Abstract
Hypercholesterolaemia is one of the risk factors in coronary heart disease. Hence, this research was designed to investigate the effect of iru on cholesterol levels in hypercholesterolaemic rats. The fermented condiment iru was produced naturally and with starter cultures of Lactobacillus plantarum, Bacillus subtilis, and Leuconostoc sp.. The hypercholesterolaemic rats were fed a diet supplemented with iru for 28 days, and total cholesterol, triglyceride (TG), high density lipoproteins (HDL), and low density lipoproteins (LDL) were determined before and after feeding. Cholesterol levels in hypercholesterolaemic rats (100.80 mg/dL) were reduced to 56.99∼80.21 mg/dL after feeding with iru supplementation while rats not placed on the iru diet had cholesterol levels of 119 mg/dL. There were also significant reductions (P<0.05) in serum TG (78.77∼32.57 mg/dL) and LDL (28.43∼6.63 mg/dL) levels in rats fed the iru diet compared with the control (63.36 mg/dL). Higher and significantly different (P<0.05) HDL was found in rats fed with iru fermented with L. plantarum (44.01 mg/dL) while the least was found in the untreated group (28.93 mg/dL). The results from this study suggest that supplementing the diet with iru obtained by the fermentation of Parkia biglobosa seeds may significantly reduce cholesterol level in the blood stream.
Keywords: cholesterol, hypercholesterolaemia, iru, Lactobacillus plantarum
INTRODUCTION
Cholesterol is a waxy substance made by animal liver and also supplied in diet through animal products such as meats, poultry, fish, and dairy. Cholesterol is needed in the body to insulate nerves, make cell membranes, and produce certain hormones, and it is an important lipid in some membranes. However, cholesterol plays a major role in human heart health and high cholesterol is a leading risk factor for human cardiovascular disease (CVD) such as coronary heart disease and stroke (Ma and Shieh, 2006). World Health Organization reported that 17.3 million people died from CVD in 2008. It has been estimated that 23.6 million people a year will die from CVD by 2030 (Kobayashi et al., 2012; Napoli et al., 2012). Studies have shown that a high serum total cholesterol (TC) or low-density lipoprotein (LDL) cholesterol is a risk factor for new or recurrent coronary event (Aronow, 2013).
Historically, fermented foods have been produced to extend the shelf life of raw materials and increase their safety as well as serve as probiotics. During production, microorganisms play vital and essential roles, contributing to improvement of the physiochemical, sensory, and safety characteristics of final products (Adelekan and Nwadiuto, 2012). Iru is a fermented condiment, prepared from the seed of African locust beans (Parkia biglobosa). This condiment is mainly consumed in West Africa, and very popular among the Yoruba people of Nigeria. It is often used in cooking traditional dishes, such as melon soups, okra soups, and other vegetable soups (Aderibigbe et al., 2011).
Fermented foods have been reportedly used in treating some illnesses. Aderiye and Laleye (2003) reported that iru is not only consumed as a flavouring additive for soup and a cheap meat substitute amongst poor families, but also used as a local remedy in the treatment of eye infection.
Statins are synthetic drugs generally used in lowering hypercholesterolaemia, however, these drugs are expensive and often elicits some side effects. The side effects of cholesterol-lowering drugs include: muscle aches, abnormal liver function, allergic reaction (skin rashes), heartburn, dizziness, abdominal pain, constipation, and decreased sexual desire (Ma and Shieh, 2006). Based on the side effects associated with drugs used in lowering hypercholesterolaemia as well as the cost, it is imperative to investigate the effect of naturally produced condiment in reducing the body’s cholesterol. This may eventually serve not only as a natural means for reducing cholesterol in the body but also as a cheaper remedy source for hypercholesterolaemia. This research was therefore designed to investigate the hypocholesterolaemic effect of fermented seeds of iru, a condiment produced from the fermentation of P. biglobosa seed.
MATERIALS AND METHODS
Source of P. biglobosa seeds
Raw P. biglobosa seeds were bought from a local market (Oja Oba) in Omuo-Ekiti, Southwestern Nigeria (7° 46’ 0” North 5° 43’ 0” East).
Seed processing
The seeds were processed based on the method (Fig. 1) earlier described by Atere et al. (2019).
Fig. 1.

