Abstract
Cudrania tricuspidata has been used as an East Asian folk remedy to treat various symptoms. Recently, scientific evidence of the efficacy of C. tricuspidata has emerged. The objective of this study was to elucidate protective role of C. tricuspidata in the gastric mucosa using pylorus-ligated Sprague-Dawley rats and primary parietal cells. C. tricuspidata ethanol extracts attenuated gastric mucosal damage, secretion, and juice acidity in pylorus-ligated rats; however, it did not affect expression of gastric acid-related genes [muscarinic acetylcholine receptor M3 receptor (M3R), histamine H2-receptors (H2R), and cholecystokinin-2/gastrin receptors (CCK2R)] or serum gastrin concentrations. Furthermore, extracts greatly reduced levels of gastric cyclic adenosine monophosphate (cAMP) and significantly increased mRNA levels of gastric-type mucins (MUC5AC and MUC6). To identify the mode of action of C. tricuspidata extract in regulating gastric acid secretion, intracellular cAMP and mRNA for H2R, M3R, and CCK2R were measured in primary parietal cells. mRNA levels of H2R, M3R, and CCK2R did not significantly differ following treatment with C. tricuspidata extract, whereas cAMP induced by the H2R-specific agonist was significantly decreased. C. tricuspidata may therefore reduce gastric acid secretion by inhibiting H2R activity rather than regulating mRNA expression. These finding suggest that ethanol extracts of C. tricuspidata inhibit H2R-related gastric acid secretion and increase gastric mucus to help prevent gastric mucosal damage. Therefore, C. tricuspidata extract has potential to be used in foods and medicines to prevent diseases related to gastric mucosal damage, such as gastritis and functional dyspepsia.
Keywords: Cudrania tricuspidata, gastric acid, gastric injury, gastritis, mucin
INTRODUCTION
Gastritis refers to inflammation caused by damage to the epithelium of the stomach. The most common symptoms of gastritis include upper abdominal pain and nausea, vomiting, burping, and weight loss (Glickman and Antonioli, 2001). Chronic gastritis is associated with Helicobacter pylori infection, stress, or autoimmune diseases. Moreover, some types of gastritis caused by H. pylori infection progress to gastric ulcers and tumors (Kandulski et al., 2008). Alternatively, acute gastritis is usually caused by excessive alcohol intake, long-term use of nonsteroidal anti-inflammatory drugs, and stress (Franke et al., 2005; Srivastava and Lauwers, 2007). Such irritation increases gastric acid secretion and damages the gastric mucosa (Bujanda, 2000; Liu and Cho, 2000). Gastric secretion pathways include activation of histamine H2 receptors (H2R) by histamine, activation of muscarinic acetylcholine receptor M3 (M3R) by acetylcholine, and activation of cholecystokinin 2 receptor (CCK2R) by gastrin. Activated H2R, M3R, and CCK2R increase cyclic adenosine monophosphate (cAMP) concentrations in parietal cells and activate H+/K+ ATPases. This increases the concentration of hydrochloric acid in gastric fluids, leading to gastric mucosal damage (Mårdh et al., 1987; Helander and Keeling, 1993). Antacids, H2 receptor antagonists and proton pump inhibitors are used to treat gastritis by helping to minimize gastric mucosal damage caused by excessive gastric acid secretion (Berardi et al., 1987; Szabo and Bynum, 1988; Fullarton et al., 1989). However, the side effects and long-term burden of using these drugs are of increasing concern; therefore, there is a growing interest in promoting stomach health through healthier foods and natural compounds.
Mucins are the main components of mucus on the stomach’s inner surface. Mucins are secreted though various mechanisms throughout the gastrointestinal, respiratory, female genital, and conjunctival mucosa systems (Strous and Dekker, 1992). Mucins have several physiological functions, including cell protection, mechanical protection, maintenance of viscosity in secretion, and cell recognition (Toribara et al., 1993). The structure of mucins have different compositional ratios depending on tissue type and disease (Ho et al., 1993; Tytgat et al., 1994; Ho et al., 1995; Kim and Gum, 1995). Previous studies have shown that the stomach expresses a unique mucin that provides protection against damage by low pH and protease digestion (Strous and Dekker, 1992). In addition, changes in mucin gene expression may affect functional dyspepsia (Kandulski et al., 2008). Nine mucin genes are known to exist in humans, of which gastric- type mucins (MUC1, MUC5AC, and MUC6) are expressed in the human stomach (Ho et al., 1995; Kim and Gum, 1995; Van Klinken et al., 1995).
