Abstract
The objective of this study was to investigate the structural and physicochemical properties of starch from seven sweet potato cultivars (Shinyulmi, Sinjami, Hogammi, Jeonmi, Jinyulmi, Juhwangmi, and Pungwonmi). Jeonmi and Jinyulmi had amylose contents of 40.04% and 37.39%, respectively, whereas Juhwangmi and Pungwonmihad amylose contents of 30.95% and 32.37%, respectively. As a result of amylopectin polymerization, the seven cultivars were found to have high (>48%) contents of the degree of polymerization (DP) 13∼24 fraction, whereas the DP≥37 fraction content was <3.45%. The level of resistant starch was highest in Jeonmi (>30%) and lowest in Pungwonmi (<5%). The in vitro digestibility of Pungwonmi was greater than that of the other cultivars. Starch X-ray patterns did not differ among the cultivars. The results of this study provide useful information for the food industry regarding the application of sweet potato starches.
Keywords: characteristics, cultivars, starch, sweet potato
INTRODUCTION
Starch, the major source of carbohydrates in the human diet, is produced by plants to enable energy storage. It is pervasive in food, paper, textiles, pharmaceuticals, and other materials (Vanier et al., 2017). Starch is a biopolymer consisting of amylose and amylopectin. Amylose is composed primarily of linear chains of α-1—4 linear glucosyl units, whereas amylopectin is made up of a large amount of shorter chains bound together at their reducing ends through (1→6) linkages (Guo et al., 2019b). Native starch is highly variable in structure and function, depending on its origin. The physicochemical and functional properties of starch are associated closely with its structural and physicochemical properties (Singh et al., 2007; Yong et al., 2018).
Sweet potato (Ipomoea batatas L.) is among the most important economic crops in many tropical and subtropical countries in Asia, Africa, and Latin America. Sweet potato has white, yellow, orange, purple, and red cultivars with differences in composition, including phenolic content and root tuber pigments (Wang et al., 2018). Its root tuber is rich in starch, dietary fiber, vitamin C, iron, and minerals, and is usually used as an energy source in the human diet and as an important starch resource in the food industry (Zhu et al., 2011; Guo et al., 2019a). Sweet potato has useful physiological properties, such as the regulation of blood glucose and lipid levels, improvement of immunity, and protection from cancers and oxidation; thus, it has received extensive attention in recent years (Wang et al., 2016). As a consequence, the applications of sweet potato have diversified considerably, with many studies focusing on different drying and cooking methods, and the nutritional composition and physicochemical properties of sweet potato (Hou et al., 2019).
Sweet potato starches are used widely in starchy noodles, baked goods, snack foods, and confectionery products due to their unique properties, such as low gelatinization temperature, high water-binding capacity, high paste consistency, and good paste clarity (Zaidul et al., 2007; Guo et al., 2016). The structural and functional properties of starch determine its applications. Therefore, in recent decades, many studies have been conducted to determine the properties of sweet potato starch, including its morphology, granule size, amylose content, water- binding capacity, pasting properties, and digestion properties (Zhang et al., 2018). However, few studies have examined the starch characteristics of recently developed sweet potato cultivars in Korea.
Therefore, this study investigated the physicochemical characteristics of starches isolated from Korean sweet potato cultivars that are currently used in the Korean food industry. The information generated in this study provides essential guidance for the production of food products.
MATERIALS AND METHODS
Materials
Seven cultivars of sweet potato (Shinyulmi, Sinjami, Hogammi, Jeonmi, Jinyulmi, Juhwangmi, and Pungwonmi) were grown at the National Institute of Crop Science, Rural Development Administration, Muan, Korea. The characteristics of the sweet potato cultivars are shown in Table 1. The sweet potatoes were harvested in 2017 and stored at 13±1°C (90±2% humidity) for 3 months.
Table 1.
