Abstract
Whole grain-based foods have been shown to reduce the risk of development of metabolic syndrome. In this study, we formulated whole grain-based multigrain flour and analyzed for available carbohydrate content, glycemic index (GI), and sensory evaluation. The multigrain flour composition 1 (C1) and composition 2 (C2) were formulated using defatted soya or bengal gram as a source of protein along with millets (40∼45%) and whole cereals. The proximate composition was calculated using Indian food composition tables. The microbial load and free fatty acid contents were analyzed in flour samples that were stored for different durations. The total dietary fiber, protein, and carbohydrate contents per 100 g of C1 and C2 flours were in the range of 11∼14, 13∼15, and 60 g, respectively. The available carbohydrate content in C1 and C2 were 55.4 and 62.3 g, and the in vivo GI was 63.2 and 66.2%, respectively. The acceptability scores of C1 and C2 products were in the range of 3.38 to 3.39 on the 5 points Hedonic scale. The multigrain flours were stable for 3 months based on microbial load and rancidity. The observed GIs of the multigrain flour were much lower than that of commercial refined wheat products. Therefore, these products may be recommended to regular diet plans to help prevent and/or ameliorate metabolic syndrome in the general population.
Keywords: glycemic carbohydrate, in vivo glycemic index, metabolic syndrome, multigrain flour, sensory evaluation
INTRODUCTION
There is growing evidence for the health benefits of whole grain-based cereal products, such as regulation of blood glucose, management of weight and diabetes, and prevention of certain cancers (Jacobs et al., 1998; Liese et al., 2003; Mellen et al., 2008). Whole grain products contain bran and germ fractions of cereals, which are rich in biomolecules such as micronutrients and phytochemicals and have a synergetic effect on the regulation of metabolic homeostasis (Okarter and Liu, 2010). However, refined foods are linked to the development of various chronic metabolic disorders (Giacco et al., 2003). The processing of food affects the degree of starch gelatinization and alters the available carbohydrate contents, which can result in undesired blood glucose and insulinogenic profiles in healthy individuals (Jenkins et al., 1981). Studies have shown that the glycemic index (GI) and glycemic load of food are key factors that determine the health benefits of food and diabetic management for consumers (Brand-Miller et al., 2002).
GI refers to the relative carbohydrate content of food compared with standards, such as glucose or white bread, based on their impact on blood glucose levels (50 g equivalent of carbohydrate) (Atkinson et al., 2008). Foods with GI values in the range <55, 55∼69, and >70 are considered low, medium, and high GI foods, respectively. Many studies have hypothesized that consumption of food with high GIs for a long duration enhances insulin resistance, increases insulin release, and results in hyperinsulinemia and altered lipid metabolism (Brand-Miller et al., 2002). Further, increased insulin resistance induces a postprandial increase in free fatty acids (FFAs), which further exacerbate insulin resistance and leads to type 2 diabetes (Brand-Miller et al., 2002). In addition, high GI foods induce a sequence of hormonal and metabolic changes that promote excessive food intake, resulting in increased energy intake and obesity (Venn and Green, 2007). Therefore, the concept of a GI is well recognized by the World Health Organization and American Diabetic Association for regulating hyperglycemia, obesity, and hypoglycemic shocks in patients receiving insulin therapy.
Multigrain conceptions show promising results for attenuating GIs in end products. Multigrain breads consist of millets and wheat, which contain more resistant starch and increased dietary fiber, demonstrates significantly decreased GIs in vitro with higher protein digestibility scores (Chauhan et al., 2017). Similarly, Indian multigrain pancake (Thalipeeth) (Shalini and Sonali, 2017) and healthy cookies (Tahilramani and Sengupta, 2016) consisting of wheat and whole grains demonstrate decreased GIs and are promoted as functional and nutritional foods due to their dietary fiber contents, and their protein digestibility and GIs. Many studies have demonstrated that roti prepared from multigrain flour have lower glycemic responses and GIs compared with whole wheat roti, and that consuming multigrain flour may lower dietary glycaemic load and help to prevent lifestyle-related diseases (Urooj and Puttaraj, 2000; Radhika et al., 2010; Tomer et al., 2018).
