Abstract
The race for a vaccine against SARS‐CoV‐2 has accelerated research on RNA‐based therapeutics. Beyond vaccines, RNA also shows great potential for cancer therapies.

Subject Categories: S&S: Economics & Business, Chemical Biology, RNA Biology
After a long incubation time, RNA‐based therapeutics are coming of age and offer a big hope for an urgently needed SARS‐CoV‐2 vaccine. As the COVID‐19 outbreak is ravaging communities worldwide and exacting a heavy toll in terms of lives and economic costs (https://coronavirus.jhu.edu/)—with nothing to counter its spreading but social distancing—many put their hopes on the new kid among the therapeutics: RNA. Whether targeting nucleic acids or proteins, RNA therapies are emerging in the form of a large platform rather than a single approach, with great expectations for a new wave of drugs to treat a range of ailments.
Whether targeting nucleic acids or proteins, RNA therapies are emerging in the form of a large platform rather than a single approach …
Infectious diseases, cancers, rare genetic defects, metabolic disorders, degenerative diseases, you name it: RNA seems to be the solution for each and every one. The metaphor of the Swiss army knife is often abused, but it fits RNA like a glove given the multiple roles played by this key molecule: messenger RNA (mRNA), microRNA (miRNA), or small interfering RNA (siRNA).
Slow progress and successes
The beginning of the RNA therapies universe can be safely placed in 1998, with the discovery by Andrew Fire and Craig Mello of RNA interference (RNAi) as a fundamental mechanism for the regulation of gene expression. In brief, RNAi works through gene silencing, degrading the mRNA for a specific protein through the coordinated action of double‐stranded RNA and a complex biochemical machinery it activates (Fig 1). The possibility of harnessing the newly identified gene silencing mechanism for developing innovative therapeutics was soon evident and is now finally becoming reality, notably in the form of siRNAs, synthetic RNA duplexes usually 21–25 nucleotides long.
Figure 1. RNA interference and its discovery.
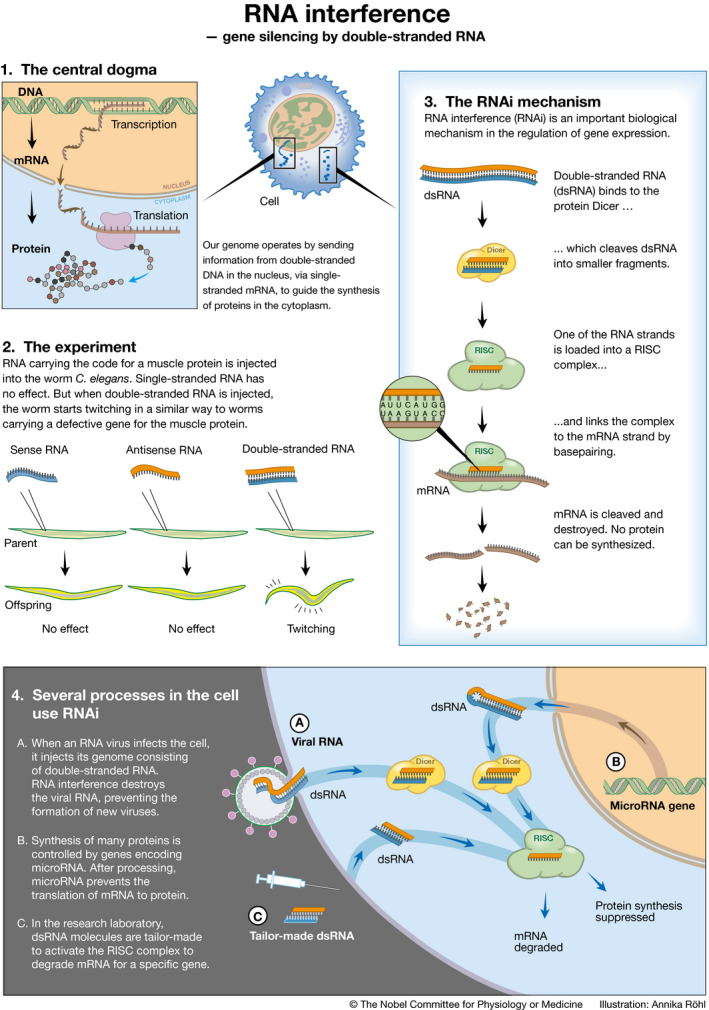
The Nobel Prize in Physiology or Medicine for 2006 was awarded jointly to Andrew Fire and Craig Mello “for their discovery of RNA interference—gene silencing by double‐stranded RNA”, a fundamental mechanism of gene regulation. © Annika Röhl/The Nobel Committee for Physiology or Medicine.