Characteristics of the sweet potato cultivars grown in Korea
| Cultivars | Developed year | Characteristics of the storage root | |||
|---|---|---|---|---|---|
| Skin color | Flesh color | Images | Yield (MT/ha)1) | ||
| Shinyulmi | 2001 | Dark red | Yellow |  |
22.4 |
| Sinjami | 1991 | Dark purple | Reddish purple |  |
22.2 |
| Hogammi | 2015 | Red | Light orange |  |
24.1 |
| Jeonmi | 2009 | Light red | Light yellow |  |
26.2 |
| Jinyulmi | 2016 | Red | Light yellow |  |
28.6 |
| Juhwangmi | 2002 | Dark red | Orange |  |
28.1 |
| Pungwonmi | 2014 | Red | Light orange |  |
24.1 |
1)Storage root yield over 50 g.
Starch isolation
Starches were isolated from the sweet potato cultivars according to the method of Wei et al. (2010) with some modification. Fresh root tubers of each cultivar were washed with tap water, peeled, cut into small cubes, and milled in a mortar. Each ground sample was treated with 0.2% sodium hydroxide solution (pH 10) for 4 h to remove protein and anthocyanin. Then, the resultant slurry was extruded through four layers of gauze and passed successively through 100- and 300-mesh sieves. The filtrate was centrifuged at 10,000 rpm for 15 min. The resultant precipitate was washed 10 times with deionized water and dehydrated with anhydrous ethanol. Finally, the resultant starch sample was dried at 40°C for 2 days, ground into powder, and passed through a 100-mesh sieve.
Amylose content and amylopectin chain length distribution
The amylose content was determined using the colorimetric method described by Juliano (1971). Each sample (100 mg) was mixed with 1 mL 95% ethanol and 9 mL 2 M NaOH. The mixture was then adjusted to 100 mL with distilled water. The solution (5 mL) was mixed with 1.0 mL CH3COOH and 2.0 mL 2% iodine solution, and the absorbance was measured at 620 nm. The amylose content was estimated by reference to a calibration curve. Amylose from potato starch (Sigma-Aldrich Co., St. Louis, MO, USA) was used as a standard.
The amylopectin chain length distribution of sweet potato starches was determined according to the method described by Lee et al. (2017). Starch (10 mg) was dissolved in 2 mL 90% dimethyl sulfoxide in a boiling water bath while stirring for 20 min. The starch solution was precipitated with absolute ethanol (6 mL) and then centrifuged (2,700 rpm for 12 min). The precipitate was dissolved with 2 mL 50 mM sodium acetate buffer (pH 3.5) and heated in a boiling water bath, with continuous stirring, for 20 min. After the solution had been equilibrated to 37°C, isoamylase (5 μL, E-ISAMY; Megazyme Inc., Bray, Ireland) was added and the starch solution was incubated at 37°C, with stirring, for 24 h. The enzyme was inactivated by boiling for 10 min. An aliquot (200 μL) of the debranched sample was diluted with 2 mL 150 mM NaOH. The sample was filtered (0.45-mm nylon syringe filter) and injected into a high-performance anion-exchange chromatography (HPAEC) system equipped with a pulse amperometric detector. The HPAEC system consisted of a Dionex ICS-5000 device (Dionex Corporation, Sunnyvale, CA, USA), an ED50 electrochemical detector, and a CarboPac PA1 column (2×250 mm; Dionex Corporation). Separation was achieved using a gradient eluent of 150 mM NaOH and 500 mM sodium acetate in 150 mM NaOH at a flow rate of 1 mL/min.
Resistant starch (RS) content and starch digestibility
The samples were incubated in a shaking water bath with pancreatic κ-amylase and amyloglucosidase (AMG) for 16 h at 37°C, during which time non-RS was solubilized and hydrolyzed to D-glucose by the combined action of the two enzymes. The reaction was completed by the addition of an equal volume of ethanol, and the RS was recovered as a pellet following centrifugation. It was then washed twice by suspension in ethanol (50%, v/v), followed by centrifugation (with free liquid removed by decantation). The RS in the pellet was dissolved in 2 M KOH by stirring in an ice-water bath over a magnetic stirrer. The solution was neutralized with acetate buffer and the starch was quantitatively hydrolyzed to glucose with AMG. The D-glucose concentration was measured with glucose oxidase/peroxidase reagent (GOPOD), and taken as a measure of the RS content of the sample. The non-RS content was determined by combining the original supernatant and the washings, adjusting the volume to 100 mL, and measuring the D-glucose content with GOPOD. The methodology used for RS determination was the Megazyme Resistant Starch Assay Procedure (Megazyme Inc.), which is essentially the same as the AACC approved method 32-40. A collaborative study verified the reproducibility of this procedure (McCleary et al., 2002).