Cudrania tricuspidata is a deciduous tree used in traditional medicine to treat eczema, mumps, pulmonary tuberculosis, allergies, and acute arthritis (Xin et al., 2017). Recent studies have reported that C. tricuspidata leaf, stem, root, and fruit extracts are effective in preventing diseases, such as liver damage, diabetes, arteriosclerosis, and obesity (Park et al., 2006; Choi et al., 2015; Jo et al., 2015; Kim et al., 2015b; Kim et al., 2016; Jo et al., 2017; Cho et al., 2019; Jo et al., 2019). In a previous study, we showed that ethanol extracts of C. tricuspidata leaves prevent reflux esophagitis in rats, which we predicted may be due to inhibition of H2R activity (Kim et al., 2019). In addition, Kim et al. (2015a) reported that C. tricuspidata leaf extract can protect against alcohol-induced gastric ulcers through their antioxidant activity. Although there is increasing evidence for the gastroprotective effects of C. tricuspidata, the molecular mechanisms underlying its biological activity remain unclear.
In the present study, we investigated the effects of C. tricuspidata ethanol extracts on gastric juice secretion, mucosal damage, and expression of acid secretion-associated genes in pylorus-ligated rats and primary parietal cells. We also measured the expression of gastric mucin genes following treatment with C. tricuspidata extracts. We aimed to provide evidence that C. tricuspidata ethanol extracts may protect gastric mucosa by inhibiting gastric cAMP and increasing mucin gene expression.
MATERIALS AND METHODS
Preparation of C. tricuspidata extract
C. tricuspidata extract powder was prepared as described in our previous study (Kim et al., 2019). Briefly, dried C. tricuspidata leaves were ground to an appropriate size. Ethanol (70%; 10 times the weight of the leaves) was added to the extraction vessel, and the mixture was stirred at 50°C. The obtained extract was concentrated using a rotary vacuum evaporator (EYELA, Tokyo, Japan). The extract was then mixed with maltodextrin (corresponding to 50% of the solid content) and lyophilized to a powder. Rutin was used as an indicator compound, as described in a previous study (Song et al., 2017). Each dried material was re-suspended in 0.5% methyl cellulose (MC) dissolved in water for animal experiments or dimethyl sulfoxide for cell experiments.
Animals and treatment
Seven-week-old male Sprague-Dawley rats were housed at a temperature of 21±2°C, relative humidity of 50±20 %, a ventilation frequency of 10∼15 times/h, and with 12 h of light per day (7:00 am to 7:00 pm). Breeding materials were disinfected before use, using a ultraviolet sterilizer. There were no abnormalities that could affect the test results during the experiment. All studies were approved by the Institutional Animal Care and Use Committee of KPC Co., Ltd. (approval number: P183005).
Rats were divided into four groups: vehicle administration group, ranitidine administration group, 5 mg/kg C. tricuspidata extract administration group, and 10 mg/ kg C. tricuspidata extract administration group. Rats were fasted for 24 h prior to receiving the test substance (C. tricuspidata extract or ranitidine), which was suspended in 0.5% MC. One hour after oral administration of the test substance, rats were anesthetized with isoflurane and the pylorus was ligated. After 8 h of operation, the rats were sacrificed with CO2 gas and stomachs were separated for experimentation. This animal study was performed using certification data for functional food approval.
Measurement of gastric secretion
Gastric juice was collected from the rat stomachs, centrifuged at 800 g for 10 min, and the amounts of gastric juice (mL) were measured in the supernatants. To measure the total acidity of the gastric secretion, gastric juice (1 mL) was dispensed into tubes and titrated to pH 7.0 with 0.1 N NaOH; the amount of 0.1 N NaOH used for the titration was recorded. Total acidity was expressed in mEq, calculated using the formula below:
Total acidity (mEq)=amount of titrated NaOH (mL)× total gastric volume (mL)×0.1 N (correction of NaOH) ×50 (acidity factor)/ligation time (h)
Measurement of gastric mucosal damage
Stomachs drained of gastric juice were opened by incision at the greater curvature and photographed using a digital camera (Coolpix P5100, Nikon, Tokyo, Japan). Areas of gastric mucosal injury were measured using imaging software (Image Pro ver. 6.3, Media Cybernetics, Inc., Rockville, MD, USA).
Measurement of serum gastrin and histamine
Blood was collected before rats were sacrificed and centrifuged at 800 g for 30 min to extract the serum. Serum gastrin and histamine levels were measured using the rat gastrin I enzyme-linked immunosorbent assay (ELISA) kit and histamine ELISA kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions.
Measurement of gastric cAMP
Gastric mucosa isolated from rats was washed twice with phosphate buffer saline (PBS) and collected by centrifugation at 800 g. The gastric mucosa was treated with cAMP assay cell lysis buffer (R&D Systems, Minneapolis, MN, USA) and ground using a tissue homogenizer. The gastric mucosa suspension was centrifuged at 2,000 g and the supernatant was used to measure cAMP levels using a rat/mouse cAMP assay kit (R&D Systems).
Measurement of gene expression in gastric tissues
Total RNA was extracted from gastric mucosa using TRIzol reagent (Sigma-Aldrich Co., St. Louis, MO, USA) according to the manufacturer’s instructions. cDNA was synthesized using the Reverse Transcription System (QIAGEN Sciences Inc., Germantown, MD, USA). The synthesized cDNA was subjected to real-time polymerase chain reaction (PCR) using the TaqMan Universal PCR master mix (Applied Biosystems, Foster City, CA, USA) and a QuantStudio 6 Real-Time PCR system (Applied Biosystems). TaqMan probe (Thermo Fisher Scientific, Waltham, MA, USA) used for mRNA expression analysis are presented in Table 1.