However, the road to success was a bumpy one. After the initial excitement, with lots of biotech companies sprouting around RNAi discovery, it became soon evident that it was not an easy task. Delivering intact siRNAs into target cells and tissues proved to be particularly challenging. A whirl of disinvestments, mergers, and discounted acquisitions followed (Lawrence, 2018). Despite all the setbacks, the first therapy based on RNAi gene silencing was approved in 2018 by the FDA. The key molecule is patisaran (marketed as Onpattro), a double‐stranded siRNA to treat a rare and often fatal hereditary condition known as transthyretin‐mediated amyloidosis (hATTR). This progressive degenerative disease is caused by the misfolding of the liver protein transthyretin (TTR), which leads to the accumulation of amyloid deposits in the peripheral nervous system but also in the heart, kidneys, eyes, and gastrointestinal tract. The drug uses lipid nanoparticles as delivery system and acts by inhibiting the synthesis of TTR, thus reducing the resultant amyloid deposits in the tissues (Akinc et al, 2019).
Despite all the setbacks, the first therapy based on RNAi gene silencing was approved in 2018 by the FDA.
While the approval of Onpattro was a major breakthrough, other RNA‐based therapeutics had made it to the clinic even before. At the end of 2004, the RNA aptamer pegaptanib, developed by Eyetech Pharmaceuticals and Pfizer under the brand name Macugen, received green light by the FDA for the treatment of age‐related macular degeneration (AMD) (Wu & Turnbull, 2018). Aptamers are oligonucleotides able to bind proteins with high affinity, and pegaptanib is specifically directed against VEGF‐165, an isoform of the vascular endothelial growth factor that plays a key role in AMD vascular pathology. Two other RNA drugs gained FDA approval in 2016, nusinersen (Spinraza) and eteplirsen (Exondys 51), used to treat spinal muscular atrophy and Duchenne muscular dystrophy, respectively. Both are examples of antisense RNA, single‐stranded RNA that binds to the mRNA coding a protein involved in disease pathology, and blocks mRNA translation or alters its splicing (Wu & Turnbull, 2018).
Cost is an issue
The future of RNA drugs might be bright, but it will be expensive. The cost of treating hATTR with Onpattro is estimated at US$ 450,000 per patient per year, and, since it is not a curative agent, the treatment will need to be maintained for lifetime. Exondys 51, that benefitted from an accelerated approval process by the FDA, can cost up to US$ 1 million a year. “One of the top explanations that drug companies give for the high cost of their products is that it's expensive to get drugs through clinical trials and across the regulatory finish line. When those requirements are truncated, the resulting drugs ought to cost less, not more”, commented the Editorial Board of The New York Times in an opinion article, asking for price caps for drugs approved with an expedite pathway (https://www.nytimes.com/2019/08/13/opinion/novartis-drug-cost.html). On the other hand, one could plausibly expect prices to fall when more therapeutics come into practice. “Advances on the scale of RNA therapies do not come cheap, and we should not underestimate the value of an effective therapy to the family of a child with spinal muscular atrophy”, noted Lorna Harries, a molecular geneticist at the University of Exeter, UK (Harries, 2019).
Money will not be a problem for developing a vaccine against SARS‐CoV‐2.
Vaccines against SARS
Money will not be a problem for developing a vaccine against SARS‐CoV‐2 (Fig 2). As the pandemic spreads across the globe, funds are pouring from both public and independent sources. The Coalition for Epidemic Preparedness Innovations (CEPI) estimated the cost to develop such a vaccine within the next 12–18 months at US$ 2 billion (https://cepi.net/covid-19/). The usual time frame to develop a vaccine is between 6 and 10 years; to get a COVID vaccine to market, so rapidly will therefore require regulatory compression, like shortening animal tests. In mid‐June this year, the World Health Organization (WHO) issued a “draft landscape of COVID‐19 candidate vaccines”, listing scores of products (the number is growing by the day) at various stages of clinical development (https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines). The aspirant vaccines were grouped in “platforms”, including protein subunits, DNA, RNA, and non‐replicating viral vectors.