The starch digestibility of sweet potato starch was determined following the method of Englyst et al. (1992), with modifications (You et al., 2015). Each sample (100 mg) was weighed in a screw-capped tube, and 15 glass beads (4-mm diameter) and 4 mL sodium acetate buffer (0.5 M, pH 5.2) were added to each tube. The sample tubes were incubated with porcine pancreatic κ-amylase (P-7545; Sigma-Aldrich Co.) and AMG (A-9913; Sigma-Aldrich Co.) in a shaking water bath (170 rpm) at 37°C. Aliquots (0.1 mL) were collected at certain intervals and mixed with 1 mL 50% ethanol. After centrifugation (2,000 rpm for 10 min), the glucose released into the supernatant from each sample was measured using a GOPOD kit. The results of this analysis were expressed as the glucose concentration/reaction time.
X-ray diffraction (XRD)
An XRD analysis was performed with an X-ray diffractometer (D8 ADVANCE with DAVINCI; Bruker, Hamburg, Germany) operated at 40 kV and 40 mA. Diffractograms were obtained from 4° to 30° (2q) at a scan rate of 0.5 s/step.
Pasting characteristics
The pasting characteristics of sweet potato starch were measured according to Woo et al. (2018) using a rapid viscosity analyzer (Model RVA-3D; Newport Scientific PTY Ltd., Warriewood, Australia). Each sample (3 g) was placed in an aluminum container and dispersed in 25 mL distilled water. It was kept at 50°C for 1 min, and the temperature was then increased from 50°C to 95°C for 3.48 min and maintained at 95°C for 2.05 min. Thereafter, the sample was cooled to 50°C for 3.48 min and its viscosity characteristics were assessed. The total duration of the experiment was about 13 min. After the experiment, the peak, trough, breakdown, final, and setback viscosities were measured and compared.
Statistical analysis
The data were analyzed using SPSS software (ver. 18.0; SPSS Inc., Chicago, IL, USA) and one-way analysis of variance. When significant differences were detected, the differences among mean values were determined using Duncan’s multiple comparison test at a confidence level of P<0.05. Mean values and standard errors of the mean are reported.
RESULTS AND DISCUSSION
Amylose content and amylopectin chain length distribution
Amylose content affects the intrinsic quality and application of starch. The amylose contents of starches isolated from the seven sweet potato cultivars ranged from 30.95 % in Juhwangmi to 40.31% in Jeonmi (Table 2). Han et al. (2014) showed that the amylose contents of sweet potato starch grown in Korea ranged from 16.0% to 23.4 %. Chung et al. (2011) reported that the amylose content strongly affected the physicochemical properties of starches. The amylose content is influenced by the genetic background, growing environment, and measurement method (Zhang et al., 2018).
Table 2.
Amylose and resistant starch contents of sweet potato starch in the cultivars in grown in Korea
| Cultivars | Amylose | Resistant starch |
|---|---|---|
| Shinyulmi | 35.68±0.17c | 18.55±0.29c |
| Sinjami | 35.65±0.24c | 11.39±0.32a |
| Hogammi | 35.57±0.23c | 13.46±0.28d |
| Jeonmi | 40.31±0.20a | 30.75±0.34a |
| Jinyulmi | 37.31±0.24b | 27.75±0.34b |
| Juhwangmi | 30.95±0.22e | 6.46±0.25f |
| Pungwonmi | 32.37±0.25d | 1.76±0.05g |
Different letters (a-g) in the same column indicate significant differences (P<0.05).
