Abstract
Results of previous studies regarding the effect of L-carnitine on lipid profiles in the patients with liver diseases are contradictory. This meta-analysis was performed to assess the effect of L-carnitine on serum levels of low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride (TG), and total cholesterol (TC) in overweight patients with liver diseases. A systematic search was carried out using the Web of Science, PubMed, Scopus, and Cochrane library databases to identify articles published before April 2019 investigating the effects of L-carnitine supplementation on patients with liver disease. There was no language or time limitation for the studies. A meta-analysis was carried out using both the random and fixed effects model where appropriate, and I2 index were used to evaluate heterogeneity. These results indicated that L-carnitine supplementation significantly reduces blood levels of TC and TG in patients with liver disease, whereas carnitine had no effect on the levels of HDL and LDL. The reducing effect of L-carnitine on both TC and TG was found following long-term carnitine supplementation (≥24 weeks), supplementation with doses less than or equal to 2,000 mg/d, and in patients with chronic hepatitis C. This meta-analysis indicates the beneficial effect of L-carnitine on TC and TG in overweight patients with liver disease, particularly patients with chronic hepatitis C, in both long-term and low doses.
Keywords: high-density lipoprotein, L-carnitine, low-density lipoprotein, total cholesterol, triglyceride
INTRODUCTION
It is well known that the liver plays an important role in lipogenesis, gluconeogenesis, and cholesterol metabolism (Ponziani et al., 2015). Over the last decade, it has been shown that metabolic syndrome and obesity promote pathophysiological changes that can cause liver damage and ultimately lead to liver disease, such as non-alcoholic fatty liver disease (NAFLD), the most common liver disease in Western societies (Bechmann et al., 2012). Lipid accumulation and oxidation of fatty acids play an important role in damage, repair, and regeneration of the liver due to autosomal induction and production of reactive oxygen species (ROS) (Bechmann et al., 2012). Dyslipidemia is prevalent amongst people with NAFLD and has an important role in the development of cardiovascular disease, which is the leading cause of mortality for these patients (Speliotes et al., 2018). Dyslipidemia in patients with NAFLD is typically characterized by increased levels of low-density lipoproteins (LDL) and hypertriglyceridemia, and decreased levels of high-density lipoproteins (HDL) (Speliotes et al., 2018). Furthermore, it has been shown that treatment with interferon alpha (IFNα) in combination with ribavirin in patients infected with hepatitis C virus influences lipid metabolism (Malaguarnera et al., 1995). These therapies reduce serum HDL levels and increase triglyceride (TG) levels and, consequently, the amount of fat in the liver (Naeem et al., 2001).
Exercise and diet are the first-line approaches for managing dyslipidemia (Alipour et al., 2018). When these approaches are insufficient, the first-line pharmacologic approach for managing dyslipidemia is treatment with statins, which have proven effectiveness in preventing primary and secondary cardiovascular disease (Stone et al., 2014; Amani et al., 2018). Moreover, a large number of studies have shown that L-carnitine supplementation is effective for normalizing the blood concentrations of cholesterol and TGs (Malaguarnera et al., 2009; Abbasnezhad et al., 2019). A systematic review and meta-analysis indicated that L-carnitine supplementation can significantly decrease LDL concentrations, whereas, it does not significantly affect concentrations of total cholesterol (TC), HDL, and serum TG in patients requiring hemodialysis (Huang et al., 2013). However, a recent meta-analysis, which included a trials conducted on adults with cardiovascular risk factors, demonstrated that L-carnitine supplementation significantly reduces concentrations of TC, LDL, and HDL but does not significant effect concentrations of TG (Asadi et al., 2020). Due to the contradictory results found in the previous meta-analyses regarding the effect of L-carnitine supplementation on lipid profiles in patients requiring hemodialysis and people with cardiovascular risk factors, this systematic review and meta- analysis aimed to summarize the effect of L-carnitine on the lipid profiles of patients with liver diseases.
MATERIALS AND METHODS
Search strategy
This systematic review and meta-analysis was carried out according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (Liberati et al., 2009). The extensive literature search was carried out in electronic databases, including PubMed, Scopus, Web of Science, and Cochrane library, until April 2019 with no circumscription of language or time. To include patients with liver diseases irrespective of etiology, we searched for the following search terms in the titles, abstracts, and keywords: “hepatic encephalopathy”, “liver encephalopathy”, “Non-alcoholic Fatty Liver Disease”, “Nonalcoholic Steatohepatitis”, “Alcoholic Fatty Liver”, “Hepatitis”, “Human Viral Hepatitides”, “Liver Cirrhosis”, “Hepatic Cirrhosis”, “Liver Fibroses”, “Liver Fibrosis”, “Liver Neoplasm”, “Hepatic Neoplasms”, “Cancer of Liver”, “Hepatocellular Cancer”, and “liver disease” in combination with “levocarnitine”, “Vitamin BT”, “L-carnitine”, “L carnitine”, and “carnitine”. Moreover, a manual search and reference list check of all the included studies and related reviews was performed to identify further relevant articles.