Table 1.
TagMan probes used for qPCR
| Gene name | TaqMan® probe ID | Dye |
|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Rn01775763_g1 | FAM |
| Histamin receptor H2 (HRH2) | Rn00564216_s1 | FAM |
| Muscarinic acetylcholine receptor M3 (M3R) | Rn00560986_s1 | FAM |
| Cholecystokinin 2 receptor (CCK2R) | Rn00565867_m1 | VIC |
| Mucin 1 (Muc1) | Rn01462585_m1 | VIC |
| Mucin 5AC (Muc5AC) | Rn01451252_m1 | FAM |
| Mucin 6 (Muc6) | Rn01759814_m1 | FAM |
Parietal cell isolation and culture
Gastric parietal cells were isolated following the method of Nakada et al. (2012). Isolated stomach tissues were washed twice with PBS and incubated for in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone Laboratories, Logan, UT, USA) containing 20 mM hydroxyethyl piperazine ethane sulfonicacid (HEPES), collagenase (type 4, Worthington Biochemical Corp., Lakewood, NJ, USA) 1 mg/mL and bovine serum albumin (BSA) 1 mg/ mL for 30 min at 37°C. The cell suspensions was filtered through a cell strainers with 40 μM pores (BD Biosciences, Franklin Lakes, NJ, USA) and centrifuged at 1,000 g for 10 min. Isolated cells were suspended in DMEM/F-12 (GIBCO-BRL, Grand Island, NY, USA) containing 20 mM HEPES, 0.2% BSA, 10 mM glucose, insulin-transferrin-selenium-A, and 1 mM glutamine. This isolated gastric parietal cell suspensions were transferred into CorningⓇMatrigelⓇ-coated plates (Corning Incorporated, Steuben County, NY, USA) and incubated at 37°C until use.
Measurement of gene expression in parietal cells
After parietal cells were stabilized in CorningⓇMatrigelⓇ- coated plates (Corning Incorporated) for 24 h, we added C. tricuspidata extracts or ranitidine with 100 μM of the H2R-specific agonist dimaprit (Sigma-Aldrich Co.) for 30 min. Cells were then collected to separate total RNA and synthesize cDNA. The cDNA was used as a template for real-time PCR to measure mRNA levels of H2R, M3R, and CCK2R. Reactions were carried out with TaqMan probes (Table 1) using a QuantStudio 6 Real-Time PCR system (Applied Biosystems).
Measurement of intracellular cAMP
The medium of cultured parietal cells was replaced with DMEM/F-12 medium containing 10 μM RO-201724 (Sigma-Aldrich Co.) prior to treatment with test materials. The parietal cells were pretreated with C. tricuspidata extracts or 10 μM of ranitidine hydrochloride for 5 min, followed by 10 μM of the H2R specific agonist dimaprit (Sigma-Aldrich Co.) for 30 min. After 30 min of dimaprit treatment, parietal cells were treated with cAMP assay cell lysis buffer (R&D Systems) and immediately frozen. The frozen cells were disrupted by repeatedly freezing and thawing three times, centrifuged at 800 g for 15 min, and cAMP production was measured in the supernatant. cAMP assays were performed using the Rat/Mouse cAMP assay kit (R&D Systems).
Statistical analysis
Experimental results were compared between groups using Minitab and one-way ANOVA, a parametric multiple comparison procedure. Results were expressed as mean± standard error (SE) and were considered statistically significant when P<0.05.
RESULTS
Effect of C. tricuspidata on pylorus ligation-induced gastric mucosal damage
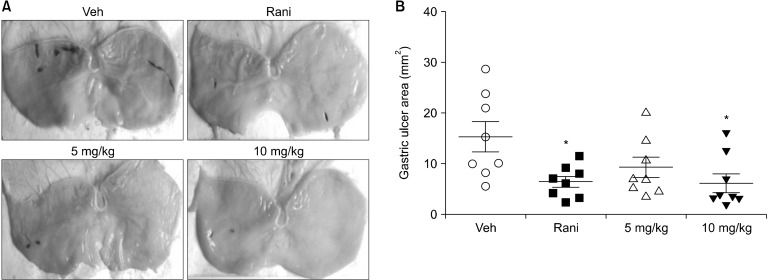
Pylorus-ligated rat models (Satyanarayana et al., 1989) were used to investigate the effects of C. tricuspidata extracts on gastric acid secretion and resulting gastric damage. In the vehicle group, gastric mucosa was damaged by gastric acid, resulting in redness. In contrast, in rats treated with ranitidine or C. tricuspidata extract there was a reduction in redness due to gastric acid secretion (Fig. 1A). For the vehicle group, the area of gastric injury was 15.46±2.92 mm2. C. tricuspidata extracts at concentrations of 5 and 10 mg/kg attenuated the esophageal injury ratio to 9.09±2.13 mm2 and 6.37±1.82 mm2, respectively. Ranitidine at 7.7 mg/kg also reduced the area of gastric injury to 6.65±1.09 mm2 (Fig. 1B). C. tricuspidata extract inhibited gastric mucosal damage in a dose-dependent manner, with the greatest reduction of gastric mucosal damage observed at 10 mg/kg.
