Abstract
The link between the gut microbiome and obesity is not well defined. Understanding of the role of the gut microbiome in weight and health management may lead to future revolutionary changes for treating obesity. This review examined the relationship between obesity and the gut microbiome, and the role of probiotics, prebiotics, and synbiotics for preventing and treating obesity. We used PubMed and Google Scholar to collect appropriate articles for the review. We showed that the gut microbiome has an impact on nutrient metabolism and energy expenditure. Moreover, different modalities of obesity treatment have been shown to change the diversity and composition of the gut microbiome; this raises questions about the role these changes may play in weight loss. In addition, studies have shown that supplementation with probiotics, prebiotics, and synbiotics may alter the secretion of hormones, neurotransmitters, and inflammatory factors, thus preventing food intake triggers that lead to weight gain. Further clinical studies are needed to better understand how different species of bacteria in the gut microbiome may affect weight gain, and to determine the most appropriate doses, compositions, and regimens of probiotics, prebiotics, and synbiotics supplementation for long-term weight control.
Keywords: gut microbiome, obesity, prebiotics, probiotics, weight loss
INTRODUCTION
The prevalence of several chronic diseases are growing worldwide; of these, obesity is the primary culprit and has been major concern for decades (Gentile and Weir, 2018; Wilkins et al., 2019). Although several methods for treating excessive weight gain are used, obesity remains a major public health problem, which requires novel nutritional and/or medical solutions. Being overweight [body mass index (BMI) between 25 and 30 kg/m2] or obese (BMI higher than 30 kg/m2), and the related comorbidities (cardiovascular diseases, diabetes, and cancer) are leading causes of death; thus, researchers strive to find novel efficient treatments for these conditions (Wilkins et al., 2019). A high-calorie diet is a causal factor for obesity and may induce changes in the function of the gut microbiome (Guirro et al., 2019). In addition to nutritional, lifestyle and genetic factors, it has been suggested that obesity may also result from perturbation of the gut microbiome, which affects metabolic function and energy homeostasis (Guirro et al., 2019).
Approximately 1014 bacteria and archaea of more than 1,000 species exist in the human gastrointestinal tract; together, these are known as the gut microbiota (Lv et al., 2019). The impact of the gut microbiome on health has been a major focus of interest for the past couple of decades, since an adequate gut microbiota and probiotic supplementation have positive effects on many health conditions, including type 2 diabetes, cardiovascular diseases, and immune and infectious diseases (Mohajeri et al., 2018; Valdes et al., 2018). The role of the gut microbiome in affecting the wellbeing of individuals is encouraging researchers to find new treatments for different health conditions, such as obesity and weight gain. A reciprocated relationship exists between the gut flora and diet, whereby dietary factors regulate the role and structure of the microbiota. Microbes in the human intestines impact the absorption, breakdown, and storage of nutrients, and have potential consequences on host physiology (Gentile and Weir, 2018). Furthermore, overuse of antibiotics has been linked to the onset of obesity (Podolsky, 2017; Leong et al., 2018). Gut dysbiosis (an imbalance of gut microbiota composition) due to dietary or environmental changes can promote overgrowth of pathogenic organisms that cause chronic inflammation, thereby playing a major role in the pathology of chronic metabolic and intestinal diseases (Turroni et al., 2014). In contrast, a healthy balance of intestinal microbiota may play a role in preventing or alleviating obesity and metabolic diseases (John and Mullin, 2016). Increases in certain beneficial bacterial species and decreases in other damaging species may impact the health and the wellbeing of the host (Fischer and Relman, 2018).
Gut microbiota may also play a role in the brain and the central nervous system, which may help explain relationship between the gut microbiota and overall health (Lankelma et al., 2015). The gut, referred to as the ‘second brain’, is composed of trillions of microorganisms that directly affect the brain and brain signals, influencing stimulants for hunger and appetite. The diversity and composition of the gut microbiome are influenced by multiple factors, such as birth mode, dietary habits, the use of antibiotics and medications, aging, and other environmental factors (Azad et al., 2016). Human microbial colonization commences at birth, and then progresses and modifies species profusion for 3 years until the microbiota grows into its adult form. The human microbiota is composed of 5 different families of microbiota (Bacteroidetes, Firmicutes, Actinobacteria, Proteobacteria, and Verrucomicrobiai, of which the Bacteroidetes and Firmicutes make up 90% of the species of bacteria (Lv et al., 2019).
This review will discuss the relationship between the gut microbiota and obesity, to increase understanding of the mechanism by which the gut microbiome affects weight gain. Moreover, this review will examine the effects of probiotics, prebiotics, and synbiotics supplementation on body weight.
MATERIALS AND METHODS
This study was undertaken using the PubMed and the Google Scholar databases in November 2019, using the descriptor Medical Subject Headings, without limiting the publication period (with emphasis on the most recent papers). Studies involving animals and obese adult subjects were included. This review did not include obese children, subjects with genetic disorders, or autoimmune diseases.
To review the relationship between the gut microbiome and obesity, articles studying gut microbiota diversity and composition in obese versus lean subjects were included as the first objective of the review. The following combinations of keywords were used: “obesity”, “weight loss”, “weight gain”, with “gut microbiome, microbiota, or microflora” (16 articles), “gut diversity” (13 articles), or “gut dysbiosis” (14 articles).
The second part of this review discussed the possible effects of probiotics, prebiotics, and synbiotics supplementation on overweight and obese individuals, alone, or combined with other treatment methods. This search included the following keywords: “obesity”, “overweight”, or “weight loss” with “probiotics” (20 articles), “prebiotics” (15 articles), or “synbiotics” (15 articles).
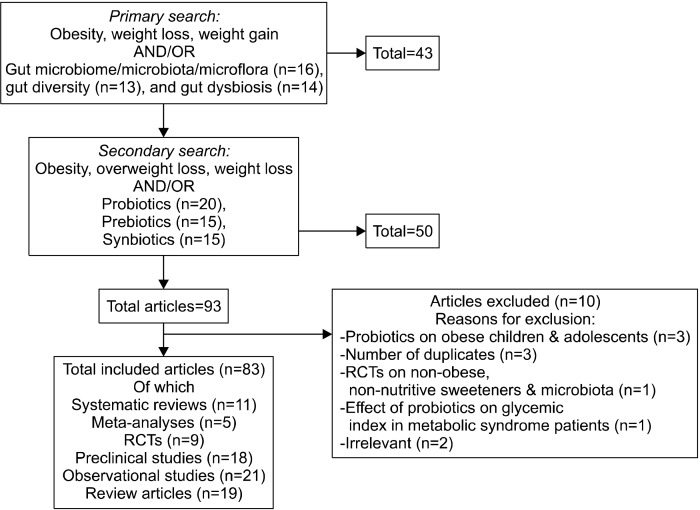
Of the 93 articles, the search identified 83 articles that we included in this review: 11 systematic reviews, 5 meta-analyses, 9 randomized controlled trials, 18 preclinical studies, 21 observational studies, and 19 review articles. This process is summarized in Fig. 1.