Study selection
After deleting duplicate articles, two independent investigators (SK and OA) screened the titles and abstracts of the remaining articles based on the inclusion and exclusion criteria. Articles were eligible for inclusion in this meta-analysis if they fulfilled the following criteria: 1) randomized controlled trials (RCTs) that examined the effects of L-carnitine supplementation on lipid profiles in patients with liver disease; 2) trials with any duration of treatment; 3) reported mean or median values [with standard deviation (SD), standard error of the mean, or 95% confidence interval (CI)] at baseline and at the end of the supplementation period in both intervention and control groups. The exclusion criteria were as follows: 1) studies with no placebo or control group; 2) case-control, cross-sectional, cohort design, and review studies; 3) studies which used a combination of other vitamins and minerals; 4) animal studies; 5) studies in which the data was not available.
Data extraction
The full texts of the selected articles were reviewed to determine whether articles qualified for inclusion. Any disagreements in study inclusion were referred to a third author (AA). The following data was extracted (by RC): first author’s name, publication year, study design, participants’ characteristics [sex, age, body mass index (BMI), and disease status], sample size, duration of intervention, and dose of the supplemented L-carnitine. In addition, we extracted the mean and SD of the blood concentrations of TG, TC, HDL, and LDL at baseline and after intervention.
Quality assessment
To assess the risk of bias, the Cochrane Collaboration’s tool was used (Higgins et al., 2011). This tool has nine items, of which each is divided into six domains of bias with three rating categories: 1) low risk of bias (alters the results significantly); 2) unclear risk of bias (raises some doubt about the results); 3) high risk of bias (seriously weakens confidence in the results). All selected articles were scored by 2 authors (SK and RC). Disagreement between the authors was resolved by a third assessor (AA).
Statistical analysis
For statistical analysis, we used the mean change and SD of the variables between baseline and post intervention for both the intervention and control groups. Reported standard error (SE) was converted to SD using the following formula: SE×√n. A random effects model was used to calculate the pooled effect size for concentrations of TG, TC, HDL, and LDL. To estimate the impact of each trial on the pooled effect size, sensitivity analysis (metaninf analysis) was performed [one-study remove (leave-one-out) approach]. Between-study heterogeneity was examined using Q tests and I-square (I2) tests. To calculate the potential sources of between-study heterogeneity, we carried out a pre-planned subgroup analysis based on the dose of L-carnitine, study duration, and type of liver disease. To evaluate publication bias, we used Egger’s regression asymmetry test. Statistical analysis was performed using Stata software (version 11.2, StataCorp, College Station, TX, USA) and P-values<0.05 were considered statistically significant.
RESULTS
Literature search
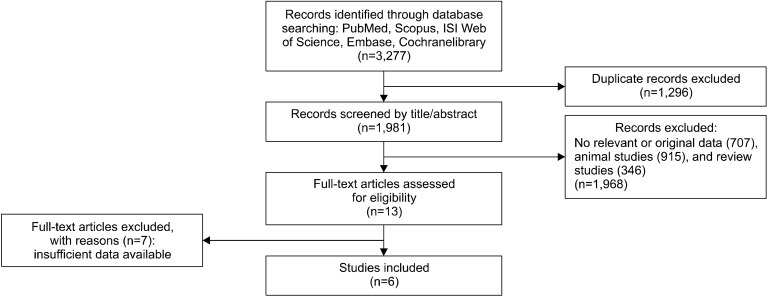
The first search yielded 3,227 results. After duplicates were removed, 1,981 articles remained. The titles and abstracts were then screened based on inclusion and exclusion criteria and 1,968 studies were excluded due to the following: 915 were animal studies, 346 were review studies, and 707 did not report relevant studies. Therefore, the full texts of 13 studies were assessed for eligibility, of which 6 studies were considered eligible for inclusion in our meta-analysis (Malaguarnera et al., 2002; Romano et al., 2008; Malaguarnera et al., 2010; Hong et al., 2014; Bae et al., 2015; Alavinejad et al., 2016). The process of study selection is shown in the flow diagram in Fig. 1.
Fig. 1.
Flow diagram of the literature search.
Study characteristics
Pooled data included a total of 234 participants in the intervention arm and 234 participants in the control arm. These studies were all carried out between 2002 and 2016. The overall age range of the participants was 47.8 ∼60 years old, and BMI of the participants ranged between 25.7 and 29.5 kg/m2. Durations of intervention ranged from 12 weeks to 48 weeks. The selected studies were conducted in three countries: Italy (Malaguarnera et al., 2002; Romano et al., 2008; Malaguarnera et al., 2010), South Korea (Hong et al., 2014; Bae et al., 2015), and Iran (Alavinejad et al., 2016). The dose of supplemented L-carnitine ranged from 900 mg to 2,472 mg. The studies included patients with chronic hepatitis C (Malaguarnera et al., 2002; Romano et al., 2008) and nonalcoholic fatty liver disease/steatohepatitis (NAFLD/ NASH) (Malaguarnera et al., 2010; Hong et al., 2014; Bae et al., 2015; Alavinejad et al., 2016) (Table 1).