Fig. 1.
Effect of Cudrania tricuspidata extracts on gastric mucosal damage. (A) Gastric lesions and (B) the area of gastric mucosal injury. Stomachs were collected immediately after rats were sacrificed and was dissected longitudinally from the greater curvature. The pylorus-ligated rats were treated with the indicated dose of C. tricuspidata extract. Veh, 0.5% methyl cellulose; Rani, 7.7 mg/kg of ranitidine; 5 mg/kg, 5 mg/kg of C. tricuspidata extract; 10 mg/kg, 10 mg/kg of C. tricuspidata extract. Values are mean±SE. Significantly different from the Veh group at *P<0.05.
Effect of C. tricuspidata on gastric acid secretion
C. tricuspidata extracts decreased the total acidity of gastric juice in a concentration-dependent manner (Table 2). Furthermore, C. tricuspidata ethanol extracts reduced gastric acid secretion after pyloric ligatures, suggesting that C. tricuspidata extracts protect the gastric mucosa.
Table 2.
Effect of Cudrania tricuspidata on gastric juice volume and total acidity
| Group | Gastric juice volume (mL) | Total acidity (mEq) | pH |
|---|---|---|---|
| Veh | 12.81±1.00 | 16.10±2.03 | 1.23±0.05 |
| Rani | 7.82±0.69** | 8.95±0.94** | 1.30±0.03 |
| 5 mg/kg | 9.82±1.03 | 11.63±1.35 | 1.35±0.12 |
| 10 mg/kg | 9.06±0.84* | 9.63±1.26* | 1.32±0.06 |
Data were expressed as mean±SE.
The results were statistically analyzed by one-way ANOVA.
Significantly different from Veh at *P<0.05 and **P<0.01.
Veh, 0.5% methyl cellulose; Rani, 7.7 mg/kg of ranitidine; 5 mg/kg, 5 mg/kg of C. tricuspidata extract; 10 mg/kg, 10 mg/kg of C. tricuspidata extract.
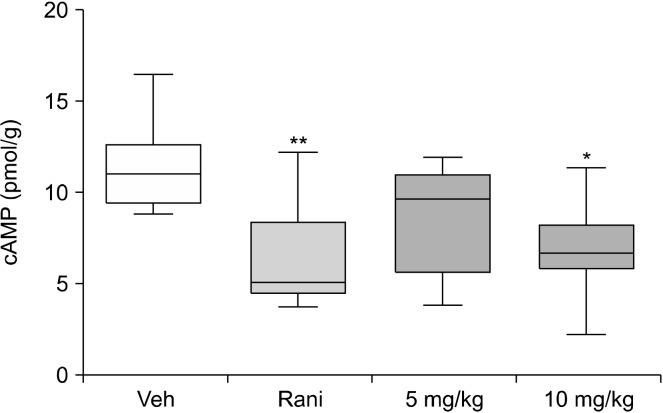
Effect of C. tricuspidata on gastric mucosa cAMP concentrations
Gastric acid secretion is caused by activation of H2R, M3R, and CCK2R, which increase cAMP concentrations in parietal cells. Therefore, cAMP was measured in gastric mucosa to determine whether the acid-lowering effect of C. tricuspid extracts may be related to gastric cAMP. C. tricuspidata ethanol extracts reduced gastric cAMP in test animals in a concentration-dependent manner (Fig. 2). Specifically, the extracts at 10 mg/kg inhibited cAMP activity to a similar extent as the H2R blocker, ranitidine.
Fig. 2.
Effect of Cudrania tricuspidata extract on gastric gastric cyclic adenosine monophosphate (cAMP) levels. Gastric mucosa was collected from dissected stomachs, and cAMP levels were measured using cAMP detection kits. Pylorus-ligated rats were treated with the indicated dose of C. tricuspidata extract. Veh, 0.5% methyl cellulose; Rani, 7.7 mg/kg of ranitidine; 5 mg/kg, 5 mg/kg of C. tricuspidata extract; 10 mg/kg of C. tricuspidata extract. Values are mean±SE. Significant differences from the Veh group at *P<0.05 and **P<0.01.