Figure 2. Coronavirus @ Molecular Landscapes.

This painting depicts a coronavirus just entering the lungs, surrounded by mucus secreted by respiratory cells, secreted antibodies, and several small immune systems proteins. The painting is based on information about the SARS virus. Illustration by David S. Goodsell, RCSB Protein Data Bank, PDB‐101 (PDB101.rcsb.org); https://doi.org/10.2210/rcsb_pdb/goodsell-gallery-019. Reproduced with permission.
Several of the entries in the WHO list, including two of the eleven in Phase 1/3—all the others are still in pre‐clinical research with a handful expected to enter clinical trials in the next months—are mRNA‐based vaccines. The spearhead is mRNA‐1273, which encodes a prefusion‐stabilized form of the spike (S) protein of SARS‐CoV‐2, developed at an unusually fast rate (first volunteer injected on Mach 16, only 65 days after the publication of the virus sequence) by Moderna, a biotech based in Cambridge, Massachusetts (https://www.modernatx.com/). In mid‐April this year, the company announced receiving US$483 million from the US Biomedical Advanced Research and Development Authority (BARDA), to speed up the development of its candidate. “If supported by safety data from the Phase 1 study, the Company intends to begin a Phase 2 study of mRNA‐1273….in the second quarter of 2020”, reads a relevant press release (https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-award-us-government-agency-barda-483-million). “Subject to data from these studies and discussions with regulators, a Phase 3 study could begin as soon as fall, 2020”. In mid‐May, Moderna announced positive interim clinical data for mRNA‐1273 (https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-positive-interim-phase-1-data-its-mrna-vaccine).
Another mRNA COVID‐19 vaccine program that has recently started human trials is developed by the German biotech company BioNTech, in collaboration with Pfizer (https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-dose-first-participants-us-part-global-covid). Four vaccine candidates will be studied, two of which include a nucleoside‐modified mRNA (modRNA), one a uridine‐containing mRNA (uRNA), while the fourth vaccine candidate utilizes self‐amplifying mRNA.
RNA‐based vaccines
The mRNA vaccines against SARS‐CoV‐2 are not the first of their type, but rather the last shoot in a rapidly growing tree (Jackson et al, 2020) (Fig 3). Although there is still no product on the shelves, a number of pre‐clinical and clinical trials have shown promising results against a range of infectious diseases, including Zika, Ebola, dengue, and papillomavirus. The main advantage of the mRNA approach to vaccine development is rapid and scalable manufacturing, a very attractive feature in times of epidemic emergency. Moreover, RNA vaccines may be able to elicit a stronger and broader immune response since they stimulate both the adaptive and innate immune systems. In addition, RNA vaccines are cell‐free, non‐infectious, and not‐integrating, thus eliminating the risks of infection and insertional mutagenesis that comes with viral vectors. Multi‐component proteins that would be impossible to target with other systems are accessible with RNA technology, as recently demonstrated by the generation of a multi‐antigenic mRNA vaccine encoding human cytomegalovirus glycoproteins gB and pentameric complex (John et al, 2018). Instability and inefficient delivery, two of the main hurdles that hampered RNA vaccines success so far, have been largely overcome by recent improvements (Pardi et al, 2020).
Figure 3. Conceptual framework for the production of mRNA vaccines.