Table 1.
Characteristic of the studies included in the meta-analysis
| References | Country | Study design | Participants | Sex | Mean age (intervention/control) | Mean BMI (intervention/control) | Trial duration (week) | Daily dose of L-carnitine (mg) | Daily dose of L-carnitine (mg) |
|---|---|---|---|---|---|---|---|---|---|
| Malaguarnera et al. (2002) | Italy | R | Patients with chronic hepatitis C | F/M | 56.8±7.2/ 57.7±6.1 | NR/NR | 24 | >2,000 | 35/35 |
| Romano et al. (2008) | Italy | R | Patients with chronic hepatitis C | F/M | 50.1±6.1/ 50.4±5.6 | 25.8/25.7 | 24/48 | >2,000 | 35/35 |
| Malaguarnera et al. (2010) | Italy | R/DB/PC | Patients with nonalcoholic steatohepatitis | F/M | 47.9±5.4/ 47.8±5.8 | 26.6/26.5 | 24 | >2,000 | 36/38 |
| Hong et al. (2014) | Korea | R/DB/PC | Patients with nonalcoholic fatty liver disease with impaired glucose metabolism | F/M | 51.5±9.4/ 52.0±9.6 | 27.2/27.0 | 12 | 900 | 26/26 |
| Bae et al. (2015) | Korea | R/DB/PC | Patients with fatty liver disease with type 2 diabetes | F/M | 50.6±9.3/ 52.0±9.4 | 28.2/26.7 | 12 | 2,472 | 39/39 |
| Alavinejad et al. (2016) | Iran | R/DB/PC | Patients with fatty liver disease with type 2 diabetes | F/M | 60±5/59±9 | 28.6/29.5 | 12 | 2250 | 28/26 |
DB, double-blinded; PC, placebo-controlled; R, randomized; NR, not reported; F, female; M, male.
Assessment of risk of bias
The RCTs risk of bias is shown in Table 2. Overall, the assessors agreed on 34 of the 42 items, resulting in an 81% agreement rate. After discussions with the consulting third assessor (AA), 100% agreement was reached. Two studies (Romano et al., 2008; Malaguarnera et al., 2010) had the lowest risk of bias and reached the highest score (6 out of 7). Overall, all the studies had a low risk of bias and reached a score of ≥4 out of 7. In all the selected studies, participants were randomization; however, in two studies, participants and study personnel were not blinded (Malaguarnera et al., 2002; Romano et al., 2008). Further details are shown in the Table 2.
Table 2.
Quality assessment (Cochrane Collaboration’s tool for assessing risk of bias)
| References | Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other bias | Total | |
|---|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Anything else, ideally pre-specified | Low on risk of bias | |
| Malaguarnera et al. (2002) | Low | Low | High | High | Low | Low | Low | 5/7 |
| Romano et al. (2008) | Low | Low | High | Low | Low | Low | Low | 6/7 |
| Malaguarnera et al. (2010) | Low | Low | Low | High | Low | Low | Low | 6/7 |
| Hong et al. (2014) | Low | High | Low | High | Low | Low | Unclear | 4/7 |
| Bae et al. (2015) | Low | Low | Low | High | High | Low | Low | 5/7 |
| Alavinejad et al. (2016) | Low | High | Low | High | Low | Low | Unclear | 4/7 |
Effect of L-carnitine supplementation on TC levels
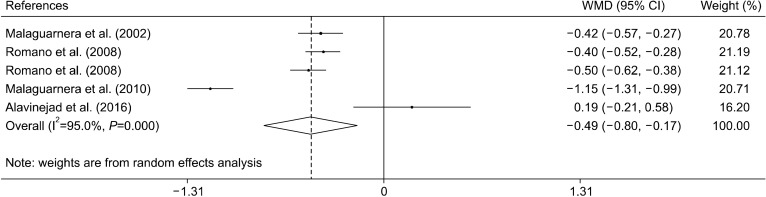
The effect of L-carnitine supplementation on TC levels were examined in four trials with five effect sizes (Alavinejad et al., 2016; Malaguarnera et al., 2010; Romano et al., 2008; Malaguarnera et al., 2002). Results of the meta- analysis indicated that L-carnitine supplementation reduces blood concentrations of TC [weighted mean difference and 95% CI: −0.49 mmol/L, (−0.80, −0.17)] (Fig. 2). The results of the subgroup analysis showed that L- carnitine was effective in reducing TC levels in trials with durations of more than 24 weeks, at doses of less than 2,000 mg/d, and in patients with chronic hepatitis C. The heterogeneity only disappeared in the chronic hepatitis C subgroup (Table 3).
