Abstract
Development of anti-inflammatory products remains in high demand due to the incidence of inflammatory diseases, including diabetes, cardiovascular disease, and neurodegenerative diseases. In this study, we examined the potential anti-inflammatory activity of the nutraceutical, Kmeria duperreana (Pierre) Dandy extract (KDE). We evaluated the ability of KDE to inhibit lipopolysaccharide (LPS)-induced inflammatory markers, including nitric oxide (NO), nuclear factor kappa-B, and mitogen-activated protein kinases, in RAW 264.7 cells. KDE suppressed LPS-induced nitrite production and inducible NO synthase (iNOS) expression in RAW 264.7 cells, but has no effect on cyclooxygenase-2 expression. KDE also suppressed LPS-induced phosphorylation of p65, IκB kinase, and p38 in RAW 264.7 cells. Through Western blot assays and immunofluorescence results, we showed that KDE suppresses LPS-induced p65 translocation from cytosol to the nucleus in RAW 264.7 cells. Moreover, KDE suppressed mRNA expression of LPS-induced interleukin (IL)-1β in RAW 264.7 cells, but had no effect on mRNA expression of IL-6 or tumor necrosis factor-a. These results demonstrate that KDE may be a promising anti-inflammatory nutraceutical. KDE may act by suppressing iNOS expression and subsequent NO production by inhibiting phosphorylation of p65 and p38 and suppressing translocation of p65 from the cytosol to the nucleus.
Keywords: inflammation, Kmeria duperreana (Pierre) Dandy, NF-κB, nitric oxide, nutraceuticals
INTRODUCTION
Chronic inflammation is implicated in the pathogenesis of a diverse array of diseases, including atherosclerosis, obesity, metabolic syndrome, diabetes, neurodegenerative diseases, and several types of cancers (Coussens and Werb, 2002; Pradhan, 2007; Lee et al., 2010). A complex web of intracellular and intercellular signaling pathways are involved in inducing inflammation. Activated monocytes and/or macrophages release a variety of inflammatory mediators, such as nitric oxide (NO) and prostaglandin, in response to lipopolysaccharide (LPS) (Wang et al., 1994). NO is an important pro-inflammatory mediator that is regulated by inducible NO synthase (iNOS) (Lu et al., 2015). Overproduction of NO leads to pronounced inflammation and tissue destruction. Therefore, inhibition of NO signaling pathways could be a promising strategy for attenuating inflammation (Sharma et al., 2007).
Nuclear factor kappa-B (NF-κB), composed of the proteins Rel and p50, is an important transcription factor involved in regulating inflammation, immunity, cell proliferation, and survival (Oeckinghaus and Ghosh, 2009). NF-κB activation involves rapid and transient activation of IκB kinase (IKK) and IκBα phosphorylation followed by IκBα degradation and translocation of NF-κB to the nucleus (Sun, 2017). In activated macrophages, NF-κB in synergy with other transcriptional activators, plays a central role in coordinating the expression of genes encoding tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and iNOS (Baeuerle and Henkel, 1994). When innate immune cells, such as macrophages, recognize LPS present on the surface of pathogens, IKK/IκB/NF-κB signaling pathways are activated to induce production of iNOS. Mitogen-activated protein kinases (MAPKs) also participate in the activation of NF-κB (Guha and Mackman, 2001).
Kmeria is a genus of tree in the family Magnoliaceae, which consist of 5 species native to the eastern Asian regions of Southern China and Indochina. The species Kmeria duperreana is known for yielding kmeriol. K. duperreana (Pierre) Dandy is a synonym of Magnolia duperreana Pierre (Dong et al., 1989). Dong et al. (1989) reported that the CHCl3-soluble fraction of a crude extract of K. duperreana exhibits cytotoxic activity in both KB and P388 tumor-cell cultures. However, the preventive activity of K. duperreana (Pierre) Dandy extract (KDE) on NO-mediated inflammation and the NF-κB signaling cascade have yet to be fully elucidated.