Fig. 1.
Process of inclusion/exclusion of studies on gut microbiome and obesity, and on the role of probiotics, prebiotics, and synbiotics in weight loss.
RESULTS AND DISCUSSION
Obesity and the gut microbiome
The prevalence of obesity has increased worldwide; sedentary lifestyles and increased consumption of food are considered the primary causes for this epidemic (Baothman et al., 2016). The main physiological functions of the gut microbiota are digestion, vitamin synthesis, and metabolism. It has been reported that the gut microbiota increases energy production from food, provides low-grade inflammation, and impacts fatty acid tissue composition. These mechanisms may link the gut microbiota with obesity (Baothman et al., 2016; Duca et al., 2018).
Animal studies: Obese subjects tend to have a greater amount of Firmicutes species, and a higher Firmicutes to Bacteroidetes ratio than individuals of normal weight (John and Mullin, 2016). Firmicutes have been shown to be negatively proportional to resting energy expenditure, whereas Bacteroides have been shown to be positively correlated with the percentage of body fat (Gomes et al., 2018).
A large depletion of Bacteroides species are present in obese mice, including Bacteroides thetaiotaomicron, which protects against adiposity. Transfer of B. thetaiotaomicron to mice that received a normal diet resulted in a significant reduction in total fat, and prevented weight gain in mice that received a high fat diet (Liu et al., 2017). Mice that received a high fat diet and were supplemented with B. thetaiotaomicron also had increased levels of bacteria of the Akkermansia genus (Liu et al., 2017). Akkermansia muciniphila have been linked to lower subcutaneous adiposity diameters, waist to hip ratios, and fasting blood glucose levels (Dao and Clément, 2018). A. muciniphila have also been shown to counteract inflammation and adiposity after consumption of a high fat diet in rodent models (Schneeberger et al., 2015). A. muciniphila produce acetate and propionate, which can be used as sources of energy by other species of bacteria (Schneeberger et al., 2015). Furthermore, this phylum may also induce a noticeable improvement in insulin sensitivity (Dao and Clément, 2018) and host metabolism (Dao et al., 2016), thus preventing and alleviating obesity.
Surprisingly, high-fat diets (HFD) and high-sucrose diets (HCD) may have different effects on the gut microbiota. Kong et al. (2019) studied obese mice that received either a HFD, HCD, or normal diet for 13 weeks. During the last 4 weeks of the intervention, mice received probiotics consisting of Lactobacillus acidophilus, Bifidobacterium longum, and Enterococcus faecalis in a 1:1:1 ratio. Mice that received the HFD or HCD showed increases in body weight during the first 9 weeks; however, once probiotics were introduced during the last 4 weeks, weight gain slowed in both groups. Furthermore, the HCD and HFD had different effects on the gut microbiota (Kong et al., 2019). The HFD tended to decrease the diversity of the gut microbiota, whereas the HCD completely changed the structure of the bacteria related to obesity. Some favorable bacteria (Allobaculum, Bifidobacterium, Olsenella, Faecalibacterium, and Ruminococcus) were reduced in the microbiota of mice that received the HFD, whereas significantly more obesity-related bacteria (Acinetobacter, Blautia, and Dorea) were observed in the HCD group. Following ingestion of probiotics, Allobaculum, Lactococcus, and Bifidobacterium were significantly increased in both groups. Kong et al. (2019) suggested that dietary sucrose impacts the obesity-induced intestinal microbiota to a greater extent than dietary fat.
Supplementation of obese mice with other species of bacteria, such as Lactobacillus plantarum, reduces adipose deposition and upregulates expression of lipid oxidative genes compared with the control group (Park et al., 2017); this suggests that these species of bacteria may be used in combination with dietary management to treat obesity. In addition, anti-obesity effects of administering specific species of Lactobacillus sakei from probiotics were observed in obese murine models (Ji et al., 2019a; Ji et al., 2019b). However, further studies are needed to better understand the mechanism by which Lactobacillus species reduce fat storage in human.
Human studies: Microbial diversity shows a preventive effect on long-term weight gain in healthy individuals (Menni et al., 2017). Microbial composition differs between people; for example, most studies have noted that the ratio of Firmicutes to Bacteroidetes is significantly higher in obese subjects (John and Mullin, 2016). Furthermore, previous studies have shown correlations between an increased amount of Bacteroidetes in stools and weight loss, and between Firmicutes and development of obesity (Ley, 2010; John and Mullin, 2016).
In addition, a study was performed to report the differences in gut microbiota between obese and non-obese Japanese subjects. Obese subjects had significantly reduced numbers of Bacteroidetes and higher Firmicutes to Bacteroidetes ratios compared with non-obese subjects. The diversity of the bacteria was also significantly greater in the obese subjects than the non-obese subjects (Kasai et al., 2015).
In a study of obese and lean Chinese college males, there was a negative correlation between BMI and gut microbiota diversity. Therefore, obese male subjects had a less diverse microbiome than individuals in the lean group. In addition, fecal microbial composition was more complex for lean males and contained a higher ratio of Bacteroides to Firmicutes (Lv et al., 2019).
Interestingly, aside from a higher number of Firmicutes in obese subjects, a recent study noted that overweight and obese individuals who start therapy with high Firmicutes/Bacteroides ratios have better health outcomes when following a high fiber and whole grain diet than those with low Firmicutes/Bacteroides ratios (Hjorth et al., 2018).
Ley (2010) suggested that obese participants receiving a low caloric diet for 12 months show increases in Bacteroidete counts associated with weight loss. However, contradictory data exists regarding Bacteroidetes species in the gut flora of obese compared with non-obese individuals, with other studies showing no correlation or even a positive correlation with obesity (Schwiertz et al., 2010). Such discrepancies between studies may be due to host physiology and/or various dietary habits, as well as the techniques used for analysis.
The remarkable effect of A. muciniphila on metabolism has also been observed in human studies. A. muciniphila have shown protective effects against gut permeability and endotoxemia, to reinforce the immune system, and to improve glucose homeostasis. Thus, individuals with higher amounts of A. muciniphila have a better status of health (Dao et al., 2016).