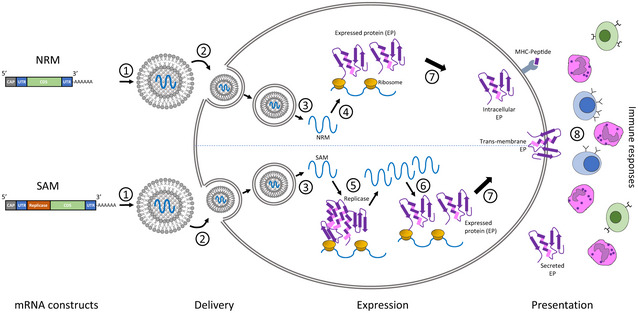
Two categories of mRNA constructs are being actively evaluated. Non‐replicating mRNA (NRM) constructs encode the coding sequence (CDS) and are flanked by 5′ and 3′ untranslated regions (UTRs), a 5′‐cap structure and a 3′‐poly‐(A) tail. The self‐amplifying mRNA (SAM) construct encodes additional replicase components able to direct intracellular mRNA amplification. (1) NRM and SAM are formulated in this illustration in lipid nanoparticles (LNPs) that encapsulate the mRNA constructs to protect them from degradation and promote cellular uptake. (2) Cellular uptake of the mRNA with its delivery system typically exploits membrane‐derived endocytic pathways. (3) Endosomal escape allows release of the mRNA into the cytosol. (4) Cytosol‐located NRM constructs are immediately translated by ribosomes to produce the protein of interest, which undergoes subsequent post‐translational modification. (5) SAM constructs can also be immediately translated by ribosomes to produce the replicase machinery necessary for self‐amplification of the mRNA. (6) Self‐amplified mRNA constructs are translated by ribosomes to produce the protein of interest, which undergoes subsequent post‐translational modification. (7) The expressed proteins of interest are generated as secreted, trans‐membrane, or intracellular protein. (8) The innate and adaptive immune responses detect the protein of interest. Reproduced from (Jackson et al, 2020), with permission.
The main advantage of the mRNA approach to vaccine development is rapid and scalable manufacturing, a very attractive feature in times of epidemic emergency.
“mRNA platforms can be designed very fast to express an optimized version of the gene of interest and their production is much easier than the traditional vaccine platforms. In addition, you can also combine mRNAs coding for multiple antigens to increase the chance of inducing both production of neutralizing antibodies and resident memory cytotoxic T cells”, explained Chantal Pichon, a molecular and cell biologist at the French National Centre for Scientific Research (CNRS) in Orléans. “This could be done through different mRNAs coding those antigens or a polycistronic mRNA coding the multiple sequences for those antigens”. However, gaps still exist in our knowledge on how different mRNAs are processed and sensed once they are injected, which will require more basic studies, Pichon warns. “The fundraising obtained by companies, mostly in the US, has invigorated both basic and translational research around this approach, which can be applied beyond vaccination”, Pichon concludes. “For sure, mRNAs hold a real promise as biopharmaceuticals for the years to come”.
However, despite the urgency dictated by the COVID‐19 pandemic and various calls for international collaboration and data sharing in order to “reduce inefficiencies and duplication of effort” (https://www.who.int/news-room/detail/13-04-2020-public-statement-for-collaboration-on-covid-19-vaccine-development), the landscape looks much fragmented, with probably too many candidate vaccines being pushed forward, and a risk of dispersing much needed resources (Berkley, 2020). The consequences of failure would be dreadful. “Novel infectious diseases resulting from RNA viruses subject to mutation and genetic recombination, as well as cross‐species transmission, will continue to present a serious global health threat, as exemplified by COVID‐19”, recently remarked Cynthia Liu and colleagues at the American Chemical Society (Liu et al, 2020). “Despite two former major outbreaks of coronavirus infections causing the SARS and MERS respiratory illnesses, the world remains underprepared to effectively manage the current COVID‐19 outbreak, as evidenced by the fact that COVID‐19 has resulted in thousands of deaths worldwide”.
The scenario of cancer RNA immunotherapeutics is rapidly expanding, with clinical studies of known molecules being carried out and new classes of compounds emerging.
Vaccinating against cancer
Another key area, where RNAs are expected to have a significant impact, is cancer therapy. Here, again, mRNA vaccines are bound to play a major role in the future. The idea behind many ongoing clinical trials is to elicit an antitumor immune response in patients by challenging them with mRNAs encoding tumor‐specific antigens (Pastor et al, 2018; Pardi et al, 2020). These mRNAs can be directly injected as naked RNA or loaded into patient‐derived dendritic cells (Fig 4). The identification of patient‐specific tumor antigens can also allow for personalized mRNA cancer vaccines. “RNA‐based cancer vaccines have the advantage of providing transient expression of tumor antigens at high levels”, said Kenneth Lundstrom, an expert in cancer vaccines at PanTherapeutics, Lutry, Switzerland. “Especially, application of RNA replicons from self‐amplifying RNA viruses provides the means for direct antigen mRNA amplification in the cytoplasm leading to extremely high levels of antigen expression for optimal induction of antibodies”.