This study investigated the effects of KDE on LPS-induced NO production and NO-producing signaling pathways. The results indicate that KDE inhibits LPS-induced iNOS/NO production and IL-1β mRNA expression via suppressing phosphorylation of p65 and p38 and translocation of p65 from the cytosol to the nucleus.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and antibiotics (penicillin/streptomycin solution) were obtained from Thermo Fisher Scientific Inc. (Logan, UT, USA). LPS from Escherichia coli O111: B4 was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Antibodies against iNOS, cyclooxygenase (COX)- 2, p-p65 (Ser536), p65, p-IκBα (Ser32), IκBα, p-IKKα/β (Ser176/180), IKKα, p-extracellular signal-regulated kinase (ERK)1/2 (Thr202/Tyr204), ERK1/2, p-stress-activated protein kinase (SAPK)/c-JUN N-terminal kinase (JNK) (Thr183/Tyr185), SAPK/JNK, p-p38 (Thr180/ Tyr182), p38, and α/β-tubulin were purchased from Cell Signaling Technologies (Danvers, MA, USA). Primary antibodies against β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-lamin B1 was obtained from Abcam (Cambridge, UK).
Sample preparation
K. duperreana (Pierre) Dandy was collected from the Gung Re, Di Linh, Lam Dong, Vietnam. Plant samples were collected and identified by Dr. Tran The Bach at the Institute of Ecology and Biological Resources (Hanoi, Vietnam). Voucher specimens recorded as KRIB 0031397 and VK 3652 were deposited at the herbarium of the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). The K. duperreana (Pierre) Dandy leaves and branches (116 g) were mixed with 99.9% MeOH (1 L) and sonicated several times at room temperature over three days. The resulting MeOH extracts (9.37 g) were filtered and evaporated at 40°C under reduced pressure to produce crude extracts.
Cell culture and viability assays
RAW 264.7 murine macrophage cells were grown and maintained in DMEM containing 5% FBS and 1% antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin) at 37°C in a 5% CO2 incubator. To assess cell viability, RAW 264.7 cells (3×105 cells/mL) were seeded in 96- well plates and incubated at 37°C in a 5% CO2 incubator. After the cells were treated with the sample, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent (Promega, Madison, WI, USA) was added to each well. After 1 h incubation, the absorbance of formazan were measured at 490 nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Nitrite assays
RAW 264.7 mouse macrophage cells were seeded (3× 105 cells/mL) in 96-well plates and incubated at 37°C for 24 h in a 5% CO2 incubator. The medium was changed with KDE and cells were incubated for 1 h. Then, cells were treated with LPS (1 μg/mL) and incubated for 24 h. To determine the level of nitrite in the media of cells, an equal volume of Griess reagent [1% sulfanilamide, 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride, and 5% phosphoric acid] was added to 100 μL of sample. After 15 min, the absorbance at 550 nm was measured using a microplate reader.
Western blot assays
RAW 264.7 cells (2×105 cells/mL) were seeded in 10 cm dishes for 24 h. Cells were pretreated with KDE for 1 h, stimulated with LPS (1 μg/mL), and then incubated for stepped time periods. Cells were collected after incubation and washed twice with cold phosphate-buffered saline (PBS). Total cell lysates were extracted with lysis buffer (Cell Signaling Technologies), mixed with a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific Inc.), and maintained on ice for 30 min whilst vortexing. The total protein contents of the cell lysates were measured using a DC Protein Assay Kit reader (Bio-Rad Laboratories, Inc.) according to the manufacturer’s instructions. Harvested proteins were separated electrophoretically on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (ImmobilonⓇ-P, Millipore, Burlington, MA, USA). The membrane was blocked in Tris-buffered saline with Tween-20 buffer containing 5% skim milk for 1 h at room temperature. Then, the membrane was incubated with specific primary antibodies at 4°C overnight. After incubation with horseradish peroxidase-conjugated secondary antibodies (Thermo Fisher Scientific Inc.) at room temperature for 1 h, protein bands were visualized using a chemiluminescence detection kit (ATTO, Tokyo, Japan) and GeneGnome XRQ NPC (Syngene, Cambridge, UK).
Cytoplasmic and nuclear fractionation
RAW 264.7 mouse macrophage cells (3×105 cells/mL) were seeded in 6 cm dishes and incubated at 37°C for 24 h in a 5% CO2 incubator. The medium was replaced with KDE for 1 h. Cells were treated with 1 mg/mL of LPS for 30 min, collected, and then washed with cold PBS. Nuclear proteins were extracted using NE-PERTM Nuclear and cytoplasmic extraction reagents (Thermo Fisher Scientific Inc.) according to the manufacturer’s protocol.