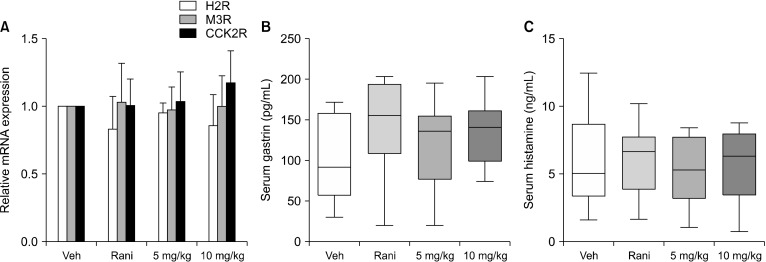
Measurement of factors promoting gastric acid secretion
To investigate factors that stimulate gastric cAMP production and gastric acid secretion, we measured mRNA levels of H2R, M3R, and CCK2R by real-time PCR, and serum gastrin levels by ELISA. Despite a slight degree of variation in the results, there were no significant differences in H2R, M3R, or CCK2R mRNA expression between the vehicle administration group and the C. tricuspidata extract administration groups (Fig. 3A). Serum gastrin levels was unchanged by administration of C. tricuspidata extracts and slightly increased by ranitidine, although the difference was not significant (Fig. 3B). The concentration of serum histamine did not vary between groups (Fig. 3C).
Fig. 3.
Measurement of factors promoting gastric acid secretion in pylorus-ligated rats. (A) mRNA levels of muscarinic acetylcholine receptor M3 receptor (M3R), histamine H2-receptors (H2R), and cholecystokinin-2/gastrin receptors (CCK2R) in gastric mucosa. (B) Serum gastrin and (C) histamine levels in pylorus-ligated rats. mRNA was measured using cDNA synthesized from isolated gastric mucosa, and serum was collected before rats were sacrificed. The pylorus-ligated rats were treated with the indicated dose of Cudrania tricuspidata extract. Veh, 0.5% methyl cellulose; Rani, 7.7 mg/kg of ranitidine; 5 mg/kg, 5 mg/kg of C. tricuspidata extract; 10 mg/kg, 10 mg/kg of C. tricuspidata extract. Values are mean±SE.
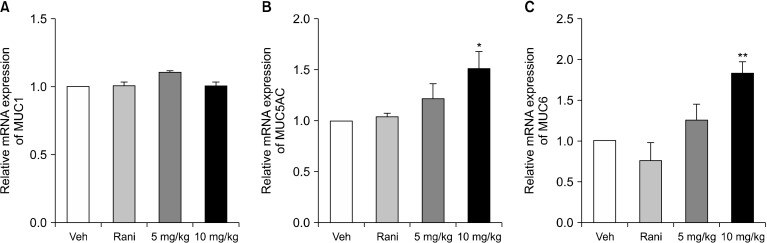
Measurement of gastric mucin genes in gastric mucosa
The gastric mucus protects stomach tissues from stimulants such as gastric acid. Indeed, it has been reported that gastric ulcers caused by injury occur when the expression of MUC5AC or MUC6 genes in the gastric mucosa are reduced by H. pylori infection (Wang and Fang, 2006; Kang et al., 2008). The effect of C. tricuspidata extract on gastric mucus was investigated by measuring mRNA expression of MUC1, MUC5AC, and MUC6. Administration of C. tricuspidata extracts had no effect on MUC1 mRNA expression (Fig. 4A), but increased mRNA expression of MUC5AC and MUC6 (Fig. 4B and 4C).
Fig. 4.
Effect of Cudrania tricuspidata extract on gastric mucin gene expression. mRNA levels of (A) MUC1, (B) MUC5AC, and (C) MUC6 in gastric mucosa from pylorus-ligated rats. Rats were treated with the indicated dose of C. tricuspidata extract. Veh, 0.5% methyl cellulose; Rani, 7.7 mg/kg of ranitidine; 5 mg/kg, 5 mg/kg of C. tricuspidata extract; 10 mg/kg, 10 mg/kg of C. tricuspidata extract. Values are mean±SE. Significantly different from the Veh group at *P<0.05 and **P<0.01.
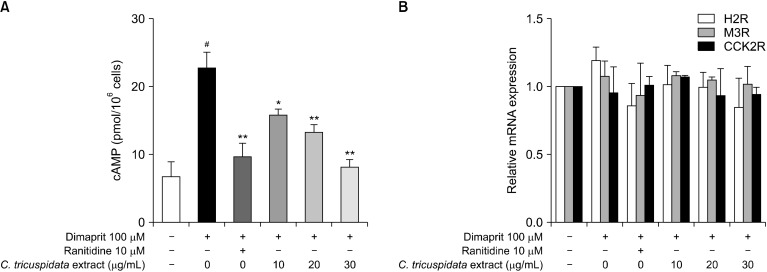
C. tricuspidata inhibits H2R-mediated cAMP production in parietal cells
As described above, we showed that C. tricuspidata ethanol extracts inhibit gastric acid secretion and gastric mucosa injury by reducing gastric cAMP levels and increasing mucin gene expression. However, the mechanism by which C. tricuspidata inhibits gastric cAMP has not been elucidated. Therefore, to identify the mode of action of C. tricuspidata extracts, we measured the expression of genes related to gastric acid secretion and changes in cAMP levels after treatment of primary rat parietal cells with H2R agonists. Treatment of parietal cells with dimaprit, a selective H2R agonist, significantly increased levels of cAMP, which were inhibited by C. tricuspidata extracts in a dose-dependent manner (Fig. 5A). However, C. tricuspidata only induced a, non-significant decrease in H2R mRNA expression (Fig. 5B) and did not alter mRNA expression of the genes M3R and CCK2R involved in gastric secretion. Thus, C. tricuspidata was shown to reduce cAMP levels by inhibiting H2R activation rather than affecting mRNA expression.