Figure 4. Personalized mRNA‐based antitumor vaccines.
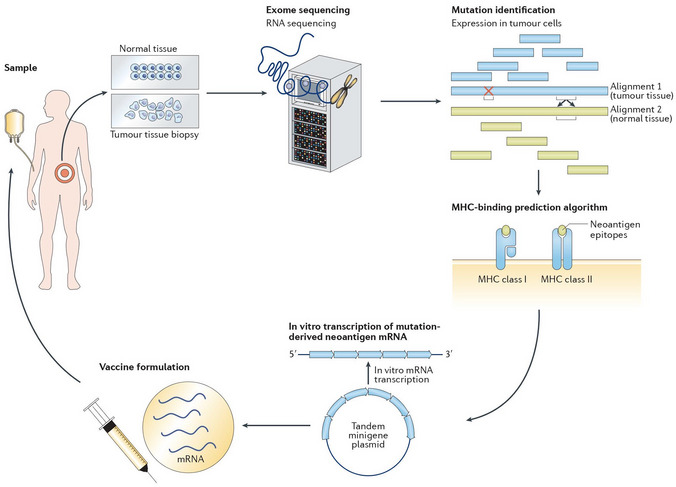
The exome of tumor cells isolated from a biopsy sample and the exome of normal cells are compared to identify tumor‐specific mutations. Point non‐synonymous mutations, gene deletions, or rearrangements can give rise to neoantigens. Several bioinformatics tools are used to predict major histocompatibility complex (MHC) class I and class II binding (necessary for recognition by T cells) and RNA expression presence of the mutated antigen among tumor cells (clonality). RNA sequencing enables verification that the gene encoding the neoantigen is actually expressed by tumor cells. A tandem gene encoding several neoantigen peptides is cloned into a plasmid and transcribed to mRNA. Finally, these mRNAs are injected as naked RNA, formulated into liposomes or loaded into dendritic cells. Reproduced from (Pastor et al, 2018), with permission.
Most such therapeutics are still in Phase 1/2, but there are promising pre‐clinical indications that this approach might trigger an anti‐cancer immune response. CV9202, for example, is a mRNA encoding six antigens usually expressed in non‐small‐cell lung cancer (NSCLC), developed by CureVac, a biotech based in Tübingen, Germany (https://www.curevac.com/), in collaboration with Boehringer Ingelheim. The mRNA has been complexed with the cationic protein protamine to protect it from degradation and increase immunogenicity; CV9202 is currently being investigated in a Phase 1/2 trial in metastatic NSCLC patients (https://clinicaltrials.gov/ct2/show/NCT03164772?term=NCT03164772&draw=2&rank=1).
Other forms of cancer are also in the crosshairs. “Melanoma seems like a good target, which has shown promise in clinical trials”, said Lundstrom. “Liposome and polymer encapsulation of RNA can further provide protection against degradation and host immune recognition and can target and improve RNA delivery”. Needless to say, success is not guaranteed. In April 2018, Argos Therapeutics discontinued a Phase 3 trial of its dendritic cells‐loaded mRNA vaccine (Rocapuldencel‐T) against metastatic renal cell carcinoma after disappointing results (https://www.targetedonc.com/view/phase-iii-rcc-trial-stopped-after-rocapuldencelt-falls-short).
Novel approaches and strategies
“mRNA is the only vaccine format that is highly robust in terms of production – any sequence can be produced with the exact same manufacturing process – and very reliable in terms of efficacy, as it naturally induces an innate immune response on its own and at the same time an adaptive immune response against the encoded protein”, commented Steve Pascolo, a molecular biologist at the University of Zurich, Switzerland. “Therefore, its current evaluation in different formats such as naked or formulated in particles, self‐amplifying or non‐replicating, or different site of injection and design (e.g., coding wild type proteins, modified antigens or epitopes), will with no doubt lead to commercialization of superlative vaccines against cancer and infectious diseases”.