Fig. 2.
Forest plot of the effect of L-carnitine supplementation on total cholesterol.
Table 3.
Subgroup analyses of L-carnitine supplementation on lipid profiles
| Variables | No. | WMD (95% CI) | P within group | P heterogeneity | I2 |
|---|---|---|---|---|---|
| Total cholesterol | |||||
| Trial duration (week) | |||||
| <24 | 1 | 0.18 (−0.20, 0.58) | 0.34 | <0.001 | − |
| ≥24 | 4 | −0.61 (−0.93, −0.29) | <0.001 | <0.001 | 0.955 |
| L-Carnitine dose (mg) | |||||
| >>2,000 | 1 | 0.18 (−0.20, 0.58) | 0.34 | <0.001 | − |
| ≤2,000 | 4 | −0.61 (−0.93, −0.29) | <0.001 | <0.001 | 0.955 |
| Type of liver disease | |||||
| NAFLD/NASH | 2 | −0.49 (−1.81, 0.82) | 0.460 | <0.001 | 0.974 |
| Chronic hepatitis C | 3 | −0.44 (−0.51, −0.37) | <0.001 | 0.492 | 0 |
| Triglycerides | |||||
| Trial duration (week) | |||||
| <24 | 3 | 0.12 (−0.30, 0.54) | 0.57 | 0.104 | 0.557 |
| ≥24 | 4 | −0.37 (−0.47, −0.27) | <0.001 | 0.993 | 0 |
| L-Carnitine dose (mg) | |||||
| >>2,000 | 2 | −0.07 (−0.28, 0.13) | 0.473 | 0.912 | 0 |
| ≤2,000 | 5 | −0.28 (−0.48, −0.07) | 0.008 | 0.026 | 0.638 |
| Type of liver disease | |||||
| NAFLD/NASH | 4 | −0.05 (−0.39, 0.28) | 0.753 | 0.007 | 0.754 |
| Chronic hepatitis C | 3 | −0.38 (−0.50, −0.26) | <0.001 | 0.96 | 0 |
| HDL-cholesterol | |||||
| Trial duration (week) | |||||
| <24 | 2 | 0.00 (−0.04, 0.06) | 0.784 | 0.896 | 0 |
| ≥24 | 4 | 0.11 (−0.00, 0.23) | 0.053 | <0.001 | 0.997 |
| L-Carnitine dose (mg) | |||||
| >>2,000 | 1 | 0.00 (−0.05, 0.06) | 0.871 | − | − |
| ≤2,000 | 5 | 0.09 (−0.00, 0.20) | 0.071 | <0.001 | 0.996 |
| Type of liver disease | |||||
| NAFLD/NASH | 3 | 0.043 (−0.02, 0.11) | 0.159 | 0.027 | 0.723 |
| Chronic hepatitis C | 3 | 0.13 (−0.11, 0.36) | 0.272 | <0.001 | 0.998 |
| LDL-cholesterol | |||||
| Trial duration (week) | |||||
| <24 | 2 | 0.06 (−0.11, 0.24) | 0.457 | 0.853 | 0 |
| ≥24 | 4 | −0.32 (−0.80, 0.15) | 0.183 | <0.001 | 0.988 |
| L-Carnitine dose (mg) | |||||
| >>2,000 | 1 | 0.08 (−0.14, 0.30) | 0.474 | − | − |
| ≤2,000 | 5 | −0.25 (−0.68, 0.16) | 0.234 | <0.001 | 0.984 |
| Type of liver disease | |||||
| NAFLD/NASH | 3 | −0.35 (−1.27, 0.57) | 0.455 | <0.001 | 0.981 |
| Chronic hepatitis C | 3 | −0.05 (−0.31, 0.21) | 0.698 | <0.001 | 0.952 |
CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; WMD, weighted mean differences; NAFLD/NASH, nonalcoholic fatty liver disease/steatohepatitis.
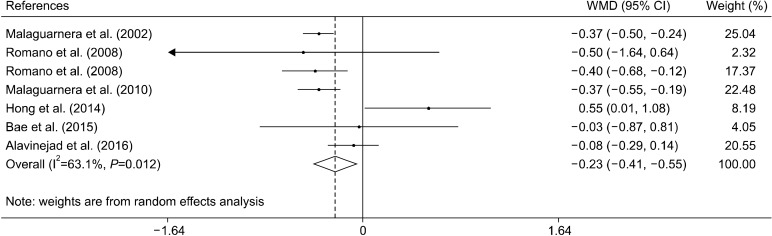
Effect of L-carnitine supplementation on TG levels
Six clinical trials with seven effect sizes evaluated the effect of L-carnitine supplementation on TG levels (Malaguarnera et al., 2002; Romano et al., 2008; Malaguarnera et al., 2010; Hong et al., 2014; Bae et al., 2015; Alavinejad et al., 2016). The results indicated that L-carnitine supplementation significantly decreased concentrations of TG [−0.23 mmol/L, (−0.41, −0.05)] (Fig. 3). Subgroup analysis indicated that L-carnitine was effective in reducing TG when supplemented for 24 weeks, at dose of less than 2,000 mg/d, and in patients with chronic hepatitis C (Table 3).
