While gastrointestinal symptoms are more and more recognized, the exact etiopathogenesis of SARS-CoV-2-associated diarrhea is still unknown [1]. It also remains unclear whether SARS-CoV-2 directly impacts enterocytes. The amino acid citrulline is not incorporated into proteins and is involved in intermediary metabolism. In humans, plasma citrulline is almost exclusively produced by enterocytes. Therefore, citrulline is used as a biomarker of small bowel enterocyte mass and function. For example, it is decreased in total villous atrophy as well as in short bowel syndrome with generally accepted thresholds of 10 µmol/L and 20 µmol/L, respectively [2]. The epithelial cells of the gastrointestinal tract express the angiotensin-converting enzyme 2 (ACE2), which is a crucial receptor for SARS-CoV2 [3,4]. Stanifer et al. provided in vitro evidence that SARS-CoV-2 could infect human intestinal cells. Moreover, they showed that the virus could replicate in intestinal epithelial cell lines as well as in human colon organoid models, therefore participating in the spread of SARS-CoV-2 with enhanced viremia [5,6].
As human evidences of a direct impact of SARS-CoV-2 on the enterocytes of the small intestine are lacking, we aimed to assess enterocytes' function in patients with SARS-CoV-2 infection by measuring plasma citrulline concentrations.
In a single academic center (Beaujon Hospital, APHP, France), during ten days (from April 14 to April 24, 2020), we prospectively enrolled consecutive patients hospitalized for a PCR-confirmed COVID-19. Following ethical committee's standard (IRB 00,006,477), we collected clinical features and performed a biological assessment, including citrulline measured by liquid chromatography-mass spectrometry and inflammatory biomarkers.
Twenty-six patients with a wide range of severity of the disease were enrolled, including five who were admitted in an intensive care unit (ICU). Studied patients had a mean age of 61.2 years (standard deviation of 13.8) and 26.7% were females. 42.3% of patients presented diarrhea (extensive clinical characteristics are presented in the Table 1 ).
Table 1.
Characteristics of patients with low or normal concentrations of plasma citrulline.
| Clinical characteristics | Citrulline < 20 µmol/L | Citrulline > 20 µmol/L | p value | |
|---|---|---|---|---|
| (n = 16) | (n = 10) | |||
| Age (mean +/- SD) | 57.8 +/−11.1 | 66.6 +/−16.6 | 0.16 | |
| Sex (female (n,%)) | 4 (25%) | 3 (30%) | 1 | |
| Time from onset of symptoms (days) | 14.8 +/−8.2 | 16.3 +/−14 | 0.77 | |
| Cardiovascular risk factors | Elevated blood pressure | 7 (43.8%) | 4 (40%) | 1 |
| Diabetes | 8 (50%) | 4 (40%) | 0.7 | |
| Dyslipediamia | 4 (25%) | 2 (20%) | 1 | |
| Cardiovascular history | 0 (0%) | 2 (20%) | 0.14 | |
| Pulmonary diseases | COPD | 1 (6.3%) | 1 (10%) | 1 |
| Asthma | 2 (12.5%) | 0 (0%) | 0.51 | |
| History of IBD | 0 (0%) | 0 (0%) | 1 | |
| Smoking | 0 (0%) | 1 (10%) | 0.38 | |
| Medications | ARB2 | 2 (12.5%) | 2 (20%) | 0.63 |
| Steroids | 0 (0%) | 0 (0%) | 1 | |
| NSAID | 0 (0%) | 0 (0%) | 1 | |
| Clinical features | Fever | 12 (75%) | 6 (60%) | 0.66 |
| Coughing | 13 (81.3%) | 8 (80%) | 1 | |
| Shortness of breath | 6 (37.5%) | 5 (50%) | 0.69 | |
| Anosmia | 5 (31.3%) | 2 (20%) | 0.67 | |
| Ageusia | 6 (37.5%) | 2 (20%) | 0.42 | |
| Arthromyalgia | 6 (37.5%) | 3 (30%) | 1 | |
| Anorexia | 10 (62.5%) | 4 (40%) | 1 | |
| Nausea/vomiting | 3 (18.8%) | 0 (0%) | 0.26 | |
| Abdominal pain | 2 (12.5%) | 0 (0%) | 0.6 | |
| Diarrhea | 9 (56.3%) | 2 (20%) | 0.11 | |
| Digestive symptoms | 10 (62.5%) | 2 (20%) | 0.05 | |
| Physical characteristics | Abdominal circonference | 100.3 +/−14.2 | 104.4 +/−12.9 | 0.5 |
| Baseline body mass index | 28.6 +/−8.3 | 27.4 +/−3.7 | 0.42 | |
| CT-scan | Lung area affected > 25% | 8 (50%) | 2 (20%) | 0.22 |
| Outcomes | Admission in ICU | 4 (25%) | 1 (10%) | 0.62 |
| Orotracheal intubation | 3 (18.8%) | 0 (0%) | 0.26 | |
| Death | 1 (6.3%) | 0 (0%) | 1 |
ARB2: angiotensin II receptor blocker; COPD: chronic obstructive pulmonary disease; CT-scan: computarized tomography scan; ICU: intensive care unit; NSAID: non-steroidal anti-inflammatory drug; SD: standard deviation.
Sixteen out of the 26 patients (61.5%) presented concentrations of plasma citrulline below 20 µmol/L and 4 (15.4%) exhibited citrulline plasma concentrations below 10 µmol/l. Interestingly, digestive symptoms (including nausea/vomiting, abdominal pain, and diarrhea) were more frequent in patients with low citrulline (below 20 µmol/L) as compared with patients with normal citrulline (62.5% versus 20%, p = 0.05, Fisher's exact test). Clinical markers of severity (lung surface area affected, ICU admission, orotracheal intubation) were all numerically higher in patients with low citrulline, although they did not reach statistical significance.
