Highlights
-
•
Helmet CPAP is effective to treat mild and moderate ARDS secondary to SARS COV 2.
-
•
Lung recruitment is not the only pathological mechanism responsible of CPAP effect.
-
•
Patients that improve PaO2/FiO2 ratio after one hour of CPAP have a lower mortality.
Keywords: COVID 19, Coronavirus, SARS COV 2
Abstract
Background
During the COVID-19 outbreak, a very high number of infected patients developed pneumonia and many of them complicated with acute respiratory distress syndrome. The optimal management of respiratory failure and the role of lung ultrasound imaging in the evaluation of efficacy of treatment are unknown.
Methods
In March 2020 we treated 18 patients with mild and moderate ARDS secondary to SARS-CoV-2 with non-invasive continuous positive airway pressure therapy (NI-CPAP). All patients underwent lung ultrasound imaging to verify the entity of lung recruitment after NI-CPAP initiation.
Results
After one hour of treatment we observed a significant improvement in PaO2/FiO2 ratio in 10 patients. Notably, only 50 % of them reached an effective improvement in lung aeration detectable with lung ultrasound. In the other 50 % or patients the improvement in PaO2/FiO2 might be related to blood redistribution and reverse of hypoxic vasoconstriction.
Conclusion
NI- CPAP is a valid therapeutic option in mild and moderate ARDS secondary SARS-CoV-2. Lung recruitment detected by means of lung ultrasound is a relevant but not the exclusive mechanism that underlies the therapeutic efficacy of NI-CPAP in this clinical setting.
1. Introduction
By the end of 2019, an outbreak of cases of unusual interstitial pneumonia named Coronavirus 19 disease (COVID-19) rapidly spread from China to the rest of the world with a very high number of infected patients that developed severe bilateral interstitial pneumonia, and many of them complicated with an acute respiratory distress syndrome (ARDS) with a high mortality rate (Rodriguez-Moralesa et al., 2020). Considering the large number of cases and the limited resources in terms of intensive care unit’ beds availability, many patients with ARDS needed to be treated with non-invasive continuous positive airways pressure (NI-CPAP) (Duca et al., 2020). NI-CPAP is an effective tool for the treatment of mild/moderate ARDS in intensive care unit and in patients without multi organ failure (Chiumello et al., 2017). From a pathophysiological standpoint, NI-CPAP promotes the recruitment of non aerated alveoli reducing extravascular lung water (EVLW), increases functional residual capacity and reduces work of breathing (Navalesi and Maggiore, 2013). In acute respiratory failure, NI-CPAP improves arterial oxygenation, reduces the rate of endotracheal intubation in comparison to the Venturi mask (Cosentini et al., 2010; Brambilla et al., 2014) and is not less effective than non-invasive pressure support ventilation (Pagano et al., 2018). Thorax Computer tomography (CT) is considered the gold standard chest imaging technique that allows to perform a quantitative analysis for lung aeration recruitment (Gattinoni et al., 2001), but its execution in critically-ill patients affected by contagious infectious disease is often not easily feasible and needs resources that prevent its routine use as a monitoring imaging tool. Moreover, there are some logistic concerns in performing lung CT during COVID-19 outbreak, mostly due to the need of a dedicated radiology and of moving unstable patients from shock room.
Thus, lung ultrasound (LUS) has been proposed as an alternative imaging technique in intensive care unit. LUS is an alternative, accurate technique to assess lung recruitment and to quantify the EVLW (Mongodi et al., 2014), and, among its most relevant advantages, it is repeatable and bedside practicable.
Aims of this study were to analyze the effects of NI- CPAP to improve oxygenation and lung aeration in patients with mild/moderate ARDS related to COVID-19, and to explore the role of LUS in the evaluation of response to NI-CPAP treatment.
2. Methods
2.1. Study population
This is an observational prospective study. Consecutive patients affected by acute respiratory failure due to COVID-19 admitted to COVID Care Unit of Santa Maria delle Grazie Hospital, Pozzuoli, Italy, from March to April 2020 were enrolled in the study. Inclusion criteria were: age > 18 years; diagnostic confirmation of COVID-19 infection through a nucleic acid test by real-time reverse transcription polymerase chain reaction; need of oxygen supplementation trough NI-CPAP with helmet Ventukit; presence of diagnostic criteria of mild to moderate ARDS (The ARDS definition task force, 2012).