Flow chart to produce iru (Atere et al., 2019).
Experimental animals
The animal study was conducted in accordance with the guidelines for animal experiments approved by the University Institutional Animal Care and Use Committee (CERAD-2018 Approved MCB-1240). Sixty albino rats (both sex) weighing between 45 and 57 g were used. The rats were acclimatized for 2 weeks. After acclimatization, the rats were separated into two groups, consisting of ten and fifty rats. The group of fifty rats were induced into hypercholesterolaemia using pork extract and pork (80 mg pork/g and 1 mL of extract/g) for 14 days (Aderiye et al., 2007). Three rats were then scarified in each group to determine and compare the level of induction.
Effect of feeding on rats
In the second phase of the experiment, the induced rats were divided into 6 groups made up of 6 rats per group as presented in Table 1 and 2. After 28 days of feeding, six rats were scarified per group. The serum was collected and analyzed for serum cholesterol and lipoproteins.
Table 1.
Different feeding groups and administration of condiments
| Sample number | Groups | Feeding composition |
|---|---|---|
| 1 | A control (uninduced) | Commercial feed |
| 2 | B induced (treated) | Commercial feed+20% iru fermented naturally |
| 3 | C induced (treated) | Commercial feed+20% iru fermented with Bacillus subtilis |
| 4 | D induced (treated) | Commercial feed+20% iru fermented with Leuconostoc sp. |
| 5 | E induced (treated) | Commercial feed+20% iru fermented with Lactobacillus plantarum |
| 6 | F induced (treated) | Commercial feed+flavostatin (standard) |
| 7 | G induced (untreated) | Commercial feed |
Table 2.
The proximate composition of the commercial feed
| Sample number | Components | Percentage |
|---|---|---|
| 1 | Crude protein | 15 |
| 2 | Crude fiber | 7 |
| 3 | Carbohydrate | 54 |
| 4 | Moisture content | 12 |
| 5 | Crude fat | 7 |
| 6 | Ash | 7 |
Serum lipid analyses
The serum lipid assay was determined using the method of Aderiye et al. (2007). Blood samples collected in the heparinized bottle were centrifuged at 3,000 rpm for 15 min. The plasma was collected and used in the determination of cholesterol using the Randox kits. TC, triglyceride (TG), and high density lipoproteins (HDL) cholesterol were determined using the enzymatic kit and standardized reagent. The LDL cholesterol and very low-density lipoprotein (VLDL) cholesterol concentrations were calculated by using the formulas below:
Statistical analysis
Statistical analysis was carried out on data obtained, using analysis of variance (ANOVA), Duncan’s multiple range test, and Statistical Package for Social Sciences (SPSS version 16.8, SPSS Inc., Chicago, IL, USA).
RESULTS
The average weight of rats after inducement was 112.4 g while the weight of the control group was 94.3 g. The cholesterol level of the rats after inducement was 100.8 mg/dL in the induced group and 51.4 mg/dL in the control group (Fig. 2). Hypercholesterolaemic rats fed the iru supplemented diet went from 100.80 mg/dL to 56.99∼ 80.21 mg/dL serum cholesterol while rats not fed the iru supplemented diet had a higher and significantly different cholesterol level (119.45 mg/dL) (Fig. 3). The control group (uninduced) had 68.56 mg/dL of cholesterol while the group fed with iru fermented with Lactobacillus plantarum had 56.99 mg/dL of cholesterol. The group administered the standard drug had 68.25 mg/dL. Fig. 4 showed the changes in TGs after treatment. The control (uninduced) group had 79.18 mg/dL TG level. The untreated had 124.57 mg/dL of TG and the least value of TG was found in the sample fermented with L. plantarum with a value of 32.56 mg/dL. The group fed with the standard drug had a value of 80.39 mg/dL of TGs.
Fig. 2.
The body weight and cholesterol level of rats after two weeks inducement.
Fig. 3.
Cholesterol level of rats after feeding with a diet supplemented by iru. Bars with different letters (a-d) are significantly different (P≤0.05).
Fig. 4.
Triglyceride (TG) level of rats after feeding with a diet supplemented by iru. Bars with different letters (a-d) are significantly different (P≤0.05).