Immunofluorescence
RAW 264.7 cells were seeded (5×104 cells/mL) on 8-well chamber slides (ibidi, Munich, Germany). After a 24 h incubation, the medium was exchanged with media containing varying amounts of KDE (50 and 100 μg/mL). After a 1 h incubation, cells were treated with LPS (1 mg/ mL) and incubated for a further 15 min. Cells were then fixed with 4% formaldehyde, permeabilized with ice-cold 100% MeOH, blocked, then incubated with specific anti- p65 antibodies (VECTASHIELDⓇ, Vector Laboratories Inc., Burlingame, CA, USA) overnight at 4°C. After washing, cells were incubated with goat anti-rabbit IgG H&L conjugated to Alexa FluoraⓇ 488 secondary antibodies (Abcam) for 1 h.
Quantitative real-time (qRT)-polymerase chain reaction (PCR)
Total RNA was isolated from RAW 264.7 cells using RNAiso Plus (Takara Bio, Dalian, China) according to the manufacturer’s instructions. cDNA was synthesized from isolated RNA using the ReverTra AceⓇ qPCR RT master mix (Toyobo, Osaka, Japan). qRT-PCR was performed using the SYBRⓇ Green Realtime PCR Master Mix (Toyobo), according to the manufacturer’s protocol. Levels of TNF-α, IL-1β, and IL-6 mRNA was examined using the CFX Real-Time PCR Detection Systems (Bio-Rad Laboratories, Inc.). Relative gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase using the 2−DDCT method.
Statistical analysis
Where appropriate, data are expressed as the mean± standard deviation (SD) of three independent experiments. Significant differences were determined using one- way analysis of variance (ANOVA). A probability value of P<0.05 was used to determine statistical significance.
RESULTS
Effect of KDE on LPS-induced nitrite production and cell viability in RAW 264.7 cells
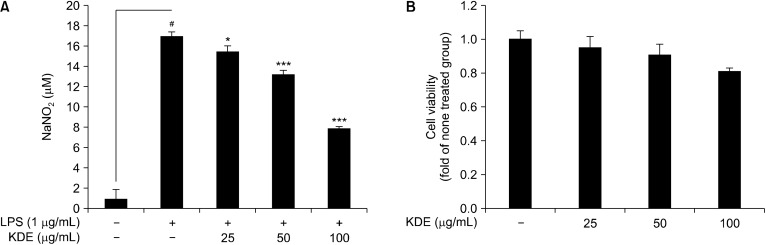
The amount of nitrite, a stable metabolite of NO, in the cell medium was used as an indicator of NO production; abnormal production was considered to be a marker for inflammation in RAW 264.7 cells (Green et al., 1982). We evaluated the effects of KDE on LPS-induced nitrite production in RAW 264.7 cells. Using the Griess assay, we showed that KDE significantly suppressed LPS-induced nitrite production in RAW 264.7 cells (Fig. 1A). The MTS assay results indicated that the tested concentration of KDE did not induce cytotoxicity of RAW 264.7 cells (Fig. 1B).
Fig. 1.
Effects of Kmeria duperreana (Pierre) Dandy extract (KDE) on lipopolysaccharide (LPS)-induced nitrite production and cell viability in RAW 264.7 cells. (A) KDE suppressed LPS-induced nitrite production in RAW 264.7 cells. Cells were pre-treated with KDE in the presence or absence of LPS (1 μg/mL) for 24 h. (B) KDE did not affect cell viability at concentrations of up to 50 mg/mL. The cells were treated with stepped concentrations of KDE for 24 h. #P<0.05 between the control group and the group exposed to LPS alone; *P<0.05 and ***P<0.001 between the LPS+KDE groups and the group exposed to LPS alone. Data are presented as mean±SD of three independent experiments.
Effect of KDE on LPS-induced iNOS and COX-2 expression in RAW 264.7 cells
We investigated the effect of KDE on expression of iNOS involved in NO production in RAW 264.7 cells. Using Western blot analysis, we showed that KDE suppressed expression of LPS-induced iNOS in a dose-dependent manner but had no impact on expression of COX-2 (Fig. 2).
Fig. 2.
Kmeria duperreana (Pierre) Dandy extract (KDE) on lipopolysaccharide (LPS)-induced iNOS and COX-2 expression in RAW 264.7 cells. (A) KDE suppressed LPS-induced inducible nitric oxide synthase (iNOS) but not cyclooxygenase (COX)-2 expression in RAW 264.7 cells. (B) Quantification of iNOS suppression by KDE. Expression levels of iNOS, COX-2, and β-actin were determined by Western blot. #P<0.05 between the control group and the group exposed to LPS alone; ***P<0.001 between the LPS+KDE groups and the group exposed to LPS alone. Data are presented as mean±SD of three independent experiments.