2.2. Non-invasive ventilation protocol
All the patients underwent to a one-hour trial of NI-CPAP with StarMed Ventukit Helmet. Positive end expiratory pressure (PEEP) was set to 10 cm H2O and fraction of inspired oxygen (FiO2) was regulated to reach a target of oxygen peripheral saturation (SpO2) greater than 93 %. An arterial sample for blood gas analysis was collected before and after the NI-CPAP trial. The oxygenation status was assessed with the ratio between the partial pressure O2 to the FiO2 ratio (PaO2/FiO2). Patients with a P/F improvement at least of 15 % after one hour of NI-CPAP were considered responders. Not-responder patients underwent to endotracheal intubation (ETI) and were treated with invasive mechanical ventilation (MV). Not-responder patients aged greater than 75 years or affected by end-stage chronic diseases were considered do-not-intubate (DNI) patients and were managed with NI-CPAP. The study was conducted in accordance with the principles of the Declaration of Helsinki.
2.3. Lung ultrasound imaging
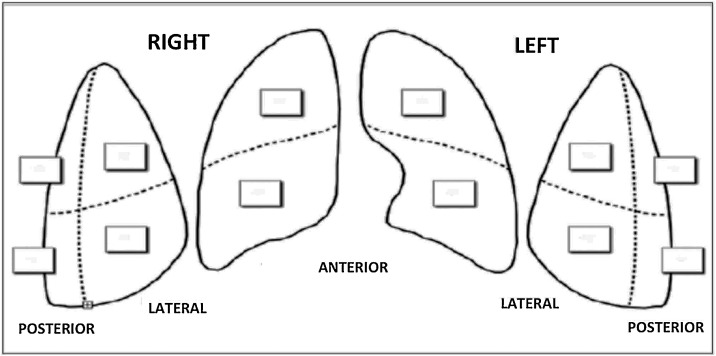
LUS was performed in each patient before and after application of NI-CPAP. Emergency physicians (P.A., B.G., A.E., P.G., S.C.) with expertise in LUS performed the examinations with a Samsung HR70A echographer, using convex probe (3,5 MHz). The chest wall was divided in three areas for each side: anterior, lateral and posterior. Each area was further divided in two sections (superior and inferior) for a total of twelve zones of examination (Fig. 1 ). Each zone was scored according to the lung ultrasound pattern as follows:
-
-
Score 0: presence of A-lines or fewer than three isolated B-lines
-
-
Score 1: presence of multiple well-spaced B-lines
-
-
Score 2: presence of coalescent B-lines with or without small subpleurical consolidations
-
-
Score 3: presence of lung consolidation.
Fig. 1.
Twelve zones’ model for LUS calculation.
The score observed in each region was recorded and their sum defined the total score (range 0–36), the higher LUS values the worse ultrasound pattern (Fig. 1) (Rouby et al., 2020).
2.4. Laboratory tests
Each patient underwent venous sample for the measurement of cell blood count, glucose, creatinine, aspartate-transamynase (AST), alanine-transamynase (ALT), lactate dehydrogenase (LDH), creatininphosphokinase (CPK), fibrinogen, C-reactive protein (CRP), procalcitonin (PCT), d-Dimer, coagulation panel.
Arterial blood samples were processed and instantly analyzed through a mobile point of care system (Cobas b 123, Roche). The radial artery was the site used for arterial puncture. Oxygenation status was assessed using partial pressure of O2 (pO2), partial pressure of CO2 (pCO2) and haemoglobin oxygen saturation (SO2). The oxygen partial pressure/oxygen inspiratory fraction (PaO2/FiO2) ratio was used to compare different values of arterial pO2 in patients receiving different FiO2 and it was obtained dividing the pO2 by the percent of FiO2 expressed as decimals. Among the other parameters, pH and bicarbonate concentration (HCO3-) were measured for the evaluation of acid-base disorders. Arterial blood lactate concentrations were also recorded.