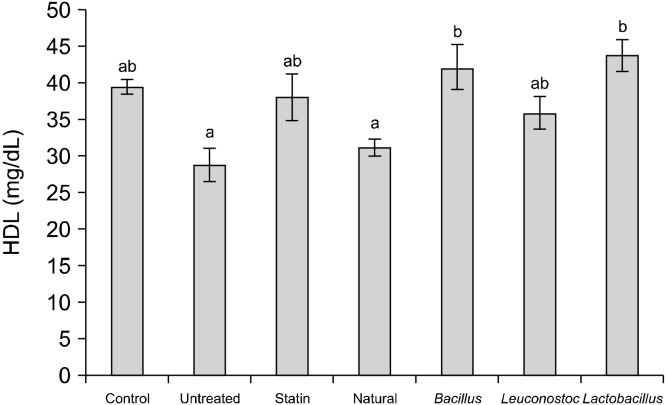
HDL was highest in the group fed with samples fermented with L. plantarum at a value of 44.01 mg/dL. The untreated group had the least value of 28.92 mg/dL while the control (uninduced) group had 39.73 mg/dL. The standard drug had 38.23 mg/dL as presented in Fig. 5.
Fig. 5.
High density lipoprotein (HDL) of rats after feeding with a diet supplemented by iru. Bars with different letters (a,b) are significantly different (P≤0.05).
Fig. 6 showed the LDL distribution for each group of rats. LDL for the group fed samples fermented with L. plantarum had the lowest value of 6.62 mg/dL. The untreated group had the highest LDL with a value of 62.36 mg/dL while the control group had 16.82 mg/dL. The group that received the standard drug had 16.32 mg/dL.
Fig. 6.
Low density lipoprotein (LDL) of rats after feeding with a diet supplemented by iru. Bars with different letters (a-c) are significantly different (P≤0.05).
VLDL was lowest in groups fed iru samples fermented with L. plantarum with a value of 6.513 mg/dL while the untreated group had 24.91 mg/dL. The group fed with sample fermented by Bacillus subtilis had 10.94 mg/dL, while the control group had 15.83 mg/dL, and the group administer with the standard drug had 16.08 mg/dL (Fig. 7).
Fig. 7.
Very low-density lipoprotein (VLDL) of rats after feeding with a diet supplemented by iru. Bars with different letters (a-d) are significantly different (P≤0.05).
DISCUSSION
Considerable cholesterol can be obtained from the diet, especially a fatty meal. Often food from animal sources are rich in cholesterol. In this study, pork and pork extract significantly increased the cholesterol level of the induced rats after 14 days. The result is in line with the report of Aderiye et al. (2007) who reported a serum TC of 109.0 mg/dL when rats were fed with pork and pork extract. The increase in serum TG and TC in the rats might have resulted from the enlargement of the intestinal absorption of these lipids (Tabuchi et al., 2003).
The rats induced had higher body weight than the uninduced. The difference in body weight may be attributed to an increase in fat and protein contents in the diets. Edem et al. (2003) earlier reported that increased fat and protein content of meals supplemented in rats often result in an increase in body weight.
With the incorporation of fermented P. biglobosa seeds into the feed of rats, a clear reduction in the cholesterol level was observed. This reduction is even lower than the reduction reported by the standard drug, statin. This result agrees with Ayo-Lawal et al. (2014) who reported a decrease in the cholesterol level of the rats fed with fermented P. biglobosa seed.
The group fed the iru sample fermented with L. plantarum had a better cholesterol lowering potential. This may have resulted from the ability of the bacteria to produce a (some) secondary metabolite, which helps in the conversion of LDL to HDL. In previous research (Rašić et al., 1992; De Rodas et al., 1996; Noh et al., 1997; Aderiye et al., 2007), Lactobacillus has been reported to have the ability of breaking cholesterol in the serum lipid. Linoleic acid (C18:2) has been reported as the most abundant fatty acid in fermented P. biglobosa seed. This fatty acid has been reported to moderately reduce serum lipids, which may have accounted for the reduction of TC in the treated groups (Aremu et al., 2015).
All the groups fed with fermented iru showed varying degree of reduction in the cholesterol level compared to the untreated. The observed high level of cholesterol in the untreated group also indicated that the condiment administered was responsible for the observed decrease in the cholesterol level after the 28 days of treatment. A report by Laleye et al. (2016) using the fermented sorghum meal ‘ogi’ demonstrated a reduction in hypercholesterolaemia; it was reported that the fermented sample reduced the cholesterol level, and this reduction was attributed to the co-precipitation of cholesterol with deconjugated bile acids.