Effect of KDE on LPS-induced NF-κB and MAPKs pathways in RAW 264.7 cells
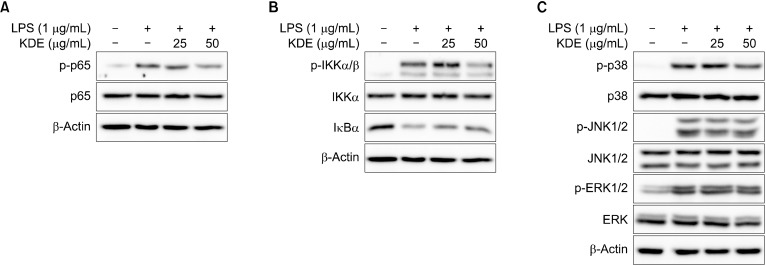
Since expression of iNOS and COX-2 are both regulated by NF-κB (Soromou et al., 2012), we further evaluated whether KDE affects LPS-induced NF-κB signaling pathways. KDE suppressed LPS-induced phosphorylation of p65 and IKK α/β (Fig. 3A and 3B). Since MAPKs are involved in LPS-induced NF-κB signaling pathways (Buchanan et al., 2010), we next investigated the effect of KDE on LPS-induced phosphorylation of MAPKs. KDE inhibited the LPS-induced phosphorylation of p38, but it did not affect the phosphorylation of ERK1/2 and JNK (Fig. 3C).
Fig. 3.
Effects of Kmeria duperreana (Pierre) Dandy extract (KDE) on lipopolysaccharide (LPS)-induced NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways in RAW 264.7 cells. (A and B) KDE inhibits the LPS-induced phosphorylation of p65 and IKK in RAW 264.7 cells. (C) KDE inhibits the LPS-induced phosphorylation of p38, but didn’t affect phosphorylation of c-JUN N-terminal kinase (JNK) 1/2 and extracellular signal-regulated kinase (ERK) 1/2 in RAW 264.7 cells. The levels of phosphorylation and expression were detected by Western blotting with specific antibodies.
Effect of KDE on LPS-induced translocation of p65 from the cytosol to the nucleus in RAW 264.7 cells
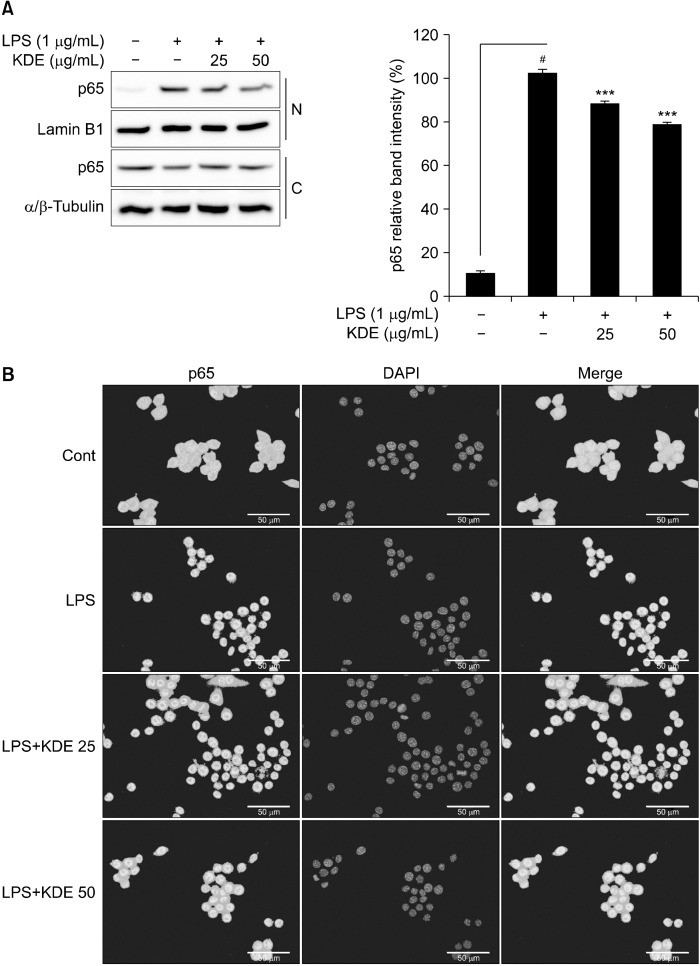
Phosphorylation of p65 at Ser536 is important for translocation of NF-κB from the cytosol to the nucleus (Brown et al., 2014). We investigated the effect of KDE on LPS- induced p65 translocation in RAW 264.7 cells. Cytosol and nuclear fractionation was shown through bands of lamin B1 and tubulin, markers of the nuclear and cytosol fractions, respectively, in the appropriate fractions (Fig. 4A). The results showed that KDE suppressed LPS-induced nuclear translocation of p65 from the cytosol to the nucleus (Fig. 4A). Also, immunofluorescence analysis showed that KDE suppressed LPS-induced nuclear translocation of p65 (Fig 4B).