Mechanism of action
Change in energy harvest and nutrient metabolism: The gut microbiome can be associated with carbohydrate and lipid metabolism. Despite a reduced food intake, transplantation of normal mouse microbiota into germ-free mice increases body fat content (by 60%) and insulin resistance within 14 days, and increases triglyceride levels through activating de novo fatty acid synthesis (Bäckhed et al., 2004). One important function of the gut microbial function is the ability to alter bile acid signaling and to produce distinct pathophysiological bile acid profiles (Sanmiguel et al., 2015). Approximately 5∼10% of bile acids are bio-transformed by anaerobic gut microbiota (Bacteroides, Eubacterium, and Clostridium), with the rest secreted in faeces. This demonstrates the role of the gut microbiome in the digestion and metabolism of dietary fat (Mazloom et al., 2019).
The gut microbiome has also been linked to metabolism of essential amino-acids (EAA). Genetic biomarkers relating to EAA metabolic pathways are significantly increased in germ-free murine recipients of the obese-twin gut microbiome. The germ-free recipients of lean-twin microbiomes were found to be richer in genes relating to the breakdown and fermentation of dietary polysaccharides (Ridaura et al., 2013). These results emphasize the major of the gut microbiome in the breakdown and metabolism of macronutrients in the human diet. Diet is a crucial contributor to microbial change; however, the exact dietary interventions that increase microbiota diversity and affect metabolic state vary between individuals (Stephens et al., 2018).
Nitrogen, a major source of dietary protein, is essential for microbial growth, assimilation of carbohydrates, and assembly of short chain fatty acids (Zhang et al., 2018). Hence it is crucial to consume a varied diet with adequate daily intake of protein from different food sources.
Short chain fatty acids (SCFAs): The gut microbiota plays a role in energy metabolism through production of SCFAs. SCFAs are produced by colonic fermentation involving anaerobic catabolism of dietary fiber and protein (Baothman et al., 2016; Zhang et al., 2018). SCFAs are microbial waste products produced by microbes to balance homeostasis of the gut (Baothman et al., 2016). SCFA, specifically acetate, propionate, and butyrate, may have an impact on human metabolism. The beneficial effects of these products have been observed on body weight, insulin sensitivity, and glucose balance. SCFAs are also able to strengthen the intestinal barrier, thus reducing inflammation, and have a positive effect on lipid metabolism (Chambers et al., 2018). SCFAs are able to regulate satiety and reduce appetite (Hamilton and Raybould, 2016; Li et al., 2018), thus playing a major role in food intake and energy consumption. Furthermore, SCFAs are able to act on free fatty acid receptor 2 to stimulate the release of the hormone peptide YY (PYY), thus playing a direct and significant role in inducing satiety (Brooks et al., 2016).
The quantities of SCFA differ in overweight or obese subjects compared with lean subjects: obese individuals had higher amounts of (Fernandes et al., 2014) fecal butyrate, acetate, and propionate produced by Bacteroidetes species (Baothman et al., 2016) compared with lean individuals. Previous studies have shown that dietary supplementation with butyrate decreases insulin resistance (via increasing mitochondrial function and energy expenditure) and is protective against obesity caused by high calorie diet (Lin et al., 2012; Baothman et al., 2016). Consumption of higher-quality whole grains or bran can increase butyrate production, thus helping increase the biodiversity of the gut microbiota (McClave and Martindale, 2019).
In summary, SCFA are known for their advantageous modulatory effects on human immunity, gastrointestinal epithelial cell integrity, lipid metabolism, glucose homeostasis, and appetite (Tandon et al., 2019).
Inflammation: Gut bacteria may alter inflammatory factors by modulating inflammatory cytokine secretion. High levels of interleukin (IL)-6, tumor necrosis factor (TNF), and C-reactive protein are biomarkers for inflammation and appear to be associated with obesity (Rastelli et al., 2018; Singer-Englar et al., 2019). Probiotic studies have shown that a decrease in inflammatory factors (IL-6 and TNF) lower inflammation, which makes this pathway clearer for interpreting the impact of probiotics on obesity. Moreover, Chang et al. (2019) has stated that studies have identified several potential next generation probiotics due to rapid development of advanced genetic sequencing tools: Bacteroides fragilis decreases inflammation and Parabacteroides goldsteinii is a potential anti-obesity probiotic.
Chang et al. (2019) and Wu et al. (2019) have shown that water extract from Hirsutella sinensis, a medicinal mushroom, decreases obesity, insulin resistance, and inflammation in HFD fed mice. The isolated polysaccharides within the water extract also reduced body weight by 50%. Analysis of the gut microbiota showed that Gram-negative P. goldsteinii was decreased in the microbiome of HFD-fed mice, whereas this bacterium was elevated in mice treated with mushroom polysaccharides. These results demonstrated the effectiveness of mushroom polysaccharides for mediating including the recently identified probiotic P. goldsteinii and anti-inflammatory, anti-obesity, and anti-insulin resistance biomarkers (Chang et al., 2019; Wu et al., 2019). H. sinensis polysaccharides and the gut bacterium P. goldsteinii may be described as prebiotics and probiotics useful for the treatment of obesity.
Antibiotic use and dysbiosis: Use of antibiotics is correlated with an increased risk of developing multiple inflammatory disorders (Le Roy et al., 2019a) and has been linked to alterations in the gut microbiome (Hamilton and Raybould, 2016). Antibiotic-induced dysbiosis induces weight gain and increases very low-density lipoprotein/high-density lipoprotein ratios in animal studies (Le Roy et al., 2019b). Moreover, exposure to antibiotics at 2 years of age increases the likelihood of developing obesity later in life (Stark et al., 2019), and alterations to the gut microbiota after use of antibiotic may lead to weight gain (Podolsky, 2017; Leong et al., 2018; Stark et al., 2019). However, how antibiotic-induced changes to the microbiota increase the risk of inflammation and weight gain in humans is currently uncertain.
Hormone and neurotransmitter secretion: Bacterial dysbiosis, related to increases in the Firmicutes species (Clostridium, and Eubacterium rectale, Clostridium coccoides, Lactobacillus reuteri, A. muciniphila, Clostridium histolyticum, and Staphylococcus aureus) has been linked to alterations in gastrointestinal peptides (gastrin, cholecystokinin, somatostatin, and ghrelin). Eventually, these changes may result in decreased satiety and increased appetite and food intake (Gomes et al., 2018).
Probiotics and prebiotics effect on obesity and weight gain
Use of probiotics and prebiotics as a prevention and treatment for different chronic diseases has largely increased in the last decade (Cerdó et al., 2019). Synbiotics (supplements including probiotics and prebiotics that may have a beneficial effect on health) are now often used as functional foods (nutraceutical supplemental additives for healthy eating). Emerging evidence has demonstrated that probiotics may be organic compounds of the microbiome and may ameliorate its function (Wang et al., 2019b).