The scenario of cancer RNA immunotherapeutics is rapidly expanding, with clinical studies of known molecules being carried out and new classes of compounds emerging. The potential of RNA aptamers (see above), to target cancer‐specific proteins, has been exploited by NOXXON Pharma (Berlin, Pharma). Their NOX‐A12 (Olaptesed pegol), a pegylated L‐oligoribonucleotide that binds and neutralizes CXCL12, a chemokine promoting tumor proliferation, yielded encouraging results from a Phase 1/2 trial in metastatic pancreatic and colorectal cancer and is now being tested for other oncological applications (https://www.noxxon.com/downloads/pressrel/2020-04-24_GBM_Cohort_1_DSMB_PR_EN.pdf).
London‐based MiNA therapeutics, a biotech graduated from the Imperial College's Translation & Innovation Hub, is on a more innovative path. The company's technology hinges around small activating RNAs (saRNA), short oligonucleotides similar in structure to siRNAs, that upregulate the expression of their target gene. MiNA's lead, MTL‐CEBPA, promotes the expression of CEBPα, the product of a tumor suppressor gene that is downregulated in various cancers. This approach is being tested in patients with hepatocarcinoma and is now entering a Phase 1/1b clinical study as a treatment for advanced solid tumors. “MTL‐CEBPA has shown real promise as a combination agent in patients with liver cancer and pre‐clinical studies suggest that MTL‐CEBPA may also be an attractive agent to enhance the benefits of checkpoint inhibitors”, commented Ruth Plummer, Newcastle University Centre for Cancer, and leading scientist of the new study (https://minatx.com/wp-content/uploads/2020/03/2020303_MiNA_Timepoint-Enrolment_FINAL.pdf). “We are looking forward to evaluating this highly innovative combination treatment in the upcoming Phase I trial. We hope this combination could become an effective new treatment option for patients with solid tumour cancers”.
With the first results from anti‐SARS‐CoV‐2 mRNA vaccines just weeks way, RNA therapeutics have their once‐in‐a‐lifetime opportunity to ultimately prove their value. To expand our possibilities to treat a variety of serious diseases, and to be ready, for when the next virus will spill over into humans.
EMBO Reports (2020) 21: e51013
References
- Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du MJ, Hope MJ, Madden TD et al (2019) The Onpattro story and the clinical translation of nanomedicines containing nucleic acid‐based drugs. Nat Nanotechnol 14: 1084–1087 [DOI] [PubMed] [Google Scholar]
- Berkley S (2020) COVID‐19 needs a big science approach. Science 367: 1407 [DOI] [PubMed] [Google Scholar]
- Harries L (2019) It's time for scientists to shout about RNA therapies. Nature 574: S15 [DOI] [PubMed] [Google Scholar]
- Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F (2020) The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines 5: 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S, Yuzhakov O, Woods A, Deterling J, Hassett K, Shaw CA, Ciaramella G (2018) Multi‐antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell‐mediated immunity. Vaccine 36: 1689–1699 [DOI] [PubMed] [Google Scholar]
- Lawrence J (2018) RNA interference therapies could be on the cusp of success. Pharm J 300: 7913 [Google Scholar]
- Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, Smoot J, Gregg AC, Daniels AD, Jervey S et al (2020) Research and development on therapeutic agents and vaccines for COVID‐19 and related human coronavirus diseases. ACS Cent Sci 6: 315–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Hogan MJ, Weissman D (2020) Recent advances in mRNA vaccine technology. Curr Opin Immunol 65: 14–20 [DOI] [PubMed] [Google Scholar]
- Pastor F, Berraondo P, Etxeberria I, Frederick J, Sahin U, Gilboa E, Melero I (2018) An RNA toolbox for cancer immunotherapy. Nat Rev Drug Discov 17: 751–767 [DOI] [PubMed] [Google Scholar]
- Wu X, Turnbull AP (2018) Current trends in RNA‐based therapeutic development. Drug Development World, https://www.ddw-online.com/media/32/133161/(2)-current-trends-in-rna.pdf