Fig. 5.
Effect of Cudrania tricuspidata extract on intracellular gastric cyclic adenosine monophosphate (cAMP) levels and gastric acid secretion related genes. (A) Intracellular cAMP levels in primary parietal cells stimulated by histamine H2-receptors (H2R) agonists. (B) H2R, muscarinic acetylcholine receptor M3 receptor (M3R), and cholecystokinin-2/gastrin receptors (CCK2R) mRNA expression in parietal cells treated with H2R agonists. Isolated primary rat parietal cells were pretreated with 10 μM of ranitidine or C. tricuspidata extracts for 5 min. Thereafter, cells were dstimulated with 100 μM Dimaprit. The control group was treated with the same amount of dimethyl sulfoxide. Values are mean±SE. #P<0.01 indicates significant difference between the control group and the Dimaprit group. Significant difference are presented between the Dimaprit group and the C. tricuspidata extract groups at *P<0.05 and **P<0.01.
DISCUSSION
C. tricuspidata is known to be effective for treating gastrointestinal diseases in folk remedies. Many studies have reported the effects of C. tricuspidata on allergies, inflammation, liver damage, diabetes, and obesity (Park et al., 2006; Lee et al., 2012; Jo et al., 2015; Kim et al., 2015b; Kim et al., 2016; Jo et al., 2017; Cho et al., 2019; Jo et al., 2019), however the mechanism of action for treating gastroenteritic conditions such as gastritis remains unknown. Although the effect of C. tricuspidata extract on alcoholic gastric injury has previously been reported, only the antioxidant and anti-inflammatory effects of C. tricuspidata were described (Kim et al., 2015a). In our previous study, we showed that C. tricuspidata extracts inhibit increases in cAMP induced by H2R selective agonists in U937 monocytic cells (Kim et al., 2019). However, the effects of C. tricuspidata on cAMP levels and expression of genes involved in gastric secretion in gastric mucosa and parietal cells were not confirmed. Therefore, in this study, we investigated whether C. tricuspidata extracts can protect gastric mucosa by reducing H2R-mediated cAMP production and increasing mucin genes in gastric mucosa of pylorus-ligated rats and primary parietal cells.
Oral administration of a single dose of C. tricuspidata ethanol extract reduced gastric acid-induced mucosa damage by reducing gastric cAMP and increasing mucin genes in pylorus-ligated animals (Fig. 1, Fig. 2, Table 2, and Fig. 4). However, we did not observe any effects of C. tricuspidata on serum gastrin levels or gastric acid secretion-related genes, including H2R, M3R, and CCK2R (Fig. 3A). Although serum gastrin appeared to increase following treatment with the H2R inhibitor ranitidine, it did not significantly differ between groups (Fig. 3B). Treatment with H2R inhibitors increased serum gastrin, which is a reported side effect of inhibitors such as ranitidine and famotidine (Decktor et al., 1989; Ohsawa et al., 2002). In addition, serum histamine levels were not affected by the treatment (Fig. 3C). These results suggest that reductions of gastric cAMP induced by C. tricuspidata extracts are not associated with mRNA expression of genes related to gastric acid secretion or levels of serum gastrin and histamine.
We therefore investigated whether C. tricuspidata inhibits H2R-mediated cAMP production in gastric parietal cells. In addition, we measured the effect of C. tricuspidata on mRNA expression of H2R, M3R, and CCK2R. We showed that C. tricuspidata ethanol extracts significantly inhibit cAMP production induced by H2R agonists, although only for a limited time (Fig. 5A). However, this was not accompanied by any significant differences in the mRNA expression of H2R, M3R, or CCK2R (Fig. 5B). Furthermore, C. tricuspidata ethanol extracts did not affect mRNA expression of the H+/K+ ATPase (data not shown). Overall, these results suggest that inhibition of gastric acid secretion by C. tricuspidata ethanol extract occurs by blocking binding of histamine to H2R.
In our previous and current studies, we showed that C. tricuspidata and its extracts are promising candidates for treatment of diseases associated with excessive gastric acid secretion, such as gastritis, gastric ulcer, and reflux esophagitis, acting via selectively inhibit H2R. Although several studies have been conducted to investigate the mechanism by which C. tricuspidata regulates gastric acid, none have investigated the increase in gastric mucin expression. Therefore, further studies are required to investigate the effect of C. tricuspidata on changes in mucin gene expression in mucosal tissues, including the stomach and intestines.