The LDL values were lowered by the treatment compared with the untreated group. LDL is a product of the liver through the packaging of TGs with cholesterol in cells (Paré et al., 2005). Very high levels of LDL may result in the hardening of the arteries by sticking to the lining of the blood vessels. The observed reduction in LDL was earlier reported by Aderiye and David (2013), where iru reduced the LDL in induced hypercholesterolaemic rats. A similar result was also reported by Laleye et al. (2016) during feeding trials with sorghum meal on hpercholesterolaemic rats.
TG content is also lowered by the feed supplemented with the fermented condiment, iru. Davignon and Cohn (1996) describes the link between LDL and TGs. TGs are regarded as the determinant of LDL composition and cell specific binding. This relationship is termed to be directly proportional. The observed reduction in TGs in this study is in line with the reduced level of LDL since both the TG and LDL are interdependent.
The observed increase in HDL for all treated groups is an indication of how effective the treatment has proven. This observed increase in HDL level in rats agrees with reports of Laleye et al. (2016) and Ayo-Lawal et al. (2014). Liong and Shah (2005) reported that high levels of HDL in animal and human blood protect against heart attack. Burtis and Ashwood (1986) reported that HDL increases the catabolism of TGs. This may be an explanation for the decrease in LDL, VLDL, and TGs, since they are interdependent.
Iru fermented with L. plantarum displayed the best hypocholesterolaemic effect in this study. Production of iru with this strain of bacteria may ultimately be a new method of hypercholesterolaemia control. This study also showed that the group treated with iru fermented with different cultures also had varying degrees of reduction in cholesterol and even lower than the conventional anti-cholesterol agent statin. Regular intake of a good quality fermented P. biglobosa seeds may be useful in protecting the human body against high levels of cholesterol, LDL, and TGs. This condiment is advantageous since it is relatively cheap, natural, and easily accessible. It may serve as a new alternative to synthetic drugs used in controlling lipid metabolism disorders, which are expensive and have varying side effects.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Adelekan AO, Nwadiuto E. bacterial succession studies during fermentation of African locust bean (Parkia biglobosa) to iru using molecular methods. Br Biotechnol J. 2012;2:49–59. doi: 10.9734/BBJ/2012/586. [DOI] [Google Scholar]
- Aderibigbe EY, Visessanguan W, Sumpavapol P, Kongtong K. Sourcing starter cultures for Parkia biglobosa fermentation I: Phylogenic grouping of Bacillus species from commercial 'iru' samples. Int J Biotechnol Mol Biol Res. 2011;2:121–127. [Google Scholar]
- Aderiye B, Laleye S. Relevance of fermented food products in southwest Nigeria. Plant Foods Hum Nutr. 2003;58:1–16. doi: 10.1023/B:QUAL.0000040315.02916.a3. [DOI] [Google Scholar]
- Aderiye BI, David OM. In vivo evaluation of hypolipidemic potentials of Bacillus species isolated from fermented locust bean (Parkia fillicoides Welw) seeds (Iru) Br Microbiol Res J. 2013;3:574–584. doi: 10.9734/BMRJ/2013/5026. [DOI] [Google Scholar]
- Aderiye BI, Laleye SA, Odeyemi AT. Hypolipidemic potential of Lactobacillus and Streptococcus sp. from some Nigerian fermented foods. Res J Microbiol. 2007;2:538–544. doi: 10.3923/jm.2007.538.544. [DOI] [Google Scholar]