Fig. 4.
Effects of Kmeria duperreana (Pierre) Dandy extract (KDE) on lipopolysaccharide (LPS)-induced p65 nuclear translocation in RAW 264.7 cells. (A and B) KDE inhibits the LPS-induced p65 nuclear expression in RAW 264.7 cells. Protein expression was detected by Western blotting with specific antibodies. N, nucleus; C, cytosol. Nucleus were stained with DAPI. #P<0.05 between the control group and the group exposed to LPS alone; ***P<0.001 between the LPS+KDE groups and the group exposed to LPS alone. Data are presented as mean±SD of three independent experiments.
Effect of KDE on LPS-induced IL-1β mRNA expression in RAW 264.7 cells
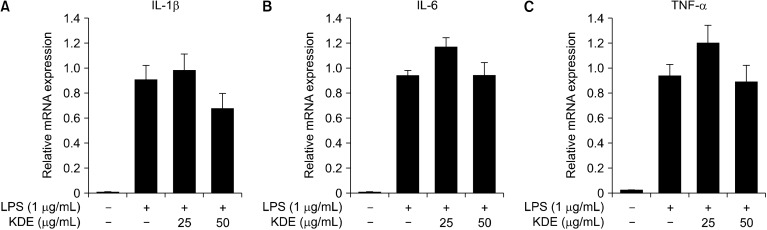
Activation of NF-κB results in activation of genes encoding pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α (Luyendyk et al., 2008). Using qRT-PCR, we investigated the effect of KDE on LPS-induced mRNA expression of IL-1β, IL-6, and TNF-α in RAW 264.7 cells. The results showed that KDE suppressed LPS-induced IL-1β mRNA expression (Fig. 5A) but did not induce expression of IL-6 or TNF-α (Fig. 5B and 5C).
Fig. 5.
Effects of Kmeria duperreana (Pierre) Dandy extract (KDE) on lipopolysaccharide (LPS)-induced inflammatory cytokines in RAW 264.7 cells. (A) KDE inhibits the LPS-induced interleukin (IL)-1β mRNA expression in RAW 264.7 cells. (B and C) KDE did not affect IL-6 or tumor necrosis factor (TNF)-α mRNA expression in RAW 264.7 cells, determined by quantitative real-time-polymerase chain reaction as described in the MATERIALS AND METHODS.
DISCUSSION
Since the discovery of the importance of inflammation in the development of chronic metabolic disease including diabetes, atherosclerosis, and neurodegenerative diseases (Hotamisligil, 2006), many trials have focused on developing anti-inflammatory nutraceuticals; however, more anti-inflammatory functional food materials are still needed (Fang et al., 2019; Jung et al., 2019). Therefore, the aim of this study was to develop novel anti-inflammatory materials. Of the 48 botanical extracts, we selected KDE since it had the highest inhibitory effect on LPS-induced NO production in RAW 264.7 cells (data not shown). We further evaluated the effect of KDE on LPS- induced inflammatory signaling pathways and expression of the pro-inflammatory cytokines IL-1β, TNF-α, and IL-6 in RAW 264.7 cells.
We confirmed that KDE suppressed LPS-induced iNOS expression and subsequent nitrite production in a dose- dependent manner in RAW 264.7 cells. Many published results have suggested that LPS-mediated inflammation and suppression of NO may be used as representative markers for the anti-inflammatory effect of botanicals (Xu et al., 2015). Based on independent experiments, Epimedium brevicornum water extract, Acanthopanax senticosus extract, and Moutan Cortex have been shown to exert inhibitory effects on nitrite production by 25, 30, and 50%, respectively (Chun et al., 2007; Lin et al., 2008; Yuk et al., 2010). Therefore, KDE exhibits a relatively high anti- inflammatory effect by inhibiting nitrite production by 50%.