The amylopectin chain length distributions and average chain length of amylopectin of sweet potato starches are presented in Table 3. Average chain of amylopectin was had the highest Hogammi and the lowest Juhwangmi. Hizukuri (1986) categorized the amylopectin unit chains as A, B, and C, with the B chains subclassified into B1, B2, and B3+ according to the number of clusters. According to the amylopectin branch chain lengths from the HPAEC chromatogram, A chains [degree of polymerization (DP) 6∼12], B1 chains (DP13∼24), B2 chains (DP 25∼36), and B3+ chains (DP≥37) were identified (Hanashiro et al., 1996). As a result of amylopectin polymerization, the seven cultivars had high (>48%) contents of the DP13∼24 fraction, whereas the DP≥37 fraction content was >3.47% (Table 3). Pungwonmi had a larger proportion of A chains (DP6∼12) and a smaller proportion of B3 chains (DP≥37) than did the other sweet potato starches. Jeonmi had a larger proportion of B1 chains (DP13∼24) and a smaller proportion of B3 chains (DP6∼12) than did the other sweet potato starches. Amylopectin chain length distributions of sweet potatoes was influenced not only by cultivars and environmental factors but also soil temperature and fertilizer (Guo et al., 2014).
Table 3.
Amylopectin chain length distribution of sweet potato starch in the cultivars grown in Korea
| Cultivars | Average chain length of amylopectin | Amylopectin chain length distribution | |||
|---|---|---|---|---|---|
| DP6∼12 | DP13∼24 | DP25∼36 | DP≥37 | ||
| Shinyulmi | 29.27±0.78b | 24.43±0.29d | 51.67±0.23b | 17.47±0.62b | 6.44±0.67ab |
| Sinjami | 30.38±0.05b | 25.77±0.48cd | 51.15±0.23b | 17.41±0.65b | 4.68±0.06bc |
| Hogammi | 32.96±0.88a | 24.75±0.44cd | 48.66±0.73c | 19.17±0.11a | 7.43±0.40a |
| Jeonmi | 28.22±0.71e | 24.19±0.78d | 54.86±0.21a | 16.12±0.60cd | 4.85±0.39c |
| Jinyulmi | 26.66±0.12d | 25.08±0.16cd | 54.88±0.12a | 15.83±0.04de | 4.23±0.30c |
| Juhwangmi | 25.22±0.51e | 27.97±0.09b | 51.22±0.08b | 15.80±0.21cd | 5.02±0.22c |
| Pungwonmi | 27.45±0.11c | 33.50±0.88a | 48.65±0.69c | 14.40±0.86e | 3.46±0.70d |
Different letters (a-e) in the same column indicate significant differences (P<0.05).
RS content and hydrolysis of sweet potato starches
RS has recently gained importance as a food additive due to several reported physiological effects, such as the prevention of colonic cancer, hypoglycemic action, reduction of gallstone formation, hypocholesterolemic effect, and control of obesity (Menon et al., 2015). The RS contents of sweet potato starches ranged from 1.76% in Pungwonmi to 30.75% in Jeonmi (Table 2). The RS content and, similarly, the amylose content are influenced by the starch structure, genotype, and growing environment (Zhang et al., 2018).
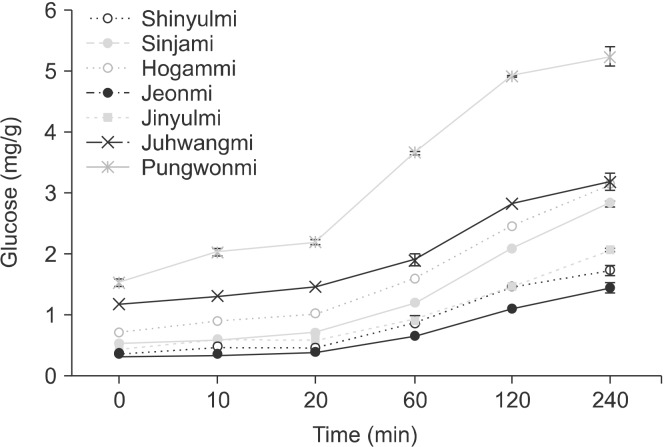
In vitro starch digestibility of foods containing starch is of great interest to predict glycemic response sine in vivo evaluations are invasive, expensive, slow, and require specialized skills and facilities (Lehmann and Robin, 2007). The starch digestibility in the seven sweet potato cultivars was analyzed (Fig. 1). Several researches have reported that in vitro digestibility of native starches in influenced by many factors such as starch botanical source, amylose content, granule size, relative crystallinity, amylose-lipid complexes, molecular architecture of amylopectin, and amylose chain length (Chung et al., 2006; Jeong et al., 2019). Chung et al. (2011) showed the in vitro digestibility of four rice starches with different amylose contents and reported that the starch with lower amylose content had higher rapidly digestible starch. The rate of conversion of sweet potato starches to monosaccharide glucose differed clearly among cultivars. This effect was related to the cultivars’ RS contents.