2.5. Statistical analysis
Collected data were evaluated by performing parametric and non-parametric tests when appropriate. Student’s t-test and Mann–Whitney U test, ANOVA and linear correlation were performed to compare the differences between groups of continuous variables. The chi-square test with Yates correction or Fisher-exact-test were used to compare categorical variables. Statistical significance was defined for p < 0.05 in a two-tailed test with a 95 % confidence interval.
3. Results
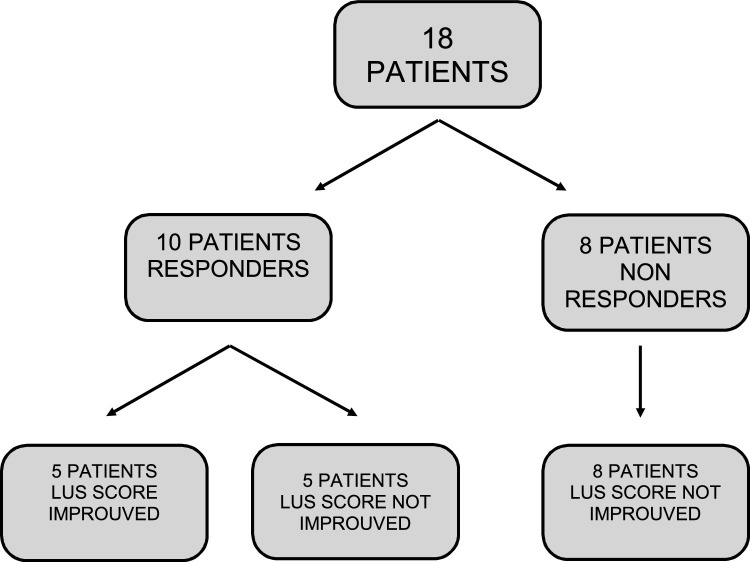
Eighteen patients with mild/moderate ARDS due to COVID-19 infection were enrolled. Among these, 13 were male, and the mean age was 69 years. At baseline, the mean PaO2/FiO2 was 153.2 ± 82 and LUS score was 17 ± 7. After one-hour-trial of NI-CPAP, 10 patients (55.5 %) were considered responders, while 8 patients were non-responders. Among the latter, 4 patients (22.2 %) did not receive ETI and were treated with MV, the others were considered as DNI and continued the treatment with NI-CPAP. There were not statistical differences in PaO2/FiO2 ratio and LUS score between responder and not-responder patients (PaO2/FiO2 ratio 143 ± 91 vs 167 ± 72, p-value = 0.5; LUS score 16 ± 7 vs 17 ± 8, p-value = 0.8). Among responders, 5 patients (27.7 %) showed an improvement in LUS score after one-hour-trial of NI-CPAP. Not-responders did not show any improvement in LUS score (Fig. 2 ). No statistical difference between the two groups was found in CRP, PCT, LDH, d-Dimer, lymphocytes, renal function (Table 1 ). Eleven patients died (61 %), 4 among the NI-CPAP responders group, and 7 in non-responders group (40 % versus 87 %, p-value = 0.004).
Fig. 2.
PaO2/FiO2 and ultrasound to CPAP.
Table 1.
Characteristic of patients at baseline.