Nitric oxide production can be induced by iNOS expression, which is the target gene of the transcription factor NF-κB (López-Collazo et al., 1998). Environmental stress, including exposure to bacteria, virus, and parasites, rapidly activate NF-κB in macrophages; once activated, NF- kB plays an important role in inflammation through its ability to induce transcription of pro-inflammatory genes (Jobin et al., 1999; Chi et al., 2012). MAPK pathways are composed of ERK, JNK/SAPK, and p38. MAPK signaling can be activated by Toll-like receptor 4, which stimulates nuclear translocation of NF-κB (Ma et al., 2015). Additionally, MAPKs are involved in NF-κB-dependent or -independent NO production in RAW 264.7 cells. Therefore, we evaluated the effect of KDE on LPS-induced NF-κB signaling pathways and phosphorylation of MAPK components. KDE suppressed LPS-induced phosphorylation of IKK α/β and p65 and only impacted phosphorylation of p38, not JNK1/2 or ERK1/2 in RAW 264.7 cells. These results indicate that suppression of NF-κB is a major mechanism of action of KDE on LPS-induced iNOS expression and NO production in RAW 264.7 cells.
Activated NF-κB translocates from the cytosol to the nucleus and binds to specific promoter regions to induce transcription of target genes, including iNOS, COX-2, and the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α. Our Western blot assay results showed that KDE suppresses p65 translocation from the cytosol to the nucleus. Immunofluorescence results support the finding that KDE increases levels of p65 in the cytosol, when compared to treatment with LPS alone. Inhibition of p65 phosphorylation subsequently suppressed LPS-induced p65 translocation from the cytosol to the nucleus.
Based on these results, we suspected that inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, are regulated by KDE. Furthermore, that, KDE slightly inhibits transcription of IL-1β, but not of IL-6 or TNF-α. Stimulation of IL-6, IL-8, and prostaglandin E2 production by LPS and IL-1β is downregulated by endogenously produced NO (Henrotin et al., 1998). Therefore, we hypothesized that KDE-induced suppression of nitrite production prevented downregulation of IL-6. Further investigations are required to clarify the effects of KDE on the expression of IL-6 and TNF-α.
Chronic inflammation causes a diverse array of diseases, such as metabolic syndrome, cardiovascular disease, nervous system disease, and digestive system disease (Libby, 2006; Chiurchiù and Maccarrone, 2011; Landskron et al., 2014). Therefore, substances that prevent inflammation may prevent metabolic diseases caused by chronic inflammation. Taken together, our results show that KDE may be promising anti-inflammatory nutraceutical, acting by suppressing LPS-induced NO production and iNOS expression, and production of IL-1β inflammatory cytokines. Since KDE suppressed the inflammatory response, it can be expected to have a preventive effect on metabolic diseases caused by chronic inflammation.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018 R1D1A1B07050031).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Brown JD, Lin CY, Duan Q, Griffin G, Federation A, Paranal RM, et al. NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell. 2014;56:219–231. doi: 10.1016/j.molcel.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan MM, Hutchinson M, Watkins LR, Yin H. Toll-like receptor 4 in CNS pathologies. J Neurochem. 2010;114:13–27. doi: 10.1111/j.1471-4159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi DS, Lin TC, Hall K, Ha T, Li C, Wu ZD, et al. Enhanced effects of cigarette smoke extract on inflammatory cytokine expression in IL-1β-activated human mast cells were inhibited by baicalein via regulation of the NF-κB pathway. Clin Mol Allergy. 2012;10:3. doi: 10.1186/1476-7961-10-3. doi: 10.1186/1476-7961-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiù V, Maccarrone M. Chronic inflammatory disorders and their redox control: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:2605–2641. doi: 10.1089/ars.2010.3547. [DOI] [PubMed] [Google Scholar]
