kidney and other tissues including dendritic cells (DC). Through paracrine and intracrine action, it regulates dendritic cells and inhibits proliferation of T helper cells (Th1), skewing cytokine production towards T helper 2 (Th2) cells. It has endocrine action on activation of antigen presenting cells like monocytes and on mobility of neutrophils contributing more to its antibacterial activity.
Vitamin D deficiency in diabetes is more prevalent in India and South East Asia. A study reported more common vitamin D deficiency among south Asians with T2DM living in the UK compared with the control group, which consisted of subjects without T2DM (83 vs 70%, p = 0.07, respectively) [17]. Braun et al. [18]showed that out of the total 1765 participants, the type 2 diabetes cases (50.2%) had a significantly higher prevalence of Vitamin D deficiency (83.5%) when compared to non-diabetic patients (68%). Bhatt et al. [19] observed a reduction in blood glucose both in fasting and 2 – hour post glucose challenge state, and in HbA1c in overweight/obese prediabetic vitamin D deficient Asian Indian women after 78 weeks of vitamin D supplementation.
5. Vitamin D and COVID -19
More and more evidences emerged linking vitamin D deficiency to COVID -19 morbidity and mortality. Agyun et al. [20] in a review opined that COVID-19 infectioninduced multiple organ damage which might be prevented by vitamin D supplementation. Interestingly, Hastie et al. [21] showed that out of 48,598 UK Biobank participants, 449 had confirmed COVID-19 infection and vitamin D deficiency invariably (OR = 0.99; 95% CI 0.99–0.999; p = 0.013) but they concluded that there was no evidence to support a potential role for 25-OH vitamin D concentration to explain susceptibility to COVID-19 infection either in overall subjects or in various ethnic groups.
A study [22] had shown that treatment with high dose of vitamin D to the tune of 2,50,000–5,00,000 IU was safe in general in critically ill mechanically ventilated patients and was associated with decreased hospital stay. Till now no randomized controlled trial evaluating the efficacy of vitamin D supplementation in sick COVID-19 patients with diabetes is available. Such study cannot be undertaken for technical reasons in the prevailing scenario. Considering the range of beneficial immune effects ascribed to vitamin D, its proven safety and ease of administration, vitamin D supplementation as an adjuvant therapeutic intervention in COVID-19 critically ill patients could be of paramount clinical and economic significance.
6. Conclusion
Diabetes is one of the most important co morbidities linked to the severity of COVID-19 infection. There is sufficient evidence of a shared pathophysiologic and mechanistic link between diabetes and COVID-19 infection which is more evident in the presence of vitamin D levels below 10 ng/ml. Health care providers need to ensure optimal metabolic control for all diabetic patients infected with COVID-19 virus and ensure adequate level of vitamin D as its supplementation offers a relatively safe, cheap and simple adjuvant therapy in such desperate situation.
Declaration of competing interest
I am submitting the manuscript entitled “COVID-19 and Diabetes” for publication as a review article of topical interest to the readers in your esteemed journal. All the authors have consented to submit the manuscript in the present form. This is also to be noted that we do not have any conflict of interest.
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel corona virus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of corona virus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel corona virus–infected pneumonia in wuhan, China. J Am Med Assoc. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J., Dong X., Cao Y., Yuan Y., Yang Y., Yan Y., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, ChinaAllergy. 2020 Feb 19. doi: 10.1111/all.14238. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 6.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes D.A., Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158:20–25. doi: 10.1111/j.1365-2249.2009.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwalfenberg G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2010;55:96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- 9.Vupputuri M.R., Goswami R., Gupta N., Ray D., Tandon N., Kumar N. Prevalence and functional significance of 25-hydroxyvitamin D deficiency and vitamin D receptor gene polymorphisms in Asian Indians. Am J Clin Nutr. 2006;83:1411–1419. doi: 10.1093/ajcn/83.6.1411. [DOI] [PubMed] [Google Scholar]
- 10.Odegaard J.I., Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harbor Perspect Med. 2012;2 doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiwari S., Pratyush D.D., Gupta S.K., Singh S.K. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br J Nutr. 2014;112:1938–1943. doi: 10.1017/S0007114514003018. [DOI] [PubMed] [Google Scholar]
- 12.Wellen K.E., Hotamisligil G.S. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drucker D.J. Coronavirus infections and type 2 diabetes—shared pathways with therapeutic implications. Endocr Rev. 2020:41. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riachy R., Vandewalle B., Moerman E., Belaich S., Lukowiak B., Gmyr V., et al. 1,25-dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the fas receptor. Apoptosis. 2006;11:151–159. doi: 10.1007/s10495-006-3558-z. [DOI] [PubMed] [Google Scholar]
- 15.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin N Am. 2010;39:365–379. doi: 10.1016/j.ecl.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhalla A.K., Amento E.P., Serog B., Glimcher L.H. 1,25- Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133(4):1748–1754. [PubMed] [Google Scholar]
- 17.Tahrani A.A., Ball A., Shepherd L., Rahim A., Jones A.F., Bates A. The prevalence of vitamin D abnormalities in South Asians with Type 2 diabetes mellitus in the UK. Int J Clin Pract. 2009;64(3):351–355. doi: 10.1111/j.1742-1241.2009.02221.x. [DOI] [PubMed] [Google Scholar]
- 18.Braun T.R. Vitamin D deficiency and cardio-metabolic risk in a north Indian community with highly prevalent type 2 diabetes. J Diabetes Metabol. 2012;3 doi: 10.4172/2155-6156.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatt S.P., Mishra A., Gulati S., Singh N., Pandey R.M. Lower vitamin D levels are associated with higher blood glucose levels in Asian Indian women with pre – diabetes: a population based cross – sectional study in North India. BMJ. Open. Diabetes Res Care. 2018;15(6) doi: 10.1136/bmjdrc-2017-000501. e 000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aygun H. Vitamin D can prevent COVID-19 infection-induced multiple organ damage2020 Jul;393(7):1157-1160. Naunyn-Schmiedeberg’s Arch Pharmacol. 2020;393(7):1157–1160. doi: 10.1007/s00210-020-01911-4. Epub 2020 May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hastie C.E., Mackay D.F., Ho F., et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. 2020;14(4):561–565. doi: 10.1016/j.dsx.2020.04.050. [published online ahead of print, 2020 May 7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han J.E., Jones J.L., Tangpricha V., Brown M.A., Brown L.A.S., Hao L., et al. High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial. J Clin Transl Endocrinol. 2016;4:59–65. doi: 10.1016/j.jcte.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]