Fig. 1.
Glucose contents determined by the starch digestibility in the sweet potato cultivars grown in Korea.
The hydrolysis of raw starches is an important factor to consider when evaluating their usefulness in a diverse range of food applications (Guo et al., 2014). The variability in starch hydrolysis might be due to the environmental conditions associated with the crop growth location, such as temperature, precipitation, and soil. The inter- and intra-molecular hydrogen bonds between starch chains are disrupted during starch gelatinization, leading to more rapid degradation of the gelatinized starch than of native starch (Rocha et al., 2010). A high proportion of amylopectin has been shown to result in great resistance to enzymatic degradation because the lightly bonded double helices of amylopectin are resistant to enzymatic and chemical attacks (Guo et al., 2014). You et al. (2014) indicated that the rapidly digestible starch content was negatively correlated with the proportion of long-branch chain and positively correlated with the proportion of DP 6∼12.
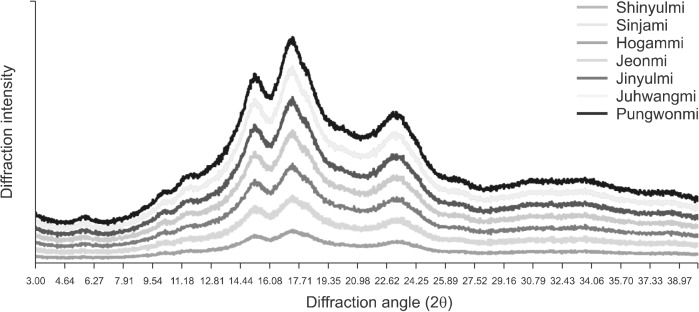
XRD
XRD was used to study the presence and characteristics of the crystalline structure of the starch granules (Kaur et al., 2010). The XRD patterns of sweet potato starches are shown in Fig. 2. According to these patterns, the starches were divided into types A∼C. Type C starch consisted of type-A and -B crystallinities and could be subclassified as CA, CC, and CB according to the proportions of these crystallinities (He and Wei, 2017). Type CC starch had typical diffraction peaks at 5.6°, 15°, 17°, and 23° 2q, and type CA starch had shoulder peaks at about 22° and 24° 2q (He and Wei, 2017). All seven sweet potato starches showed the type CA XRD pattern. These results were similar to those reported previously (Lee and Lee, 2017; Guo et al., 2019a). However, Kim et al. (2013) showed that some white and purple sweet potatoes had type CB starches. Genkina et al. (2003) reported that orange sweet potato has a type A starch when grown in soil at 33°C and a type CC starch when grown in soil at 15°C, indicating that the growing temperature has a significant effect on crystalline structure. A traditional view that cereal starches generate A-type diffraction patterns; tubers, and high amylose starches generate B-type patterns; and legume, roots, and some fruits and stem starches show C-type patterns (a mixture of A- and B-type) (Guo et al., 2014).
Fig. 2.
X-ray diffraction pattern of sweet potato starch in the cultivars grown in Korea.
Pasting characteristics
The pasting parameter of starches is generally recognized to be influenced by the amylose content, amylopectin molecular structure, and molecular weight (Woo et al., 2018). The pasting characteristics of sweet potato starches were determined proportionally, and the peak, trough, breakdown, final, and setback viscosities were measured (Table 4). Peak viscosity means the maximum swelling value of starch granules during heating process and breakdown indicates a measure of the degree of disintegration of granules (Liu et al., 2006). The peak and trough viscosities ranged from 448.44 to 639.58 rapid visco units (RVU) and from 169.25 to 280.72 RVU, respectively. The breakdown viscosities were 242.22∼366.72 RVU, and the cultivar with the lowest value was Jinyulmi. The final viscosity reflects a process in which heating is stopped and cooling takes place, which enables starch particles, such as amylose, to recombine and increase the viscosity. The final viscosities were 252.48∼379.42 RVU, and the cultivar with the highest value was Sinjami. The setback viscosities of sweet potato starches ranged from -276.00 to -171.11 RVU, and the cultivar with the lowest value was Hogammi. The setback viscosity reflects the aging tendency of starch, and higher values reflect faster aging (Lee et al., 2009). The setback viscosity was obtained by subtracting the peak viscosity from the final viscosity, which is related to the aging of starch. Higher values reflect faster aging processes and lower values reflect slower aging rates, and thus longer retention of the desired taste (Woo et al., 2018).