Probiotics: Probiotics consist of live bacteria, typically Lactobacilli and Bifidobacteria, beneficial for improving the composition of colonic microbiota and promoting health of the host (Okeke et al., 2014; Qian et al., 2019).
Animal studies: In a previous study, obese mice were fed one or more strains of probiotics (Lactobacillus rhamnosus, Bifidobacterium breve, and Lactobacillus paracasei) or placebo. Mice receiving probiotics showed decreased levels of serum lipopolysaccharides (LPS), neutral lipids in the liver and IL-6 (Plaza-Diaz et al., 2014). Deconjugation of bile acids by SCFA may explain reductions in fat mass observed in individuals consuming probiotics, due to fermentation of non-digestible carbohydrates (Brusaferro et al. 2018). These findings indicate probiotics are highly effective for improving BMI and body weight and for increasing fat loss, and suggest that altering the gut microbiome with probiotics is more predictable.
Chemerin has recently been recognized as an adipokine that plays a major role in the metabolism of adipocytes and increases adipogenesis. Çelik and Ünlü Söğüt (2019) evaluated the impact of probiotic supplementation on levels of chemerin, inflammation, and metabolic syndrome parameters in obese rats. Rats were fed either a control diet, a HFD, or a HFD supplemented with probiotics after induction of obesity. Weight and changes in weight and BMI were statistically different in the obese intervention group compared with the control group. Rats in the obese group also exhibited increased fasting plasma glucose, insulin and insulin resistance, inflammatory markers, and leptin compared with the control groups. Treatment with probiotics decreased weight gain significantly; these positive effects were observed at the level of insulin and fasting blood glucose, inflammatory markers, leptin, and chemerin levels (Çelik and Ünlü Söğüt, 2019).
Human studies: Use of probiotics has been shown to reduce BMI and total body fat, specifically visceral fat (Osterberg et al., 2015; Mazloom et al., 2019). A clinical study conducted by Osterberg et al. (2015) showed that probiotics prevented increases in body weight and body fat in 20 healthy males who consumed a high fat diet for 4 weeks.
A meta-analysis was conducted to examine the effect of probiotics on body weight and glycaemic control in overweight or obese adults. Adults who received probiotics had significant reductions in body weight, BMI, waist circumference, fat mass percentage compared with adults in the control group (Wang et al., 2019b). A greater reduction in fat mass was observed with 1) use of high versus low doses of probiotics, 2) probiotics of a single strain versus multiple strains, and 3) probiotics administered in the form of foods compared with capsules or powder. In addition, when compared with the control group, individuals in the probiotic groups had reduced insulin levels. Overall, Wang et al. (2019b) and Koutnikova et al. (2019) claim that use of probiotics both has a positive effect on weight loss and improves other metabolic parameters.
Probiotics containing Lactobacillus and Bifidobacterium appear to have some benefit for gram negative gut bacteria, thus preventing overgrowth of pathogenic bacteria in the flora (Okeke et al., 2014). In addition, probiotics can improve the intestinal epithelial barrier and reduce gut permeability, which is a major factor in preventing inflammation and endotoxemia due to transfer of foreign bodies into blood (He and Shi, 2017). SCFA play a key role in this process. Moreover, since dietary therapy may alter the function and composition of microbes found in the gut, it has an influential benefit on the host (Koutnikova et al., 2019; Qian et al., 2019). In a study done by Qian et al. (2019), subjects were randomly assigned to four groups: HFD; dietary intervention (DI), in which the HFD was replaced by a low-fat diet; HFD with probiotics (L. acidophilus, B. longum, and E. faecalis); or DI with probiotics. Fecal samples were obtained after 4 months and 16S rDNA sequencing was carried out to pinpoint how probiotics with DI change the microbiota composition. Results showed that the combination of probiotics with DI raised microbial diversity, as compared with individual application, of the two butyrate-producing families Ruminococcaceae and Lachnospiraceae. Moreover, they observed a reduction in the ratio of Bacteroidetes/Firmicutes in those with HFD-obesity, which may be inversely increased by diet adaptation. Various studies have indicated that this ratio is not a causal factor in human obesity but may be related to dietary intake. Thus, intake of probiotics with DI appeared to expand the phyla of beneficial species, and reduce that of certain harmful species, providing a natural possibility to treat HFD-obesity (Cerdó et al., 2019; Qian et al., 2019). Studies conducted on probiotics and their effect on obesity causing factors are briefly summarized in Table 1.
Table 1.
Studies of the effects of probiotics on indicators of obesity
| References | Subject of study | Study population | Duration | Results |
|---|---|---|---|---|
| Wang et al. (2019b) | Effect of probiotics on body weight and glycaemic control | Meta-analysis of 12 studies (416 subjects given placebo and 405 given probiotics) | 8 to 24 weeks | Significant ↓ in BW, BMI, FM (%), and insulin levels in the probiotic group |
| Çelik and Ünlü Söğüt (2019) | Impact of probiotic supplementation on chemerin level, inflammation, and metabolic syndrome parameters | 3 groups: control, obese rats fed high-fat diet, and obese intervention given probiotics post obesity induction | 16 weeks | ↓ weight gain, and beneficial effects on insulin, fasting blood glucose, inflammatory markers, leptin, and chemerin levels |
| Qian et al. (2019) | Dietary therapy may alter the function and composition of microbes | 4 groups: HFD, DI low-fat diet, HFD with probiotic (Lactobacillus acidophilus, Bifidobacterium longum, and Enterococcus faecalis), and DI with probiotic groups | 4 months | In the group probiotics with DI: ↑ 2 Butyrate producing families (Ruminococcaceae and Lachnospiraceae) |
| Krumbeck et al. (2018) | Bifidobacterium and galacto-oligosaccharides | 114 subjects | 3 weeks | ↓ inflammation and improvement in intestinal permeability |
| Park et al. (2017) | Lactobacillus plantarum | mice | 12 weeks | ↓ adiposity through lipid oxidation |
| Liu et al. (2017) | Bacteroides thetaiotaomicron | mice and human obese and control groups | 7 weeks | ↓ total fat mass and weight gain in HFD with probiotic |
| Brooks et al. (2016) | Fermentable carbohydrate inulin | mice | 14 weeks | Suppression of peptide YY by 87% |
| Dao et al. (2016) | Akkermansia muciniphila | 49 obese adults | 6 weeks | Improvement of insulin resistance and metabolism |
| Schneeberge et al. (2015) | Akkermansia muciniphila | mice | 16 weeks | ↓ inflammation and adiposity |
| Osterberg et al. (2015) | Mixture of Lactobacilli, Streptococcus, and Bifidobacteria | 20 healthy males in 2 groups: HFD versus HFD with probiotics | 4 weeks | Less weight and fat mass gain in HFD with probiotics |
| Plaza-Diaz et al. (2014) | Lactobacillus paracasei, Bifidobacterium breve, and Lactobacillus rhamnosus or mixture | mice | 30 days | ↓ triacylglycerol liver content (mixture with Lactobacillus rhamnosus, Bifidobacterium breve), ↓ serum LPS levels |
↓, decrease; ↑, increase; BW, body weight; BMI, body mass index; DI, dietary intervention; FM, fat mass; HFD, high-fat diet; LPS, serum levels of lipopolysaccharide.