- Chun SC, Jee SY, Lee SG, Park SJ, Lee JR, Kim SC. Anti-inflammatory activity of the methanol extract of moutan cortex in LPS- activated Raw264.7 cells. Evid Based Complement Alternat Med. 2007;4:327–333. doi: 10.1093/ecam/nel093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Mondranondra IO, Che CT, Fong HS, Farnsworth NR. Kmeriol and other aromatic constituents of Kmeria duperreana. Pharm Res. 1989;6:637–640. doi: 10.1023/A:1015969902200. [DOI] [PubMed] [Google Scholar]
- Fang ZJ, Zhang T, Chen SX, Wang YL, Zhou CX, Mo JX, et al. Cycloartane triterpenoids from Actaea vaginata with anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages. Phytochemistry. 2019;160:1–10. doi: 10.1016/j.phytochem.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/S0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Henrotin YE, Zheng SX, Deby GP, Labasse AH, Crielaard JM, Reginster JY. Nitric oxide downregulates interleukin 1beta (IL-1beta) stimulated IL-6, IL-8, and prostaglandin E2 production by human chondrocytes. J Rheumatol. 1998;25:1595–1601. [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Jobin C, Bradham CA, Russo MP, Juma B, Narula AS, Brenner DA, et al. Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. J Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- Jung S, Lee MS, Choi AJ, Kim CT, Kim Y. Anti-inflammatory effects of high hydrostatic pressure extract of mulberry (Morus alba) fruit on LPS-stimulated RAW264.7 cells. Molecules. 2019;24:1425. doi: 10.3390/molecules24071425. doi: 10.3390/molecules24071425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Han SB, Nam SY, Oh KW, Hong JT. Inflammation and Alzheimer's disease. Arch Pharm Res. 2010;33:1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- Lin QY, Jin LJ, Cao ZH, Xu YP. Inhibition of inducible nitric oxide synthase by Acanthopanax senticosus extract in RAW264.7 macrophages. J Ethnopharmacol. 2008;118:231–236. doi: 10.1016/j.jep.2008.04.003. [DOI] [PubMed] [Google Scholar]
- López-Collazo E, Hortelano S, Rojas A, Boscá L. Triggering of peritoneal macrophages with IFN-α/β attenuates the expression of inducible nitric oxide synthase through a decrease in NF-κB activation. J Immunol. 1998;160:2889–2895. [PubMed] [Google Scholar]
- Lu G, Zhang R, Geng S, Peng L, Jayaraman P, Chen C, et al. Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization. Nat Commun. 2015;6:6676. doi: 10.1038/ncomms7676. doi: 10.1038/ncomms7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyendyk JP, Schabbauer GA, Tencati M, Holscher T, Pawlinski R, Mackman N. Genetic analysis of the role of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. J Immunol. 2008;180:4218–4226. doi: 10.4049/jimmunol.180.6.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JQ, Li Z, Xie WR, Liu CM, Liu SS. Quercetin protects mouse liver against CCl4-induced inflammation by the TLR2/4 and MAPK/NF-κB pathway. Int Immunopharmacol. 2015;28:531–539. doi: 10.1016/j.intimp.2015.06.036. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr Rev. 2007;65:S152–S156. doi: 10.1111/j.1753-4887.2007.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 2007;15:252–259. doi: 10.1007/s10787-007-0013-x. [DOI] [PubMed] [Google Scholar]
- Soromou LW, Zhang Z, Li R, Chen N, Guo W, Huo M, et al. Regulation of inflammatory cytokines in lipopolysaccharide-stimulated RAW 264.7 murine macrophage by 7-O-methyl-naringenin. Molecules. 2012;17:3574–3585. doi: 10.3390/molecules17033574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. 2017;17:545–558. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Rossignol DP, Christ WJ, Geller DA, Freeswick PD, Thai NL, et al. Suppression of lipopolysaccharide-induced macrophage nitric oxide and cytokine production in vitro by a novel lipopolysaccharide antagonist. Surgery. 1994;116:339–447. [PubMed] [Google Scholar]
- Xu D, Huang P, Yu Z, Xing DH, Ouyang S, Xing G. Efficacy and safety of Panax notoginseng saponin therapy for acute intracerebral hemorrhage, meta-analysis, and mini review of potential mechanisms of action. Front Neurol. 2015;5:274. doi: 10.3389/fneur.2014.00274. doi: 10.3389/fneur.2014.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk SS, Lim EM, Lee JY, Lee YJ, Kim YS, Lee TH, et al. Antiinflammatory effects of Epimedium brevicornum water extract on lipopolysaccharide-activated RAW264.7 macrophages. Phytother Res. 2010;24:1781–1787. doi: 10.1002/ptr.3161. [DOI] [PubMed] [Google Scholar]