| Responders | Not responders | ||
|---|---|---|---|
| Age | 70 ± 11 | 68 ± 14 | p > 0,5 |
| PO2/FiO2 ratio | 143 ± 91 | 167 ± 72 | p = 0,5 |
| LUS score | 16 ± 7 | 17 ± 8 | p > 0,5 |
| CRP | 21,3 ± 9 | 14,5 ± 5 | p = 0,1 |
| PCT | 1,3 ± 3 | 0,5 ± 0,5 | p = 0,5 |
| LDH | 465 ± 196 | 570 ± 190 | p = 0,3 |
| D Dimer | 4908 ± 11587 | 1033 ± 784 | p = 0,5 |
| Lymphocytes | 1035 ± 691 | 1342 ± 694 | p > 0,5 |
| Serum creatinine | 1,45 ± 0,6 | 1 ± 0,2 | p > 0,5 |
| APACHE II score | 13 ± 4 | 13 ± 7 | p > 0,5 |
4. Discussion
In our study we used NI-CPAP for treatment of mild-to-moderate ARDS due to COVID-19 infection. This indication is supported by literature in patients without multiorgan failure (Patel et al., 2016) and justified by outbreak of COVID-19 because of the lack of available ICU beds. When NI-CPAP was used in this setting, a proper multiparametric monitoring is critical, because the clinical worsening may happen quickly and a delayed ETI is associated to increased mortality (Kangelaris et al., 2016). Our COVID Care Unit has five ICU beds with availability of multiparametric monitoring specifically dedicated to not-invasive ventilation of patients affected by acute respiratory failure. The whole medical and nursing staff is continuously trained and constantly updated on the most recent advances in the monitoring and treatment of such clinical setting.
In this study NI-CPAP failed in 45 % of the patients. This is comparable to previous results reported from Patel and colleagues (Patel et al., 2016), that enrolled patients with mild-to-moderate ARDS not secondary to COVID-19 infection, while in a recent study the rate of NIV failure in 99 patients affected by SARS-CoV-2 was 87.1 % (Duca et al., 2020). Such difference could be explained considering the presence of a lower percentage of patients with severe ARDS in our study population (31 %). it can be hypothesized that the early use of NI-CPAP might have contributed to the lower rate of NIV failure that we recorded. Duca and Colleagues used NI-CPAP in patients with PaO2 <60 mmHg and/or respiratory rate >30/min after a 15-min-trial of O2 non-rebreather mask set at 15 L/min. In our study we did not apply such stringent criteria for selection of patients undergoing NI-CPAP, but, conversely, we treated our patients with NI-CPAP in a very early timing, from primary survey in emergency department, regardless respiratory rate or dyspnea.
NI-CPAP improves oxygenation in ARDS through lung tissue reaeration (Gattinoni et al., 2006). In this regard, LUS is a valid technique to asses lung recruitment (Gattinoni et al., 2020) that is useful for the evaluation of EVLW (Mongodi et al., 2014) and its improvement after application of PEEP. LUS score simplifies the analysis trough serial exams. Notably, in our study, only 5 responder patients reached an improvement in LUS score after one hour of NI-CPAP. Therefore, it is reasonable to hypothesize that the improvement of PaO2/FiO2 obtained in these patients is mostly related to lung recruitment and increase in ventilation/perfusion ratio. In 5 patients, PaO2/FiO2 improved without showing a LUS score reduction. This observation suggests that there are other pathophysiological mechanisms that underlie the improvement in the oxygenation, that may be specific of SARS-CoV-2 infection. Gattinoni and Colleagues hypothesized that COVID-19 pneumonia is an atypical form of ARDS (Gattinoni et al., 2020). In early stage, hypoxemia is due to hypoxic vasoconstriction with loss of vascular tone regulation and reduction of ventilation/perfusion ratio. In this stage of the disease, lung is poorly recruitable with PEEP. In these patients, the improvement of PaO2/FiO2 ratio could be due to higher and more stable FiO2 provided by NI-CPAP helmet and its effect on hypoxic vasoconstriction and blood redistribution in response to PEEP administration.
In our study mortality rate was 61 %, that is in accordance with the results of Duca and Colleagues. A good response to NI-CPAP has a beneficial impact on prognosis. Indeed, non responders have a double increase of mortality in comparison to responders. A delay in ETI could be one cause of this excess of mortality, but both the close monitoring of patients and the short term of NI-CPAP trial make this explanation unlikely.
There are some limitations that need to be accounted for the interpretation of our results, mainly related to the low number of patients enrolled and the monocenter and observational design of the study. Nevertheless, there are also some strengths that should be considered. Firstly, from a methodological standpoint, we made a strict selection of a subset of COVID-19 ARDS patients as described by the inclusion criteria in spite of the fact that the study was conducted during the outbreak emergency. Furthermore, the observations obtained using LUS raised interesting insights in the pathogenesis of SARS-CoV-2 infection, whose pulmonary complications seem to be different from the typical ARDS.