Consumption of rice and wheat has significantly increased in India, mainly due to the green revolution and the established public distribution system (Pathak et al., 2000). Based on observations and scientific data, many dieticians and endocrinologists have suggested that impaired food patterns resulting from loss of diversification and increases in consumption of wheat and rice-based refined foods, are linked with an increase in the prevalence of metabolic diseases in India (Mohan et al., 2010). Further studies have shown that foods with high GIs induce more eating disorders in obese people than those of normal weight (Ludwig et al., 1999). To combat these effects, millets are prescribed by dieticians since they promote a healthy diet due to their micronutrient profile and high fiber content (Pathak et al., 2000). Many commercial products are entering the Indian market with high dietary fiber and protein contents, such as multigrain flour. However, there is not much data available in respect to the carbohydrate profile and GIs of products and the extent of wheat flour blending. In this study, we blended whole wheat with varied combinations of millets to enhance the nutritional and carbohydrate profiles. Thus, the mixed flour maybe promoted as a functional or therapeutic food in the management of diabetes and obesity.
MATERIALS AND METHODS
Ingredients including wheat, sorghum, ragi, foxtail millet, defatted soya flour, bajra, maize, barnyard, and barley were purchased from the local market. Heat stable α-amylase, protease, and amyloglucosidase were procured from Sigma Aldrich Chemicals Pvt Ltd. (Bangalore, India). The GOD-POD glucose kit was procured from BioSystems Diagnostics Pvt Ltd. (Tamil Nadu, India) and glucose strips were purchased from local distributors (Accu-Chek India, Mumbai, India). The nutrient agar, Sabouraud dextrose agar (SDA), protease peptone, and phenolphthalein indicator were procured from HiMedia Leading BioSciences Co. (Mumbai, India). Ethanol, petroleum ether, and the other chemicals used in study were of analytical grade and procured from Sigma Aldrich Chemicals Pvt Ltd..
Preparation of flour and products (roti)
The multigrain flours [composition 1 and 2 (C1 and C2, respectively)] were prepared using a cyclone mill with the different combinations of whole grains, as described in Table 1 and 2. The test foods were prepared as described in Table 3. Approximately 50 g carbohydrate equivalent flour C1 (90.24 g) and C2 (80.17 g) were added to warm water and a pinch of salt in a ratio of 1:1.5, to prepare dough weighing 210 and 188 g, respectively. Dough was kneaded into small dough balls (70 and 63 g) and rolled on a rolling machine to prepare the rotis (30∼35 g). Rotis were roasted on both sides using a griddle, and were used for the analysis of the carbohydrate contents and GIs of the products.
Table 1.
Proximate composition of the multigrain flours
| Protein(g/100 g) | Fat(g/100 g) | TDF(g/100 g) | SDF(g/100 g) | Carbohydrate(g/100 g) | Energy(kJ) | |
|---|---|---|---|---|---|---|
| Composition 1 | ||||||
| Wheat1) | 3.18 | 0.44 | 3.37 | 0.48 | 19.42 | 96.6 |
| Foxtail2) | 2.60 | 1.08 | 2.13 | − | 15.43 | 86.0 |
| Defatted soya1) | 5.00 | 0.10 | 1.80 | 0.69 | 3.20 | 33.7 |
| Bajra1) | 1.96 | 0.54 | 1.49 | 0.23 | 6.18 | 36.9 |
| Barley1) | 0.55 | 0.06 | 0.80 | 0.28 | 3.06 | 15.8 |
| Oats3) | 0.59 | 0.47 | 0.50 | 0.19 | 3.43 | 20.4 |
| Maize1) | 0.44 | 0.19 | 0.61 | 0.05 | 3.24 | 16.7 |
| Ragi1) | 0.36 | 0.10 | 0.56 | 0.08 | 3.34 | 16.0 |
| Jowar1) | 0.50 | 0.09 | 0.51 | 0.09 | 3.38 | 16.7 |
| Total | 15.18 | 3.07 | 11.77 | 2.09 | 60.68 | 333.8 |
| Composition 2 | ||||||
| Wheat1) | 3.71 | 0.51 | 3.93 | 0.56 | 22.65 | 112.68 |
| Bengal gram1) | 5.39 | 1.33 | 6.31 | 0.62 | 11.68 | 82.28 |
| Foxtail2) | 1.04 | 0.43 | 0.85 | − | 6.17 | 34.40 |
| Jowar1) | 1.00 | 0.18 | 1.02 | 0.17 | 6.76 | 33.40 |
| Kodo1) | 0.90 | 0.25 | 0.64 | 0.21 | 6.62 | 33.17 |
| Barnyard4) | 0.56 | 0.19 | 0.63 | 0.21 | 3.44 | 19.9 |
| Bajra1) | 0.98 | 0.27 | 0.75 | 0.12 | 3.09 | 18.45 |
| Total | 13.58 | 3.16 | 14.13 | 1.89 | 60.41 | 334.28 |
3)USDA, 1999.