Fig. 3.
Forest plot of the effect of L-carnitine supplementation on triglyceride levels.
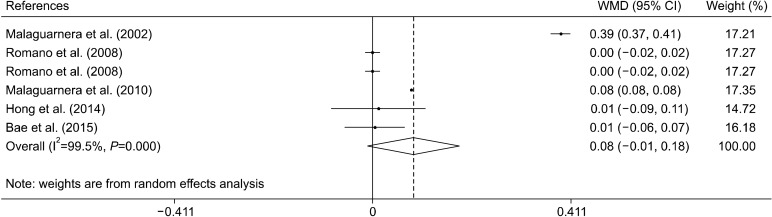
Effect of L-carnitine supplementation on HDL levels
The effect of the L-carnitine supplementation on HDL levels was examined in 5 clinical trials with 6 effect sizes (Malaguarnera et al., 2002; Romano et al., 2008; Malaguarnera et al., 2010; Hong et al., 2014; Bae et al., 2015). L-carnitine supplementation had no significant effect on concentrations of HDL [0.08 mmol/L, (−0.01, 0.18)] (Fig. 4). Subgroup analysis based on L-carnitine dose, trials duration and type of liver disease indicated that L- carnitine does not affect HDL levels in any of these subgroups (Table 3).
Fig. 4.
Forest plot of the effect of L-carnitine supplementation on high-density lipoprotein levels.
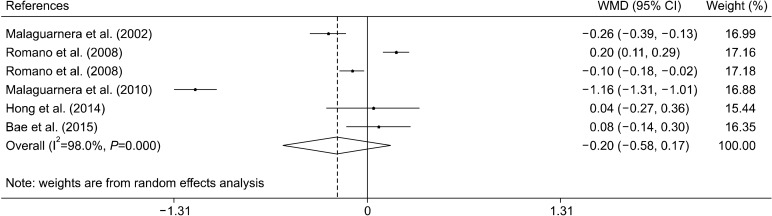
Effect of L-carnitine supplementation on LDL levels
The effect of the L-carnitine supplementation on LDL levels was examined in 5 clinical trials with 6 effect sizes (Malaguarnera et al., 2002; Romano et al., 2008; Malaguarnera et al., 2010; Hong et al., 2014; Bae et al., 2015). Overall, the meta-analysis did not show any beneficial effect of L-carnitine supplementation on concentrations of LDL [−0.20 mmol/L, (−0.58, 0.17)] (Fig. 5). Furthermore, subgroup analysis did not find any beneficial effect of L-carnitine supplementation on LDL (Table 3).
Fig. 5.
Forest plot of the effect of L-carnitine supplementation on low-density lipoprotein levels.
Sensitivity analysis and publication bias
We next tested the robustness of our findings through sensitivity analysis. These results demonstrated that overall estimates were not affected by elimination of any one study. Egger’s test was conducted to assess publication bias. There was no evidence of publication bias for any studies examining the effect of L-carnitine supplementation on levels of TC (P=0.283), HDL (P=0.077), and LDL (P=0.821); however, there was evidence of publication bias for studies examining the effect of L-carnitine supplementation on levels of TG (P=0.024).
DISCUSSION
This systematic review and meta-analysis indicated that L-carnitine supplementation significantly reduces blood levels of TC and TG in patients with liver disease, but is not effective in reducing levels of HDL or LDL. The reducing effect of L-carnitine on levels of both TC and TG was shown following long-term supplementation (≥24 weeks), with doses less than or equal to 2,000 mg/d, and in patients with chronic hepatitis C.
L-Carnitine plays an important role in the metabolism of lipids (Carter et al., 1995). L-Carnitine is implicated in beta oxidation of fatty acids and reduces conversion of free fatty acids to triglycerides (Carter et al., 1995). Several studies have shown that supplementation with L-carnitine and its analogues acetyl and propionyl L-carnitine are effective in regulating plasma concentrations of carnitine, cholesterol, and triglyceride in rats (Irat et al., 2003). A further investigation has shown that carnitine administration could prevent development of atherosclerotic lesions in hypercholesterolemic rabbits (Sayed-Ahmed et al., 2001). Recently, a large number of RCTs have evaluated the effect of L-carnitine supplementation on lipid profile in various diseases, however, the results are inconsistent. In 2016, Alavinejad et al. (2016) reported that L-carnitine supplementation has no significant effect on serum levels of cholesterol and TG. However, another study indicated that patients treated with L-carnitine showed significant improvements in serum concentrations of TC, LDL, HDL, and TG (Malaguarnera et al., 2010).