Table 4.
Pasting characteristics of sweet potato starch in the cultivars grown in Korea (unit: RVU)
| Cultivars | Pasting characteristics | ||||
|---|---|---|---|---|---|
| Peak viscosity | Trough viscosity | Break down1) | Final viscosity | Set back2) | |
| Shinyulmi | 475.92±2.32d | 169.25±2.43g | 306.67±0.59e | 252.48±0.50f | −223.44±2.36b |
| Sinjami | 621.47±4.46b | 280.72±3.18a | 340.75±1.46c | 379.42±2.74a | −242.06±2.94c |
| Hogammi | 557.14±6.52c | 197.17±1.04f | 359.97±5.48b | 281.14±4.98e | −276.00±2.68f |
| Jeonmi | 548.00±6.71c | 221.56±3.74d | 326.44±3.11d | 293.28±3.34d | −254.72±4.20d |
| Jinyulmi | 448.44±5.38e | 206.22±2.82e | 242.22±2.64f | 277.33±1.80b | −171.11±3.86a |
| Juhwangmi | 639.58±3.26a | 272.86±2.78b | 366.72±1.89a | 367.86±2.46e | −271.72±1.35ef |
| Pungwonmi | 622.33±7.66b | 264.81±2.71c | 357.53±6.30b | 353.92±3.94c | −268.42±3.88e |
Different letters (a-g) in the same column indicate significant differences (P<0.05).
RVU, rapid visco units.
1)Peak viscosity minus the through viscosity.
2)Final viscosity minus the peak viscosity.
The starch characteristics of sweet potato cultivars grown in Korea were studied. Significant differences in physicochemical properties were found among the starches of sweet potato cultivars. The starch in the Jeonmi cultivar of sweet potato had higher amylose and RS contents than did the other sweet potato starches. The Hogammi cultivar had substantially higher values for measures of pasting characteristics, especially setback viscosity. These results provide useful information to the food industry for the application of sweet potato starches.
ACKNOWLEDGEMENTS
This study was supported by a Grant (PJ01196302) from the AGENDA Program, Rural Development Administration, Republic of Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Chung HJ, Lim HS, Lim ST. Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch. J Cereal Sci. 2006;43:353–359. doi: 10.1016/j.jcs.2005.12.001. [DOI] [Google Scholar]
- Chung HJ, Liu Q, Lee L, Wei D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011;25:968–975. doi: 10.1016/j.foodhyd.2010.09.011. [DOI] [Google Scholar]
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46:S33–S50. [PubMed] [Google Scholar]
- Genkina NK, Noda T, Koltisheva GI, Wasserman LA, Tester RF, Yuryev VP. Effects of growth temperature on some structural properties of crystalline lamellae in starches extracted from sweet potatoes (Sunnyred and Ayamurasaki) Starch. 2003;55:350–357. doi: 10.1002/star.200300145. [DOI] [Google Scholar]
- Guo J, Liu L, Lian X, Li L, Wu H. The properties of different cultivars of Jinhai sweet potato starches in China. Int J Biol Macromol. 2014;67:1–6. doi: 10.1016/j.ijbiomac.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Guo K, Liu T, Xu A, Zhang L, Bian X, Wei C. Structural and functional properties of starches from root tubers of white, yellow, and purple sweet potatoes. Food Hydrocoll. 2019a;89:829–836. doi: 10.1016/j.foodhyd.2018.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Hu J, Zhang J, Du X. The role of entanglement concentration on the hydrodynamic properties of potato and sweet potato starches. Int J Biol Macromol. 2016;93:1–8. doi: 10.1016/j.ijbiomac.2016.08.075. [DOI] [PubMed] [Google Scholar]