Possible mechanism of probiotics: Supplementation with probiotics may increase SCFA-providing bacteria, reduce quantitative LPS producers, and reduce tissue loss and organic inflammation caused by LPS. Opportunistic pathogens with their metabolites (trimethylamine, LPS, and indole) are also reduced by probiotics. Probiotics may also inhibit fat accumulation, reduce inflammation and insulin resistance, and regulate neuropeptides and gastrointestinal peptides (Sivamaruthi et al., 2019; Wang et al., 2019b).
Prebiotics: Traditionally, prebiotics were considered to be non-metabolized food ingredients, which reach the intestinal lumen and are selectively utilized by beneficial microbes in the host (Tandon et al., 2019; Wiese et al., 2019). Inulin, galacto-oligosaccharides (GOS), and fructo-oligosaccharides (FOS) are the most frequently analysed prebiotic supplements in recent studies (Wiese et al., 2019). Fermentable carbohydrate inulin is able to increase the density of cells that produce the appetite suppressing hormone PYY by 87%, thus showing its role in reducing food intake and enhancing obesity treatment (Brooks et al., 2016). Supplementation with GOS in healthy individuals have been shown to raise the number of Bifidobacterium spp. and decrease the number of Bacteroides (Davis et al., 2011). Further, studies examining prebiotics have indicated that these compounds promote changes in both the composition and function of the gut microbiota. Prebiotics increase the number of Bifidobacterium species and other butyrate producers, leading to improvements in metabolic outcomes and the gut barrier against pathogens (Beserra et al., 2015). In other studies, this increase in Bifidobacterium has been shown to be accompanied by a rise in Lactobacillus species following addition of FOS, resulting in a decline in ghrelin levels, PYY, and food intake (Gomes et al., 2018).
FOS is known for its bifidogenic capabilities (Gomes et al., 2018; Wiese et al., 2019). In a randomized, double-blinded, placebo-controlled trial, human subjects received FOS at three dose levels (2.5, 5, and 10 g/d) or placebo (maltodextrin 10 g/d) with microbial DNA extracted from fecal samples at specific time-points (Wiese et al., 2019). FOS consumption increased the phyla of Bifidobacterium and Lactobacillus. Moreover, higher doses of FOS promoted selective proliferation of phyla belonging to Lactobacillus. However, withdrawal of prebiotic consumption reduced the abundance of this phyla. Furthermore, there was a significant change in certain butyrate-producing microbes (Faecalibacterium, Ruminococcus, and Oscillospira). These results indicate that prebiotic consumption reinforces microbial diversity and has beneficial effects on the health of the host (Wiese et al., 2019).
In addition, high fat diet-induced leptin resistance is improved by a single dose of prebiotic treatment. Bifidobacterium and GOS, studied alone and as synbiotics, showed no additional benefit when used together (Krumbeck et al., 2018). Clinical trials investigating their use in the treatment of obesity are still on-going. Several studies have reported that cocoa flavanols, dark chocolate (DC), and lycopene have a systemic effect on gut microbiota and a prebiotic potential. A study of obese subjects (Wiese et al., 2019) showed regular intake of lycopene for one-month, with or without DC, resulted in significant alterations in the composition of the gut microbiome. The lycopene formulations prompted a dose-depended rise in mainly Bifidobacterium species. Whereas in the DC formulation, there was a rise in Lactobacillus. In addition, these formulations led to a significant decrease in Bacteroidetes (Wiese et al., 2019). In conclusion, sufficient data on the effect of these prebiotics on weight reduction are still unavailable.
Obesity treatment methods and gut microbiome
Dietary intervention: Different diets have different influence on intestinal microbial composition and diversity (Oriach et al., 2016), which can contribute to onset of inflammatory diseases during adulthood. The effect of different dietary interventions for treatment of obesity on the gut microbiome are briefly summarized in Table 2. The correlation between different diets and their effects on obesity may be interpreted by the consequence of each diet on the gut microbiota and different mechanisms by which gut bacteria may affect body weight.
Table 2.
The influence of different modalities of obesity treatment on gut microbiome diversity and composition
| Different dietary patterns | Microbiota changes |
|---|---|
| Western diet (Simpson and Campbell, 2015) | ↓ Microbial diversity |
| ↑ Firmicutes and Enterobacteriaceae | |
| ↓ Bacteroidetes | |
| Mediterranean diet (Zhang et al., 2018) | ↑ Bacteroidetes |
| ↑ Clostridium clusters | |
| ↓ Proteobacteria | |
| ↓ Bacillaceae | |
| Vegetarian diet (Baothman et al., 2016) | ↑ Bacteroidetes |
| ↓ Pathobionts | |
| ↓ Bacteroides fragilis | |
| High fiber diet (Simpson and Campbell, 2015) | ↑ Bifidobacteria |
| ↑ Microbial diversity | |
| ↑ Firmicutes/Bacteroidetes ratio | |
| High fat diet (Baothman et al., 2016) | ↓ Bacteroidetes |
| ↑ Firmicutes | |
| High protein diet (Zhang et al., 2018) | ↑ Microbial diversity (mainly with exercise) |
| ↑ Bile tolerant microorganisms (Alistipes, Bilophila, and Bacteroides) | |
| ↑ Firmicutes (Roseburia, Eubacterium rectale, and Ruminococcus bromii) | |
| Exercise (Santacruz et al., 2009) | ↑ Microbial diversity |
| ↓ Firmicutes /Bacteroidetes/ ratio | |
| Medication (metformin) (Maniar et al., 2017) | ↑ Akkermansia muciniphila |
| Bariatric surgeries (Liu et al., 2017) | ↑ Bacteroidetes |
↑, increase; ↓, decrease.
Westernization of dietary habits has led to microbial dysbiosis (Noble et al., 2017). Studies have revealed that African children who consume low fat/high fiber diets have a higher diversity of intestinal microbes and fewer pathogenic bacteria. These children also have larger amounts of Bacteroidetes than European children, who have higher amounts of Firmicutes and Enterobacteriaceae (Shigella and Escherichia) (De Filippo et al., 2010). In contrast, a high fat/low fiber diet has been shown to reduce intestinal microbial diversity (Simpson and Campbell, 2015), protective gut bacteria and SCFA (Agus et al., 2016). Dietary intake of high fiber foods (e.g. fruits, vegetables, and legumes) increased microbial diversity (Simpson and Campbell, 2015; Zhang et al., 2018), and was associated with reduced weight gain in humans, independently of energy intake (Menni et al., 2017).