In conclusion, our data show that C. tricuspidata extracts can inhibit H2R and protect the gastric mucosa, thereby protecting against diseases such as gastritis and reflux esophagitis. Future studies should be conducted to confirm the effectiveness of C. tricuspidata in human clinical trials of individuals with heartburn and abdominal pain associated with excessive gastric acid. Moreover, this study may be used as a basis for further research on other effects of C. tricuspidata in the gastrointestinal tract.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Berardi RR, Savitsky ME, Nostrant TT. Maintenance therapy for prevention of recurrent peptic ulcers. Drug Intell Clin Pharm. 1987;21:493–501. doi: 10.1177/106002808702100602. [DOI] [PubMed] [Google Scholar]
- Bujanda L. The effects of alcohol consumption upon the gastrointestinal tract. Am J Gastroenterol. 2000;95:3374–3382. doi: 10.1111/j.1572-0241.2000.03347.x. [DOI] [PubMed] [Google Scholar]
- Cho SS, Yang JH, Seo KH, Shin SM, Park EY, Cho SS, et al. Cudrania tricuspidata extract and its major constituents inhibit oxidative stress-induced liver injury. J Med Food. 2019;22:602–613. doi: 10.1089/jmf.2018.4322. [DOI] [PubMed] [Google Scholar]
- Choi DJ, Lee YJ, Kim YK, Kim MH, Choi SR, Kim SS, et al. Physicochemical properties and antioxidant activities of different parts of Kkujippong (Cudrania tricuspidata Bureau) from Miryang. Korean J Food Cook Sci. 2015;31:510–514. doi: 10.9724/kfcs.2015.31.4.510. [DOI] [Google Scholar]
- Decktor DL, Pendleton RG, Kellner AT, Davis MA. Acute effects of ranitidine, famotidine and omeprazole on plasma gastrin in the rat. J Pharmacol Exp Ther. 1989;249:1–5. [PubMed] [Google Scholar]
- Franke A, Teyssen S, Singer MV. Alcohol-related diseases of the esophagus and stomach. Dig Dis. 2005;23:204–213. doi: 10.1159/000090167. [DOI] [PubMed] [Google Scholar]
- Fullarton GM, McLauchlan G, Macdonald A, Crean GP, McColl KE. Rebound nocturnal hypersecretion after four weeks treatment with an H2 receptor antagonist. Gut. 1989;30:449–454. doi: 10.1136/gut.30.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman JN, Antonioli DA. Gastritis. Gastrointest Endosc Clin N Am. 2001;11:717–740. doi: 10.1016/S1052-5157(18)30044-8. [DOI] [PubMed] [Google Scholar]
- Helander HF, Keeling DJ. Cell biology of gastric acid secretion. Baillière's Clin Gastroenterol. 1993;7:1–21. doi: 10.1016/0950-3528(93)90029-R. [DOI] [PubMed] [Google Scholar]
- Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, Gum ET, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641–651. [PubMed] [Google Scholar]
- Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, et al. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995;55:2681–2690. [PubMed] [Google Scholar]
- Jo YH, Choi KM, Liu Q, Kim SB, Ji HJ, Kim M, et al. Anti-obesity effect of 6,8-diprenylgenistein, an isoflavonoid of Cudrania tricuspidata fruits in high-fat diet-induced obese mice. Nutrients. 2015;7:10480–10490. doi: 10.3390/nu7125544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Kim SB, Ahn JH, Turk A, Kwon EB, Kim MO, et al. Xanthones from the stems of Cudrania tricuspidata and their inhibitory effects on pancreatic lipase and fat accumulation. Bioorg Chem. 2019;92:103234. doi: 10.1016/j.bioorg.2019.103234. doi: 10.1016/j.bioorg.2019.103234. [DOI] [PubMed] [Google Scholar]
- Jo YH, Kim SB, Liu Q, Hwang BY, Lee MK. Prenylated xanthones from the roots of Cudrania tricuspidata as inhibitors of lipopolysaccharide-stimulated nitric oxide production. Arch Pharm. 2017;350:e1600263. doi: 10.1002/ardp.201600263. doi: 10.1002/ardp.201600263. [DOI] [PubMed] [Google Scholar]
- Kandulski A, Selgrad M, Malfertheiner P. Helicobacter pylori infection: a clinical overview. Dig Liver Dis. 2008;40:619–626. doi: 10.1016/j.dld.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Kang HM, Kim N, Park YS, Hwang JH, Kim JW, Jeong SH, et al. Effects of Helicobacter pylori infection on gastric mucin expression. J Clin Gastroenterol. 2008;42:29–35. doi: 10.1097/MCG.0b013e3180653cb7. [DOI] [PubMed] [Google Scholar]
- Kim DH, Lee S, Chung YW, Kim BM, Kim H, Kim K, et al. Antiobesity and antidiabetes effects of a Cudrania tricuspidata hydrophilic extract presenting PTP1B inhibitory potential. Biomed Res Int. 2016;2016:8432759. doi: 10.1155/2016/8432759. doi: 10.1155/2016/8432759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Jang SS, Lee JL, Sim JH, Shim JJ. Cudrania tricuspidata extract protects against reflux esophagitis by blocking H2 histamine receptors. Prev Nutr Food Sci. 2019;24:159–164. doi: 10.3746/pnf.2019.24.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim OK, Nam DE, Jun W, Lee J. Anti-Inflammatory and gastroprotective activities of Cudrania tricuspidata leaf extract against acute HCl/ethanol-induced gastric mucosal injury in Sprague- Dawley rats. 2015a;39:508–516. doi: 10.1111/jfbc.12149. [DOI] [Google Scholar]