- Guo L, Tao H, Cui B, Janaswamy S. The effects of sequential enzyme modifications on structural and physicochemical properties of sweet potato starch granules. Food Chem. 2019b;277:504–514. doi: 10.1016/j.foodchem.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Han SK, Song YS, Ahn SH, Yang JW, Lee HU, Lee JS, et al. Physicochemical characteristics of sweetpotato (Ipomoea batatas (L.) Lam) starch depending on cultivation periods. Korean J Food Sci Technol. 2014;46:750–756. doi: 10.9721/KJFST.2014.46.6.750. [DOI] [Google Scholar]
- Hanashiro I, Abe JI, Hizukuri S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr Res. 1996;283:151–159. doi: 10.1016/0008-6215(95)00408-4. [DOI] [Google Scholar]
- He W, Wei C. Progress in C-type starches from different plant sources. Food Hydrocoll. 2017;73:162–175. doi: 10.1016/j.foodhyd.2017.07.003. [DOI] [Google Scholar]
- Hizukuri S. Polymodal distribution of the chain lengths of amylopectins, and its significance. Carbohydr Res. 1986;147:342–347. doi: 10.1016/S0008-6215(00)90643-8. [DOI] [Google Scholar]
- Hou F, Mu T, Ma M, Blecker C. Optimization of processing technology using response surface methodology and physicochemical properties of roasted sweet potato. Food Chem. 2019;278:136–143. doi: 10.1016/j.foodchem.2018.11.034. [DOI] [PubMed] [Google Scholar]
- Jeong D, Han JA, Liu Q, Chung HJ. Effect of processing, storage, and modification on in vitro starch digestion characteristics of food legumes: a review. Food Hydrocoll. 2019;90:367–376. doi: 10.1016/j.foodhyd.2018.12.039. [DOI] [Google Scholar]
- Juliano BO. A simplified assay for milled-rice amylose. Cereal Sci Today. 1971;16:334–340. [Google Scholar]
- Kaur M, Sandhu KS, Lim ST. Microstructure, physicochemical properties and in vitro digestibility of starches from different Indian lentil (Lens culinaris) cultivars. Carbohydr Polym. 2010;79:349–355. doi: 10.1016/j.carbpol.2009.08.017. [DOI] [Google Scholar]
- Kim J, Ren C, Shin M. Physicochemical properties of starch isolated from eight different varieties of Korean sweet potatoes. Starch. 2013;65:923–930. doi: 10.1002/star.201200217. [DOI] [Google Scholar]
- Lee BH, Lee YT. Physicochemical and structural properties of different colored sweet potato starches. Starch. 2017;69:1600001. doi: 10.1002/star.201600001. doi: 10.1002/star.201600001. [DOI] [Google Scholar]
- Lee JS, Woo KS, Chun AE, Na JY, Kim KJ. Waxy rice variety-dependent variations in physicochemical characteristics of Sogokju, a Korean traditional rice wine. Korean J Crop Sci. 2009;54:172–180. [Google Scholar]
- Lee S, Lee JH, Chung HJ. Impact of diverse cultivars on molecular and crystalline structures of rice starch for food processing. Carbohydr Polym. 2017;169:33–40. doi: 10.1016/j.carbpol.2017.03.091. [DOI] [PubMed] [Google Scholar]
- Lehmann U, Robin F. Slowly digestible starch—its structure and health implications: a review. Trends Food Sci Technol. 2007;18:346–355. doi: 10.1016/j.tifs.2007.02.009. [DOI] [Google Scholar]
- Liu Q, Donner E, Yin Y, Huang RL, Fan MZ. The physicochemical properties and in vitro digestibility of selected cereals, tubers and legumes grown in China. Food Chem. 2006;99:470–477. doi: 10.1016/j.foodchem.2005.08.008. [DOI] [Google Scholar]
- McCleary BV, McNally M, Rossiter P. Measurement of resistant starch by enzymatic digestion in starch and selected plant materials: collaborative study. J AOAC Int. 2002;85:1103–1111. doi: 10.1093/jaoac/85.3.665. [DOI] [PubMed] [Google Scholar]