Finally, Astaxanthin, an antioxidant derivative of carotenoids, and the medicinal mushroom Antrodia cinnamomea has been shown to enhance gut microbiota in obese mice induced by a HFD; both prevented weight gain, improved lipid and glucose metabolism, and regulated the gut microbiota (Chang et al., 2018; Wang et al., 2019a), through optimising Bacteroides to Firmicutes ratios and enhancing Akkermansia (Wang et al., 2019a).
Exercise: Behavioural and environmental changes can regulate the activity and structure of the gut microbiota. Studies have shown that aerobic exercise enhances microbial variation (largely bacteria producing butyrate) and increases the turnover of macromolecules, specifically carbohydrates and proteins, in the gut microbiome of athletes (Allen et al., 2018). A cross sectional study performed by Whisner et al. (2018) compared changes to gut microbial diversity and composition in young adults who undertake moderate-to-vigorous physical activity (MVPA). The study found increases in microbial diversity, especially in participants who received an adequate amount of fiber per day in addition to MVPA. This change has been observed in other studies; furthermore, an increase in Bacteroidetes and decrease in Firmicutes has been reported in obese adults following aerobic moderate-to-intense physical activity (Santacruz et al., 2009). In a study by Allen et al. (2018), sedentary lean and obese subjects participated in a 6-week endurance exercise program, which included 30 to 60 min of moderate-to-vigorous workouts. Fecal samples were taken at baseline and post intervention. Results showed that physical activity altered the gut microbiome, and that these changes were dependent on obesity status. The regimen increased fecal levels of SCFA (acetate and butyrate) in lean subjects but not in obese subjects. However, exercise did reduce body fat percentage in both groups. In summary, exercise induced compositional and functional changes to the human gut microbiota dependent on obesity status and exercise sustainment, and independent of diet.
To conclude, it is currently unclear whether exercise confers benefits for health by altering the gut microbiota as there is a lack of well-designed and prospective controlled trials.
Other therapeutic methods:Obesity non-nutritive therapies have been shown to alter the diversity and composition of the gut microflora. The medication metformin has been shown to induce increases in A. muciniphila (Maniar et al., 2017). Moreover, bariatric surgeries induced changes to the gut microbiome that could last for a decade, and induced a significant increase in Bacteroides species (Liu et al., 2017). Patients who undergo Rouex-en-Y gastric bypass (RYGB) experience enhanced metabolism that cannot be explained by caloric restriction and weight loss alone.
Alterations to the gut microbiome play a role in shifting bacterial composition (Baothman et al., 2016). This has been demonstrated by Liou et al. (2013) whom demonstrated that transplantation of fecal matter from RYGB- treated mice to germ-free mice caused a decrease in weight and fat mass in the latter.
Furthermore, Aron-Wisnewsky et al. (2019) performed a study on severely obese subjects who were candidates for bariatric surgery. In these subjects, improvements in metabolism and inflammation were observed following microbiome modification. The effects of different therapeutics that affect the gut microbiome for treating obesity are briefly summarized in Table 2.
Finally, multiple factors impact the diversity and composition of the gut microbiota and may lead to dysbiosis, which is associated with weight gain and obesity. Specifically, diet, physical activity, dietary supplementation, medications, and bariatric surgery affect the gut microbiome. The influence of the intestinal microbiome on metabolism, hormone balance, neurotransmitter function, and the brain can play a major role in weight management and treatment of obesity. Moreover, the use of probiotics and prebiotics improves gut bacterial composition, and has achieved promising outcomes for prevention and treatment of obesity.
Further studies are needed to determine the ideal formulation(s) for supplementation, identify the populations of obese patients who may benefit from gut microbiome modulation, and to assess the durability and long term impact of probiotic and prebiotic supplementation on obesity and health.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, et al. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6:19032. doi: 10.1038/srep19032. doi: 10.1038/srep19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50:747–757. doi: 10.1249/MSS.0000000000001495. [DOI] [PubMed] [Google Scholar]
- Aron-Wisnewsky J, Prifti E, Belda E, Ichou F, Kayser BD, Dao MC, et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68:70–82. doi: 10.1136/gutjnl-2018-316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123:983–993. doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baothman OA, Zamzami MA, Taher I, Abubaker J, Abu-Farha M. The role of gut microbiota in the development of obesity and diabetes. Lipids Health Dis. 2016;15:108. doi: 10.1186/s12944-016-0278-4. doi: 10.1186/s12944-016-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beserra BT, Fernandes R, do Rosario VA, Mocellin MC, Kuntz MG, Trindade EB. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin Nutr. 2015;34:845–858. doi: 10.1016/j.clnu.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Brooks L, Viardot A, Tsakmaki A, Stolarczyk E, Howard JK, Cani PD, et al. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol Metab. 2016;6:48–60. doi: 10.1016/j.molmet.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusaferro A, Cozzali R, Orabona C, Biscarini A, Farinelli E, Cavalli E, et al. Is it time to use probiotics to prevent or treat obesity? Nutrients. 2018;10:1613. doi: 10.3390/nu10111613. doi: 10.3390/nu10111613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çelik MN, Ünlü Söğüt M. Probiotics improve chemerin levels and metabolic syndrome parameters in obese rats. Balkan Med J. 2019;36:270–275. doi: 10.4274/balkanmedj.galenos.2019.2019.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdó T, García-Santos JA, Bermúdez MG, Campoy C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients. 2019;11:635. doi: 10.3390/nu11030635. doi: 10.3390/nu11030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers ES, Preston T, Frost G, Morrison DJ. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr Nutr Rep. 2018;7:198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Lin TL, Tsai YL, Wu TR, Lai WF, Lu CC, et al. Next generation probiotics in disease amelioration. J Food Drug Anal. 2019;27:615–622. doi: 10.1016/j.jfda.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Lu CC, Lin CS, Martel J, Ko YF, Ojcius DM, et al. Antrodia cinnamomea reduces obesity and modulates the gut microbiota in high-fat diet-fed mice. Int J Obes. 2018;42:231–243. doi: 10.1038/ijo.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao MC, Clément K. Gut microbiota and obesity: concepts relevant to clinical care. Eur J Intern Med. 2018;48:18–24. doi: 10.1016/j.ejim.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- Davis LM, Martínez I, Walter J, Goin C, Hutkins RW. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS One. 2011;6:e25200. doi: 10.1371/journal.pone.0025200. doi: 10.1371/journal.pone.0025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca I, Rusu F, Chira A, Dumitrascu DL. Gut microbiota and body weight-a review. Psychol Topics. 2018;27:33–53. doi: 10.31820/pt.27.1.3. [DOI] [Google Scholar]
- Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121. doi: 10.1038/nutd.2014.23. doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer N, Relman DA. Clostridium difficile, aging, and the gut: can microbiome rejuvenation keep us young and healthy? J Infect Dis. 2018;217:174–176. doi: 10.1093/infdis/jix417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018;362:776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: metabolism and perspective in obesity. Gut Microbes. 2018;9:308–325. doi: 10.1080/19490976.2018.1465157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirro M, Costa A, Gual-Grau A, Herrero P, Torrell H, Canela N, et al. Effects from diet-induced gut microbiota dysbiosis and obesity can be ameliorated by fecal microbiota transplantation: a multiomics approach. PLoS One. 2019;14:e0218143. doi: 10.1371/journal.pone.0218143. https: //doi.org/10.1371/journal.pone.0218143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MK, Raybould HE. Bugs, guts and brains, and the regulation of food intake and body weight. Int J Obes Suppl. 2016;6:S8–S14. doi: 10.1038/ijosup.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Shi B. Gut microbiota as a potential target of metabolic syndrome: the role of probiotics and prebiotics. Cell Biosci. 2017;7:54. doi: 10.1186/s13578-017-0183-1. doi: 10.1186/s13578-017-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth MF, Roager HM, Larsen TM, Poulsen SK, Licht TR, Bahl MI, et al. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int J Obes. 2018;42:580–583. doi: 10.1038/ijo.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Chung YM, Park S, Jeong D, Kim B, Holzapfel WH. Dose-dependent and strain-dependent anti-obesity effects of Lactobacillus sakei in a diet induced obese murine model. PeerJ. 2019a;7:e6651. doi: 10.7717/peerj.6651. doi: 10.7717/peerj.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Park S, Chung Y, Kim B, Park H, Huang E, et al. Amelioration of obesity-related biomarkers by Lactobacillus sakei CJLS03 in a high-fat diet-induced obese murine model. Sci Rep. 2019b;9:6821. doi: 10.1038/s41598-019-43092-y. doi: 10.1038/s41598-019-43092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GK, Mullin GE. The gut microbiome and obesity. Curr Oncol Rep. 2016;18:45. doi: 10.1007/s11912-016-0528-7. doi: 10.1007/s11912-016-0528-7. [DOI] [PubMed] [Google Scholar]
- Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100. doi: 10.1186/s12876-015-0330-2. doi: 10.1186/s12876-015-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong C, Gao R, Yan X, Huang L, Qin H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition. 2019;60:175–184. doi: 10.1016/j.nut.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Koutnikova H, Genser B, Monteiro-Sepulveda M, Faurie JM, Rizkalla S, Schrezenmeir J, et al. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2019;9:e017995. doi: 10.1136/bmjopen-2017-017995. doi: 10.1136/bmjopen-2017-017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbeck JA, Rasmussen HE, Hutkins RW, Clarke J, Shawron K, Keshavarzian A, et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome. 2018;6:121. doi: 10.1186/s40168-018-0494-4. doi: 10.1186/s40168-018-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankelma JM, Nieuwdorp M, de Vos WM, Wiersinga WJ. The gut microbiota in internal medicine: implications for health and disease. Neth J Med. 2015;73:61–68. [PubMed] [Google Scholar]
- Le Roy CI, Bowyer RCE, Castillo-Fernandez JE, Pallister T, Menni C, Steves CJ, et al. Dissecting the role of the gut microbiota and diet on visceral fat mass accumulation. Sci Rep. 2019a;9:9758. doi: 10.1038/s41598-019-46193-w. doi: 10.1038/s41598-019-46193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy CI, Woodward MJ, Ellis RJ, La Ragione RM, Claus SP. Antibiotic treatment triggers gut dysbiosis and modulates metabolism in a chicken model of gastro-intestinal infection. BMC Vet Res. 2019b;15:37. doi: 10.1186/s12917-018-1761-0. doi: 10.1186/s12917-018-1761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KSW, Derraik JGB, Hofman PL, Cutfield WS. Antibiotics, gut microbiome and obesity. Clin Endocrinol. 2018;88:185–200. doi: 10.1111/cen.13495. [DOI] [PubMed] [Google Scholar]
- Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26:5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- Li Z, Yi CX, Katiraei S, Kooijman S, Zhou E, Chung CK, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67:1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- Lin HV, Frassetto A, Kowalik EJ, Jr, Nawrocki AR, Lu MM, Kosinski JR, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240. doi: 10.1371/journal.pone.0035240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. doi: 10.1126/scitranslmed.3005687. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, et al. Gut microbiome and serum metabolome alterations in obesity and after weight- loss intervention. Nat Med. 2017;23:859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- Lv Y, Qin X, Jia H, Chen S, Sun W, Wang X. The association between gut microbiota composition and BMI in Chinese male college students, as analysed by next-generation sequencing. Br J Nutr. 2019;122:986–995. doi: 10.1017/S0007114519001909. [DOI] [PubMed] [Google Scholar]
- Maniar K, Moideen A, Bhattacharyya R, Banerjee D. Metformin exerts anti-obesity effect via gut microbiome modulation in prediabetics: a hypothesis. Med Hypotheses. 2017;104:117–120. doi: 10.1016/j.mehy.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Mazloom K, Siddiqi I, Covasa M. Probiotics: how effective are they in the fight against obesity? Nutrients. 2019;11:258. doi: 10.3390/nu11020258. https:// doi.org/10.3390/nu11020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClave SA, Martindale RG. Why do current strategies for optimal nutritional therapy neglect the microbiome? Nutrition. 2019;60:100–105. doi: 10.1016/j.nut.2018.09.024. [DOI] [PubMed] [Google Scholar]