Liver is the central organ for distributing and metabolizing carnitine. Therefore, any liver disorders can affect carnitine metabolism (Krähenbühl, 1996). Previous studies have shown that patients with liver disease have lower blood concentrations of carnitine (Selimoglu et al., 2001). The results of our meta-analysis show that L-carnitine supplementation is effective for reducing levels of both TC and TG in patients with liver disease. The beneficial effects of L-carnitine supplementation on improving lipid profiles can be explained through several mechanisms. It is well known that carnitine has an important role in mitochondrial oxidation of long-chain fatty acids. One of the most important steps in fatty acid oxidation is conversion of fatty acyl-CoA to acylcarnitine by carnitine palmitoyl transferase (CPT)-I (Hoppel, 2003). Subsequently, acylcarnitine is transported across the mitochondrial inner membrane. In the matrix, CPT-II transfers the acyl group to CoA, which results in release of carnitine. Then, acyl-CoA enters the fatty acid-oxidation pathway (Hoppel, 2003). Therefore, L-carnitine can reduce conversion of free fatty acids to triglycerides through its role in fatty acid oxidation (Carter et al., 1995). In addition, it has been reported that L-carnitine can affect cholesterol metabolism through inhibition of b-hydroxy b-methylglutaryl (HMG)-CoA reductase activity, which is involved in cholesterol synthesis (Mondola et al., 1992). However, we didn’t show a beneficial effects of L-carnitine supplementation on reducing LDL levels in patients with liver disease. Although, the beneficial effect of L-carnitine on inhibiting the activity of HMG-CoA reductase has been shown in several studies, other studies report that L-carnitine does not significantly affect LDL receptors. A recent animal study reported that administration of L-carnitine significantly decreases HMG-CoA reductase, but has no significant effect on expression of LDL receptors (Yousefinejad et al., 2015). Therefore, the results of our study showing the effect of L-carnitine supplementation on LDL concentrations could be explained through this mechanism.
Previous meta-analyses exploring the effect of L-carnitine supplementation on lipid profiles are contradictory. In 2002, a meta-analysis that included trials conducted on patients undergoing hemodialysis did not show any statistically significant differences in the serum levels of TC, any of its fractions, or TG following L-carnitine supplementation (Hurot et al., 2002). Another meta-analysis demonstrated that L-carnitine supplementation is not associated with significant reductions in serum concentrations of TC, HDL, very LDL, and TG, but can significantly reduce LDL levels in patients undergoing hemodialysis (Huang et al., 2013). Moreover, a recent meta- analysis that included trials conducted on participants with cardiovascular risk factors implied that L-carnitine supplementation significantly reduces levels of TC, LDL, and HDL, but not TG. The reasons for these discrepancy between the results of our meta-analysis and the other meta-analyses might partly be due to difference between the included subjects, or another possible mechanisms that is not fully understood. Further studies will reveal more explanations.
According to our findings, L-carnitine is effective in reducing levels of both TC and TG in patients with chronic hepatitis C. One therapeutic option with a proven efficacy for treatment of chronic hepatitis C is IFNα plus ribavirin (Lauer and Walker, 2001). These drugs have immunomodulating and antiviral actions, and can influence the metabolism of lipids (Malaguarnera et al., 1995). It has been reported that treatment with IFNα decreases serum HDL levels, increases triglyceride levels and, subsequently, increases the amount of fat in the liver (Naeem et al., 2001). Previous investigations have indicated that prolonged treatment with IFNα plus ribavirin results in better response in patients with chronic hepatitis C (Lauer and Walker, 2001). However, it has been shown that liver steatosis and progression of fibrosis reduces the effectiveness of specific antiviral therapies in these patients (Lauer and Walker, 2001). Therefore, carnitine supplementation can be considered as an adjunct therapy for improving antiviral therapies.
Our results did not support the beneficial effect of L- carnitine in patients with NAFLD/NASH. Of four RCTs included in this meta-analysis that assessed the effect of L-carnitine supplementation in patients with NAFLD/ NASH, three studies reported that L-carnitine does not significantly affect lipid profile (Hong et al., 2014; Bae et al., 2015; Alavinejad et al., 2016). The durations of L-carnitine supplementation in all of these studies were less than 24 weeks. Our results indicated that L-carnitine was effective when supplemented for more than 24 weeks. Therefore, one reason for the discrepancy in results regarding the effect of L-carnitine in NAFLD/NASH patients could be the shorter durations of supplementation in these studies. However, further studies should reveal further explanations.