- Menon R, Padmaja G, Sajeev MS. Cooking behavior and starch digestibility of NUTRIOSE® (resistant starch) enriched noodles from sweet potato flour and starch. Food Chem. 2015;182:217–223. doi: 10.1016/j.foodchem.2015.02.148. [DOI] [PubMed] [Google Scholar]
- Rocha TS, Carneiro APA, Franco CML. Effect of enzymatic hydrolysis on some physicochemical properties of root and tuber granular starches. Ciênc Tecnol Aliment. 2010;30:544–551. doi: 10.1590/S0101-20612010000200039. [DOI] [Google Scholar]
- Singh J, Kaur L, McCarthy OJ. Factors influencing the physico- chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications—a review. Food Hydrocoll. 2007;21:1–22. doi: 10.1016/j.foodhyd.2006.02.006. [DOI] [Google Scholar]
- Vanier NL, El Halal SLM, Dias ARG , da Rosa Zavareze E. Molecular structure, functionality and applications of oxidized starches: a review. Food Chem. 2017;221:1546–1559. doi: 10.1016/j.foodchem.2016.10.138. [DOI] [PubMed] [Google Scholar]
- Wang A, Li R, Ren L, Gao X, Zhang Y, Ma Z, et al. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam) Food Chem. 2018;260:124–134. doi: 10.1016/j.foodchem.2018.03.125. [DOI] [PubMed] [Google Scholar]
- Wang S, Nie S, Zhu F. Chemical constituents and health effects of sweet potato. Food Res Int. 2016;89:90–116. doi: 10.1016/j.foodres.2016.08.032. [DOI] [PubMed] [Google Scholar]
- Wei C, Qin F, Zhu L, Zhou W, Chen Y, Wang Y, et al. Microstructure and ultrastructure of high-amylose rice resistant starch granules modified by antisense RNA inhibition of starch branching enzyme. J Agric Food Chem. 2010;58:1224–1232. doi: 10.1021/jf9031316. [DOI] [PubMed] [Google Scholar]
- Woo KS, Kim HJ, Lee JH, Ko JY, Lee BW, Lee BK. Cooking characteristics and antioxidant activity of rice-barley mix at different cooking method and mixing ratio. Prev Nutr Food Sci. 2018;23:52–59. doi: 10.3746/pnf.2018.23.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong H, Wang X, Sun J, Fang Y, Liu J, Jin C. Comparison of the structural characterization and physicochemical properties of starches from seven purple sweet potato varieties cultivated in China. Int J Biol Macromol. 2018;120:1632–1638. doi: 10.1016/j.ijbiomac.2018.09.182. [DOI] [PubMed] [Google Scholar]
- You SY, Lim ST, Lee JH, Chung HJ. Impact of molecular and crystalline structures on in vitro digestibility of waxy rice starches. Carbohydr Polym. 2014;112:729–735. doi: 10.1016/j.carbpol.2014.06.065. [DOI] [PubMed] [Google Scholar]
- You SY, Oh SK, Kim HS, Chung HJ. Influence of molecular structure on physicochemical properties and digestibility of normal rice starches. Int J Biol Macromol. 2015;77:375–382. doi: 10.1016/j.ijbiomac.2015.02.054. [DOI] [PubMed] [Google Scholar]
- Zaidul ISM, Nik Norulaini NA, Omar AKM, Yamauchi H, Noda T. RVA analysis of mixtures of wheat flour and potato, sweet potato, yam, and cassava starches. Carbohydr Polym. 2007;69:784–791. doi: 10.1016/j.carbpol.2007.02.021. [DOI] [Google Scholar]
- Zhang L, Zhao L, Bian X, Guo K, Zhou L, Wei C. Characterization and comparative study of starches from seven purple sweet potatoes. Food Hydrocoll. 2018;80:168–176. doi: 10.1016/j.foodhyd.2018.02.006. [DOI] [Google Scholar]
- Zhu F, Yang X, Cai YZ, Bertoft E, Corke H. Physicochemical properties of sweetpotato starch. Starch. 2011;63:249–259. doi: 10.1002/star.201000134. [DOI] [Google Scholar]