- Kim OK, Nam DE, Jun W, Lee J. Cudrania tricuspidata water extract improved obesity-induced hepatic insulin resistance in db/db mice by suppressing ER stress and inflammation. Food Nutr Res. 2015b;59:165. doi: 10.3402/fnr.v59.29165. doi: 10.3402/fnr.v59.29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Gum JR., Jr Diversity of mucin genes, structure, function, and expression. Gastroenterology. 1995;109:999–1001. doi: 10.1016/0016-5085(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Lee H, Ha H, Lee JK, Seo CS, Lee NH, Jung DY, et al. The fruits of Cudrania tricuspidata suppress development of atopic dermatitis in NC/Nga mice. Phytother Res. 2012;26:594–599. doi: 10.1002/ptr.3577. [DOI] [PubMed] [Google Scholar]
- Liu ES, Cho CH. Relationship between ethanol-induced gastritis and gastric ulcer formation in rats. Digestion. 2000;62:232–239. doi: 10.1159/000007821. [DOI] [PubMed] [Google Scholar]
- Mårdh S, Song YH, Carlsson C, Björkman T. Mechanisms of stimulation of acid production in parietal cells isolated from the pig gastric mucosa. Acta Physiol Scand. 1987;131:589–598. doi: 10.1111/j.1748-1716.1987.tb08280.x. [DOI] [PubMed] [Google Scholar]
- Nakada SL, Crothers JM, Jr, Machen TE, Forte JG. Apical vacuole formation by gastric parietal cells in primary culture: effect of low extracellular Ca2+ Am J Physiol Cell Physiol. 2012;303:C1301–C1311. doi: 10.1152/ajpcell.00244.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa T, Hirata W, Higichi S. Effects of three H2-receptor antagonists (cimetidine, famotidine, ranitidine) on serum gastrin level. Int J Clin Pharmacol Res. 2002;22:29–35. [PubMed] [Google Scholar]
- Park KH, Park YD, Han JM, Im KR, Lee BW, Jeong IY, et al. Anti-atherosclerotic and anti-inflammatory activities of catecholic xanthones and flavonoids isolated from Cudrania tricuspidata. Bioorg Med Chem Lett. 2006;16:5580–5583. doi: 10.1016/j.bmcl.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Satyanarayana S, Kumar PP, Visweswaram D. Antiulcer activity of agnitundirasa and its comparison with cimetidine in shay rat. Anc Sci Life. 1989;8:207–211. [PMC free article] [PubMed] [Google Scholar]
- Song SH, Ki SH, Park DH, Moon HS, Lee CD, Yoon IS, et al. Quantitative analysis, extraction optimization, and biological evaluation of Cudrania tricuspidata leaf and fruit extracts. Molecules. 2017;22:1489. doi: 10.3390/molecules22091489. doi: 10.3390/molecules22091489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Lauwers GY. Pathology of non-infective gastritis. Histopathology. 2007;50:15–29. doi: 10.1111/j.1365-2559.2006.02553.x. [DOI] [PubMed] [Google Scholar]
- Strous GJ, Dekker J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992;27:57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- Szabo S, Bynum TE. Alternatives to the acid-oriented approach to ulcer disease: does 'cytoprotection' exist in man? A new classification of antiulcer agents. Scand J Gastroenterol. 1988;23:1–6. doi: 10.3109/00365528809093839. [DOI] [PubMed] [Google Scholar]
- Toribara NW, Roberton AM, Ho SB, Kuo WL, Gum E, Hicks JW, et al. Human gastric mucin. J Biol Chem. 1993;268:5879–5885. [PubMed] [Google Scholar]
- Tytgat KM, Büller HA, Opdam FJ, Kim YS, Einerhand AW, Dekker J. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Identification of a unique species by expression cloning. Gastroenterology. 1994;107:1352–1363. doi: 10.1016/0016-5085(94)90537-1. [DOI] [PubMed] [Google Scholar]
- Van Klinken BJ, Dekker J, Büller HA, Einerhand AW. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol. 1995;269:G613–G627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- Wang RQ, Fang DC. Effects of Helicobacter pylori infection on mucin expression in gastric carcinoma and pericancerous tissues. J Gastroenterol Hepatol. 2006;21:425–431. doi: 10.1111/j.1440-1746.2005.04006.x. [DOI] [PubMed] [Google Scholar]
- Xin LT, Yue SJ, Fan YC, Wu JS, Yan D, Guan HS, et al. Cudrania tricuspidata: an updated review on ethnomedicine, phytochemistry and pharmacology. RSC Adv. 2017;7:31807–31832. doi: 10.1039/C7RA04322H. [DOI] [Google Scholar]