Furthermore, we showed that L-carnitine has a beneficial effect on long-term supplementation. Our results are in line with the previous meta-analysis conducted by Huang et al. (2013), which indicated that long-term supplementation of L-carnitine is effective for improving lipid profile of patients undergoing hemodialysis (more than 16 weeks). However, another meta-analysis indicated that both long-term and short-term supplementation of L-carnitine is effective in improving lipid profiles and that long-term supplementation has beneficial effects on glycemia (Asadi et al., 2020). Moreover, our results indicated that at doses of less than or equal to 2,000 mg, L- carnitine is effective in reducing levels of both TC and TG. It should be noted that in the studies that administered higher doses of L-carnitine, the trial duration was 12 weeks (Bae et al., 2015; Alavinejad et al., 2016). It can be speculated that the reason for the lack of significant effects of high doses of L-carnitine in these studies might be the short durations of intervention.
Our systematic review and meta-analysis had several strengths. First, this is the first meta-analysis to assess the effect of L-carnitine supplementation on lipid profiles in patients with liver disease. Second, we included RCTs that examined complementary endpoints, providing a comprehensive review of this topic. Third, this review is based on an up-to-date literature search from a large number of databases. An important limitation of this meta-analysis is the low number of trials included, which limits the strength of the conclusions drawn; however, we hope malathis study will be helpful for future studies.
In conclusion, our systematic review and meta-analysis indicated beneficial effect of L-carnitine on levels of TC and TG in patients with liver disease, particularly chronic hepatitis C. These results should be useful information for clinical practice. The combination of L-carnitine with immunomodulatory and antiviral agents in patients with chronic hepatitis C seemed to be more efficacious than these agents alone, and improved the dyslipidemia associated with these drugs. Moreover, this systematic review supports the beneficial effects of L-carnitine supplementation when at doses lower than or equal to 2,000 mg/d, and during long-term supplementation (more than 24 weeks). However, it should be noted that these results are not conclusive and further well-designed studies are required.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Abbasnezhad A, Choghakhori R, Kashkooli S, Alipour M, Asbaghi O, Mohammadi R. Effect of L-carnitine on liver enzymes and biochemical factors in hepatic encephalopathy: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2019;34:2062–2070. doi: 10.1111/jgh.14765. [DOI] [PubMed] [Google Scholar]
- Alavinejad P, Zakerkish M, Hajiani E, Hashemi SJ, Chobineh M, Moghaddam EK. Evaluation of L-carnitine efficacy in the treatment of non-alcoholic fatty liver disease among diabetic patients: a randomized double blind pilot study. J Gastroenterol Hepatol Res. 2016;5:2191–2195. doi: 10.17554/j.issn.2224-3992.2016.05.662. [DOI] [Google Scholar]
- Alipour M, Malihi R, Hosseini SA, Abbasnezhad A, Ghavami A, Shahmohammadi HA, et al. The effects of catechins on related risk factors with type 2 diabetes: a review. Prog Nutr. 2018;20:12–20. [Google Scholar]
- Amani R, Abbasnezhad A, Hajiani E, Cheraghian B, Abdoli Z, Choghakhori R. Vitamin D3 induced decrease in IL-17 and malondialdehyde, and increase in IL-10 and total antioxidant capacity levels in patients with irritable bowel syndrome. Iran J Immunol. 2018;15:186–196. doi: 10.22034/IJI.2018.39388. [DOI] [PubMed] [Google Scholar]
- Asadi M, Rahimlou M, Shishehbor F, Mansoori A. The effect of L-carnitine supplementation on lipid profile and glycaemic control in adults with cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled clinical trials. Clin Nutr. 2020;39:110–122. doi: 10.1016/j.clnu.2019.01.020. [DOI] [PubMed] [Google Scholar]
- Bae JC, Lee WY, Yoon KH, Park JY, Son HS, Han KA, et al. Improvement of nonalcoholic fatty liver disease with carnitine- orotate complex in type 2 diabetes (CORONA): a randomized controlled trial. Diabetes Care. 2015;38:1245–1252. doi: 10.2337/dc14-2852. [DOI] [PubMed] [Google Scholar]
- Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Carter AL, Abney TO, Lapp DF. Biosynthesis and metabolism of carnitine. J Child Neurol. 1995;10:S3–S7. doi: 10.1177/0883073895010002S02. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong ES, Kim EK, Kang SM, Khang AR, Choi SH, Park KS, et al. Effect of carnitine-orotate complex on glucose metabolism and fatty liver: a double-blind, placebo-controlled study. J Gastroenterol Hepatol. 2014;29:1449–1457. doi: 10.1111/jgh.12536. [DOI] [PubMed] [Google Scholar]