In conclusion, we demonstrated the efficacy of NI-CPAP for the treatment of SARS-CoV-2 associated ARDS, that seems to be only in part related by the increase in lung recruitment. Further studies are needed to better clarify the mechanisms that lead to ARDS in COVID-19 infection and to expand the role of LUS in the monitoring of NI-CPAP effects in COVID-19 infection.
Funding source
None.
Declaration of Competing Interest
All authors have not conflicts of interest.
References
- Brambilla A.M., Aliberti S., Prina E., Nicoli F., Del Forno M. Helmet CPAP vs. oxygen therapy in severe hypoxemic respiratory failure due to pneumonia. Intensive Care Med. 2014;40(8):1187. doi: 10.1007/s00134-014-3325-5. [DOI] [PubMed] [Google Scholar]
- Chiumello D., Brochard L., Marini J.J., Slutsky A.S., Mancebo J. Respiratory support in patients with acute respiratory distress syndrome: an expert opinion. Crit. Care. 2017;21(1):240. doi: 10.1186/s13054-017-1820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentini R., Brambilla A.M., Aliberti S., Bignamini A., Nava S. Helmet continuous positive airway pressure vs oxygen therapy to improve oxygenation in community-acquired pneumonia: a randomized, controlled trial. Chest. 2010;138(1):114–120. doi: 10.1378/chest.09-2290. [DOI] [PubMed] [Google Scholar]
- Duca A., Memaj I., Zanardi F., Preti C., Alesi A. Severity of respiratory failure and outcome of patients needing a ventilatory support in the Emergency Department during Italian Novel Coronavirus SARS-CoV- 2 outbreak: preliminary data on the role of Helmet CPAP and Non-Invasive Ventilation. Lancet. 2020 doi: 10.1016/j.eclinm.2020.100419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Caironi P., Pelosi P. What has computed tomogra-phy taught us about the acute respiratory distress syndrome? Am. J. Respir. Crit. Care Med. 2001;164(9):1701–1711. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- Gattinoni L., Caironi P., Cressoni M., Chiumello D., Ranieri V.M. Lung recruitment in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2006;319(7):698–710. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- Gattinoni L., Coppola S., Cressoni M., Busana M., Chiummiello D. Covid-19 does not lead to a “Typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangelaris K.N., Ware L.B., Wang C.Y., Janz D.R., Zhuo H. Timing of intubation and clinical outcomes in adults with acute respiratory distress syndrome. Crit. Care Med. 2016;44(1):120–129. doi: 10.1097/CCM.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongodi S., Algieri I., Mojoli F. CT scan and ultrasound comparative assessment of lung aeration in ARDS. Crit. Care. 2014 doi: 10.1186/cc13475. [DOI] [PubMed] [Google Scholar]
- Navalesi P., Maggiore S.M. Positive end-expiratory pressure. In: Tobin M.J., editor. In Principles and Practice of Mechanical Ventilation. 3rd ed. McGraw Hill Medical; New York, NY, USA: 2013. pp. 253–302. [Google Scholar]
- Pagano A., Numis F.G., Rosato V., Russo T., Porta G. Pressure support ventilation vs continuous positive airway pressure for treating of acute cardiogenic pulmonary edema: a pilot study. Respir. Physiol. Neurobiol. 2018;255:7–10. doi: 10.1016/j.resp.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Patel B.K., Wolfe K.S., Pohlman A., Hall J.B., Kress J.P. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome. JAMA. 2016;315(22):2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Moralesa A.J., Cardona-Ospinaa J.A., Gutiérrez-Ocampoa E., Villamizar-Peñaa R., Holguin-Riveraa Y. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med. Infect. Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouby J.J., Arbelot C., Gao Y. Training for lung ultrasound score measurement in critically ill patients. Am. J. Respir. Crit. Care Med. 2020;198(3):398–401. doi: 10.1164/rccm.201802-0227LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The ARDS definition task force Acute respiratory distress syndrome. The berlin definition. JAMA. 2012;319(7):698–710. doi: 10.1001/jama.2012.5669. [DOI] [Google Scholar]