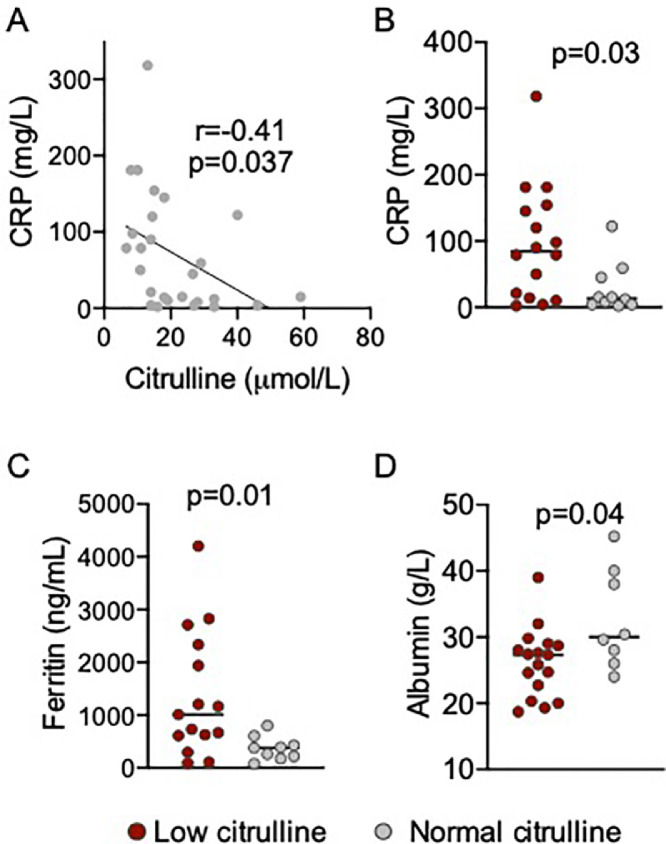
We then correlated biomarkers of systemic inflammation with citrulline. C-reactive protein concentrations were higher in patients with citrulline level below 20 µmol/L as compared to patients with normal concentrations of citrulline (84.5 versus 13.5 mg/l, p = 0.03). Similarly, ferritin concentrations, which are a hallmark of severe COVID-19 with hemophagocytic lymphohistiocytosis features, were higher in patients with low citrulline (1009 versus 379 ng/mL, p = 0.01) (Fig. 1 ).
Fig. 1.
Plasma citrulline inversely correlates with systemic inflammation in patients with COVID-19.
(A) Correlation between plasma level of citrulline and C-reactive protein (CRP). Pearson correlation test. (B) CRP levels of patients with low or normal citrulline (20 µmol/L threshold). Mann-Whitney test. (C) Ferritin level of patients with low of normal citrulline. Mann-Whitney test. (D) Albumin level of patients with low of normal citrulline. Mann-Whitney test.
In our cohort study, we showed that a majority of patients with moderate to severe COVID-19 had decreased levels of plasma citrulline, which correlated with digestive symptoms and systemic inflammation. In addition to previous indirect evidence, our study suggests that enterocytes themselves may be affected by SARS-CoV-2, leading to gastrointestinal symptoms and worsening of systemic inflammation. Low citrulline is likely involved in SARS-CoV-2-associated diarrhea by causing alteration of intestinal permeability and enterocyte malabsorption. It could also participate in the significant weight loss that is observed in patients with COVID-19.
Our study included a relatively low number of patients that limits the statistical power of the analysis. We did not include stool assessment due to safety regulations but previously published stool analyses reported that patients with COVID-19-associated diarrhea had elevated fecal calprotectin levels in patients with active diarrhea [7]. Interestingly, the level of fecal calprotectin did not correlate with the level of viral RNA in the stool but positively correlated with the level of systemic inflammation [7].
We report here for the first time, particularly low concentrations of plasma citrulline in patients with COVID-19. Citrulline is very specific of total enterocyte mass, suggesting a direct impact of SARS-CoV-2 on the enterocytes. Intestinal ischemia, through hypoxic injury and microvascular coagulopathy induced by SARS-CoV-2, could also be considered as a mechanism of enterocytes dysfunction [8].
Declaration of Competing Interest
The authors declare no competing interest related to this work.
Acknwledgement
The authors would like to thank Alexandre Nuzzo, Kahina Sadaouli and Hana Manceau for their involvement in the study as they participate in data generation, data collection and data analysis. They also critically reviewed the manuscript.
References
- 1.Zhang H., Liao Y.S., Gong J., Liu J., Xia X., Zhang H. Clinical characteristics of coronavirus disease (COVID-19) patients with gastrointestinal symptoms: a report of 164 cases. Dig Liver Dis. 2020;0 doi: 10.1016/j.dld.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crenn P., Messing B., Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Liang W., Feng Z., Rao S., Xiao C., Xue X., Lin Z. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 4.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanifer M.L., Kee C., Cortese M., Triana S., Mukenhirn M., Kraeusslich H.-.G. Critical role of type III interferon in controlling SARS-CoV-2 infection, replication and spread in primary human intestinal epithelial cells. BioRxiv. 2020.04.24;2020 doi: 10.1101/2020.04.24.059667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamers M.M., Beumer J., van der Vaart J., Knoops K., Puschhof J., Breugem T.I. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;80:eabc1669. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Effenberger M., Grabherr F., Mayr L., Schwaerzler J., Nairz M., Seifert M. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut. 2020 doi: 10.1136/gutjnl-2020-321388. gutjnl-2020-321388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]