- Hoppel C. The role of carnitine in normal and altered fatty acid metabolism. Am J Kidney Dis. 2003;41:S4–S12. doi: 10.1016/S0272-6386(03)00112-4. [DOI] [PubMed] [Google Scholar]
- Huang H, Song L, Zhang H, Zhang H, Zhang J, Zhao W. Influence of L-carnitine supplementation on serum lipid profile in hemodialysis patients: a systematic review and meta-analysis. Kidney Blood Press Res. 2013;38:31–41. doi: 10.1159/000355751. [DOI] [PubMed] [Google Scholar]
- Hurot JM, Cucherat M, Haugh M, Fouque D. Effects of L-carnitine supplementation in maintenance hemodialysis patients: a systematic review. J Am Soc Nephrol. 2002;13:708–714. doi: 10.1681/ASN.V133708. [DOI] [PubMed] [Google Scholar]
- Irat AM, Aktan F, Ozansoy G. Effects of L-carnitine treatment on oxidant/antioxidant state and vascular reactivity of streptozotocin-diabetic rat aorta. J Pharm Pharmacol. 2003;55:1389–1395. doi: 10.1211/0022357021909. [DOI] [PubMed] [Google Scholar]
- Krähenbühl S. Carnitine metabolism in chronic liver disease. Life Sci. 1996;59:1579–1599. doi: 10.1016/0024-3205(96)00343-8. [DOI] [PubMed] [Google Scholar]
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaguarnera M, Gargante MP, Russo C, Antic T, Vacante M, Malaguarnera M, et al. L-Carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis-a randomized and controlled clinical trial. Am J Gastroenterol. 2010;105:1338–1345. doi: 10.1038/ajg.2009.719. [DOI] [PubMed] [Google Scholar]
- Malaguarnera M, Giugno I, Trovato BA, Panebianco MP, Siciliano R, Ruello P. Lipoprotein(a) concentration in patients with chronic active hepatitis C before and after interferon treatment. Clin Ther. 1995;17:721–728. doi: 10.1016/0149-2918(95)80048-4. [DOI] [PubMed] [Google Scholar]
- Malaguarnera M, Maugeri D, Saraceno B, Romano M, Neri S, Rapisarda R, et al. Effects of carnitine on biochemical responses in patients with chronic hepatitis C treated with interferon-α. Clin Drug Investig. 2002;22:443–448. doi: 10.2165/00044011-200222070-00004. [DOI] [Google Scholar]
- Malaguarnera M, Vacante M, Avitabile T, Malaguarnera M, Cammalleri L, Motta M. L-Carnitine supplementation reduces oxidized LDL cholesterol in patients with diabetes. Am J Clin Nutr. 2009;89:71–76. doi: 10.3945/ajcn.2008.26251. [DOI] [PubMed] [Google Scholar]
- Mondola P, Santillo M, De Mercato R, Santangelo F. The effect of L-carnitine on cholesterol metabolism in rat (Rattus bubalus) hepatocyte cells. Int J Biochem. 1992;24:1047–1050. doi: 10.1016/0020-711X(92)90372-8. [DOI] [PubMed] [Google Scholar]
- Naeem M, Bacon BR, Mistry B, Britton RS, Di Bisceglie AM. Changes in serum lipoprotein profile during interferon therapy in chronic hepatitis C. Am J Gastroenterol. 2001;96:2468–2472. doi: 10.1111/j.1572-0241.2001.04055.x. [DOI] [PubMed] [Google Scholar]
- Ponziani FR, Pecere S, Gasbarrini A, Ojetti V. Physiology and pathophysiology of liver lipid metabolism. Expert Rev Gastroenterol Hepatol. 2015;9:1055–1067. doi: 10.1586/17474124.2015.1056156. [DOI] [PubMed] [Google Scholar]
- Romano M, Vacante M, Cristaldi E, Colonna V, Gargante MP, Cammalleri L, et al. L-Carnitine treatment reduces steatosis in patients with chronic hepatitis C treated with α-interferon and ribavirin. Dig Dis Sci. 2008;53:1114–1121. doi: 10.1007/s10620-007-9983-1. [DOI] [PubMed] [Google Scholar]
- Sayed-Ahmed MM, Khattab MM, Gad MZ, Mostafa N. L-Carnitine prevents the progression of atherosclerotic lesions in hypercholesterolaemic rabbits. Pharmacol Res. 2001;44:235–242. doi: 10.1006/phrs.2001.0852. [DOI] [PubMed] [Google Scholar]
- Selimoglu MA, Aydogdu S, Yagci RV, Huseyinov A. Plasma and liver carnitine status of children with chronic liver disease and cirrhosis. Pediatr Int. 2001;43:391–395. doi: 10.1046/j.1442-200X.2001.01423.x. [DOI] [PubMed] [Google Scholar]
- Speliotes EK, Balakrishnan M, Friedman LS, Corey KE. Treatment of dyslipidemia in common liver diseases. Clin Gastroenterol Hepatol. 2018;16:1189–1196. doi: 10.1016/j.cgh.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Yousefinejad A, Siassi F, Mirshafiey A, Eshraghian MR, Koohdani F, Javanbakht MH, et al. Effect of genistein and L-carnitine and their combination on gene expression of hepatocyte HMG- COA reductase and LDL receptor in experimental nephrotic syndrome. Iran J Public Health. 2015;44:1339–1347. [PMC free article] [PubMed] [Google Scholar]