- Aremu MO, Ibrahim H, Awala EY, Olonisakin A, Oko OJ. Effect of fermentation on fatty acid compositions of African locust bean and mesquite bean. J Chem Eng Chem Res. 2015;2:817–823. [Google Scholar]
- Aronow WS. Treatment of hypercholesterolemia. J Clin Exp Cardiol. 2013 doi: 10.4172/2155-9880.S1-006. doi: 10.4172/2155-9880.S1-006. [DOI] [Google Scholar]
- Atere AV, Oyetayo VO, Akinyosoye FA. Effects of starter culture on the proximate, antioxidant, antinutritional and mineral composition of fermented Parkia biglobosa seeds to produce iru. Int J Food Sci Nutr. 2019;4:61–65. [Google Scholar]
- Ayo-Lawal RA, Osoniyi O, Famurewa AJ, Lawal OA. Evaluation of antioxidant and hypolipidaemic effects of fermented Parkia biglobosa (Jacq) seeds in tyloxapol-induced hyperlipidaemic rats. Afr J Food Sci. 2014;8:225–232. doi: 10.5897/AJFS2013.1124. [DOI] [Google Scholar]
- Burtis CA, Ashwood ER. Tietz textbook of clinical chemistry. 3rd ed. W.B. Saunders Co.; Philadelphia, PA, USA: 1986. p. 510. [Google Scholar]
- Davignon J, Cohn JS. Triglycerides: a risk factor for coronary heart disease. Atherosclerosis. 1996;124:S57–S64. doi: 10.1016/0021-9150(96)05858-3. [DOI] [PubMed] [Google Scholar]
- De Rodas BZ, Gilliland SE, Maxwell CV. Hypocholesterolemic action of Lactobacillus acidophilus ATCC 43121 and calcium in swine with hypercholesterolemia induced by diet. J Dairy Sci. 1996;79:2121–2128. doi: 10.3168/jds.S0022-0302(96)76586-4. [DOI] [PubMed] [Google Scholar]
- Edem DO, Eka OU, Umoh IB, Udoh AP, Akpan EJ. Effect of red palm oil and refined palm olein on nutrient digestion in the rat. Pak J Nutr. 2003;2:271–278. doi: 10.3923/pjn.2003.271.278. [DOI] [Google Scholar]
- Kobayashi M, Hirahata R, Egusa S, Fukuda M. Hypocholesterolemic effects of lactic acid-fermented soymilk on rats fed a high cholesterol diet. Nutrients. 2012;4:1304–1316. doi: 10.3390/nu4091304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laleye SA, Aderiye BI, David OM. Hypolipidemic activity of fermented sorghum (Sorghum bicolor L. Moench) gruel (ogi) in hypercholesterolemic rats (Rattus nervegicus) Int J Biochem Res Rev. 2016;9:1–15. doi: 10.9734/IJBCRR/2016/19659. [DOI] [Google Scholar]
- Liong MT, Shah NP. Acid and bile tolerance and the cholesterol removal ability of Bifidobacteria strains. Biosci Microflora. 2005;24:1–10. doi: 10.12938/bifidus.24.1. [DOI] [PubMed] [Google Scholar]
- Ma H, Shieh KJ. Cholesterol and human health. J Am Sci. 2006;2:46–50. [Google Scholar]
- Napoli C, Crudele V, Soricelli A, Al-Omran M, Vitale N, Infante T, et al. Primary prevention of atherosclerosis: a clinical challenge for the reversal of epigenetic mechanisms? Circulation. 2012;125:2363–2373. doi: 10.1161/CIRCULATIONAHA.111.085787. [DOI] [PubMed] [Google Scholar]
- Noh DO, Kim SH, Gilliland SE. Incorporation of cholesterol into the cellular membrane of Lactobacillus acidophilus ATCC 43121. J Dairy Sci. 1997;80:3107–3113. doi: 10.3168/jds.S0022-0302(97)76281-7. [DOI] [PubMed] [Google Scholar]
- Paré P, Adams PC, Baik SK, Bain V, Girgrah N, Grover PT, et al. The liver. In: Shaffer EA, Thomson ABR, editors. First Principles of Gastroenterology: The Basis of Disease and an Approach to Management. 5th ed. Janssen-Ortho Inc.; Markham, ON, Canada: 2005. pp. 462–475. [Google Scholar]
- Rašić JL, Vujičić IF, Škrinjar M, Vulić M. Assimilation of cholesterol by some cultures of lactic acid bacteria and bifidobacteria. Biotechnol Lett. 1992;14:39–44. doi: 10.1007/BF01030911. [DOI] [Google Scholar]
- Tabuchi M, Asako T, Noriko Y, Tetsuo I, Masataka H, Akiyoshi H. Hypocholesterolemic effect of Lactobacillus GG in hypercholesterolemic rats. Milchwssenschaft. 2003;58:246–249. [Google Scholar]