- Menni C, Jackson MA, Pallister T, Steves CJ, Spector TD, Valdes AM. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int J Obes. 2017;41:1099–1105. doi: 10.1038/ijo.2017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri MH, Brummer RJM, Rastall RA, Weersma RK, Harmsen HJM, Faas M, et al. The role of the microbiome for human health: from basic science to clinical applications. Eur J Nutr. 2018;57:1–14. doi: 10.1007/s00394-018-1703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EE, Hsu TM, Kanoski SE. Gut to brain dysbiosis: mechanisms linking Western diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci. 2017;11:9. doi: 10.3389/fnbeh.2017.00009. doi: 10.3389/fnbeh.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeke F, Roland BC, Mullin GE. The role of the gut microbiome in the pathogenesis and treatment of obesity. Glob Adv Health Med. 2014;3:44–57. doi: 10.7453/gahmj.2014.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oriach CS, Robertson RC, Stanton C, Cryan JF, Dinan TG. Food for thought: the role of nutrition in the microbiota-gut-brain axis. Clin Nutr Exp. 2016;6:25–38. doi: 10.1016/j.yclnex.2016.01.003. [DOI] [Google Scholar]
- Osterberg KL, Boutagy NE, McMillan RP, Stevens JR, Frisard MI, Kavanaugh JW, et al. Probiotic supplementation attenuates increases in body mass and fat mass during high-fat diet in healthy young adults. Obesity. 2015;23:2364–2370. doi: 10.1002/oby.21230. [DOI] [PubMed] [Google Scholar]
- Park S, Ji Y, Jung HY, Park H, Kang J, Choi SH, et al. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl Microbiol Biotechnol. 2017;101:1605–1614. doi: 10.1007/s00253-016-7953-2. [DOI] [PubMed] [Google Scholar]
- Plaza-Diaz J, Gomez-Llorente C, Abadia-Molina F, Saez-Lara MJ, Campaña-Martin L, Muñoz-Quezada S, et al. Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036 on hepatic steatosis in Zucker rats. PLoS One. 2014;9:e98401. doi: 10.1371/journal.pone.0098401. doi: 10.1371/journal.pone.0098401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky SH. Historical perspective on the rise and fall and rise of antibiotics and human weight gain. Ann Intern Med. 2017;166:133–138. doi: 10.7326/M16-1855. [DOI] [PubMed] [Google Scholar]
- Qian L, Gao R, Huang J, Qin H. Supplementation of triple viable probiotics combined with dietary intervention is associated with gut microbial improvement in humans on a high-fat diet. Exp Ther Med. 2019;18:2262–2270. doi: 10.3892/etm.2019.7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastelli M, Knauf C, Cani PD. Gut microbes and health: a focus on the mechanisms linking microbes, obesity, and related disorders. Obesity. 2018;26:792–800. doi: 10.1002/oby.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmiguel C, Gupta A, Mayer EA. Gut microbiome and obesity: a plausible explanation for obesity. Curr Obes Rep. 2015;4:250–261. doi: 10.1007/s13679-015-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz A, Marcos A, Wärnberg J, Martí A, Martin-Matillas M, Campoy C, et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity. 2009;17:1906–1915. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- Schneeberger M, Everard A, Gómez-Valadés A, Matamoros S, Ramírez S, Delzenne NM, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5:16643. doi: 10.1038/srep16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- Simpson HL, Campbell BJ. Review article: dietary fibre-microbiota interactions. Aliment Pharmacol Ther. 2015;42:158–179. doi: 10.1111/apt.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer-Englar T, Barlow G, Mathur R. Obesity, diabetes, and the gut microbiome: an updated review. Expert Rev Gastroenterol Hepatol. 2019;13:3–15. doi: 10.1080/17474124.2019.1543023. [DOI] [PubMed] [Google Scholar]
- Sivamaruthi BS, Kesika P, Suganthy N, Chaiyasut C. A review on role of microbiome in obesity and antiobesity properties of probiotic supplements. BioMed Res Int. 2019;2019:3291367. doi: 10.1155/2019/3291367. doi: 10.1155/2019/3291367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CM, Susi A, Emerick J, Nylund CM. Antibiotic and acid-suppression medications during early childhood are associated with obesity. Gut. 2019;68:62–69. doi: 10.1136/gutjnl-2017-314971. [DOI] [PubMed] [Google Scholar]
- Stephens RW, Arhire L, Covasa M. Gut microbiota: from microorganisms to metabolic organ influencing obesity. Obesity. 2018;26:801–809. doi: 10.1002/oby.22179. [DOI] [PubMed] [Google Scholar]
- Tandon D, Haque MM, Gote M, Jain M, Bhaduri A, Dubey AK, et al. A prospective randomized, double-blind, placebo-controlled, dose-response relationship study to investigate efficacy of fructo-oligosaccharides (FOS) on human gut microflora. Sci Rep. 2019;9:5473. doi: 10.1038/s41598-019-41837-3. doi: 10.1038/s41598-019-41837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turroni F, Ventura M, Buttó LF, Duranti S, O'Toole PW, Motherway MO, et al. Molecular dialogue between the human gut microbiota and the host: a Lactobacillus and Bifidobacterium perspective. Cell Mol Life Sci. 2014;71:183–203. doi: 10.1007/s00018-013-1318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu S, Wang H, Xiao S, Li C, Li Y, et al. Xanthophyllomyces dendrorhous-derived astaxanthin regulates lipid metabolism and gut microbiota in obese mice induced by a high-fat diet. Mar Drugs. 2019a;17:337. doi: 10.3390/md17060337. doi: 10.3390/md17060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZB, Xin SS, Ding LN, Ding WY, Hou YL, Liu CQ, et al. The potential role of probiotics in controlling overweight/obesity and associated metabolic parameters in adults: a systematic review and meta-analysis. Evid Based Complementary Altern Med. 2019b;2019:3862971. doi: 10.1155/2019/3862971. doi: 10.1155/2019/3862971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisner CM, Maldonado J, Dente B, Krajmalnik-Brown R, Bruening M. Diet, physical activity and screen time but not body mass index are associated with the gut microbiome of a diverse cohort of college students living in university housing: a cross- sectional study. BMC Microbiol. 2018;18:210. doi: 10.1186/s12866-018-1362-x. doi: 10.1186/s12866-018-1362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese M, Bashmakov Y, Chalyk N, Nielsen DS, Krych Ł, Kot W, et al. Prebiotic effect of lycopene and dark chocolate on gut microbiome with systemic changes in liver metabolism, skeletal muscles and skin in moderately obese persons. Biomed Res Int. 2019;2019:4625279. doi: 10.1155/2019/4625279. doi: 10.1155/2019/4625279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins LJ, Monga M, Miller AW. Defining dysbiosis for a cluster of chronic diseases. Sci Rep. 2019;9:12918. doi: 10.1038/s41598-019-49452-y. doi: 10.1038/s41598-019-49452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TR, Lin CS, Chang CJ, Lin TL, Martel J, Ko YF, et al. Gut commensal nParabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68:248–262. doi: 10.1136/gutjnl-2017-315458. [DOI] [PubMed] [Google Scholar]
- Zhang N, Ju Z, Zuo T. Time for food: the impact of diet on gut microbiota and human health. Nutrition. 2018;51(52):80–85. doi: 10.1016/j.nut.2017.12.005. [DOI] [PubMed] [Google Scholar]