TDF, total dietary fiber; SDF, soluble dietary fiber.
Table 2.
Micronutrient profiles of the multigrain flours
| Ca (mg/100 g) | Cr (μg/100 g) | Zn (mg/100 g) | Cu (mg/100 g) | Se (μg/100 g) | Fe (mg/100 g) | Mg (mg/100 g) | Vt. B6 (μg/100 g) | |
|---|---|---|---|---|---|---|---|---|
| Composition 1 | ||||||||
| Wheat1) | 11.81 | 1.80 | 0.85 | 0.17 | 14.33 | 1.97 | 37.50 | 78.00 |
| Foxtail2) | 8.00 | 7.50 | 1.28 | 0.35 | 2.67 | 0.70 | 20.20 | 96.00 |
| Defatted soya1) | 19.50 | 0.70 | 0.38 | 0.08 | 1.69 | 0.82 | 18.90 | 45.00 |
| Bajra1) | 2.74 | 2.50 | 0.27 | 0.05 | 3.04 | 0.64 | 12.40 | 27.00 |
| Barley1) | 1.43 | 1.50 | 0.08 | 0.02 | 0.93 | 0.08 | 2.40 | 15.50 |
| Oats3) | 5.40 | 3.20 | 1.38 | 0.06 | 0.90 | 0.47 | 17.70 | 11.90 |
| Maize1) | 0.45 | 0.50 | 0.11 | 0.22 | 0.43 | 0.12 | 7.30 | 22.50 |
| Ragi1) | 18.20 | 1.60 | 0.13 | 0.34 | 0.77 | 0.23 | 7.30 | 2.50 |
| Jowar1) | 1.38 | 0.50 | 0.10 | 0.02 | 1.31 | 0.14 | 6.70 | 14.00 |
| Total | 68.91 | 19.80 | 4.58 | 1.32 | 26.07 | 5.17 | 130.40 | 312.40 |
| Composition 2 | ||||||||
| Wheat1) | 13.78 | 2.10 | 1.00 | 0.17 | 16.72 | 1.39 | 43.80 | 91.00 |
| Bengal gram1) | 11.58 | 2.00 | 0.91 | 0.21 | 12.74 | 1.52 | 29.50 | 47.50 |
| Foxtail4)5) | 3.20 | 3.00 | 0.51 | 0.14 | 0.53 | 0.28 | 8.10 | 38.40 |
| Jowar1) | 2.76 | 1.00 | 0.20 | 0.05 | 2.63 | 0.40 | 13.30 | 28.00 |
| Kodo1) | 1.53 | 2.10 | 0.17 | 0.03 | 1.41 | 0.23 | 12.20 | 7.00 |
| Barnyard4)5) | 1.40 | 4.50 | 0.15 | 0.03 | 0.13 | 0.25 | 1.90 | 19.20 |
| Bajra1) | 1.37 | 1.25 | 0.14 | 0.03 | 1.50 | 0.32 | 6.20 | 13.50 |
| Total | 35.62 | 15.95 | 3.08 | 0.66 | 35.66 | 4.39 | 115.00 | 244.60 |
Table 3.
Description of the test food
| Test foods | Test foods | Test foods |
|---|---|---|
| Available carbohydrate (g/100 g) | 55.40±0.54 | 62.30±0.89* |
| Flour required to obtain 50 gavailable carbohydrate (g) | 90.24 | 80.13* |
| Amount of water (mL) | 135 | 120 |
| Dough weight (g) | 210 | 188 |
| Dough weight (g per sample) | 70 | 63 |
| product weight (g per sample) | 35.50 | 32.00 |
The values show mean±SE (n=6).
*Statistically significant differences in the same row at P<0.05.
Proximate calculations
The proximate and micronutrient profiles of the multigrain flours were calculated per 100 g by using Indian food composition tables (Longvah et al., 2017).
Available carbohydrate
The available carbohydrate content of the multigrain products were analyzed as described previously (Devindra et al., 2017), with slight modifications. Samples (roti, 30 ∼35 g) was homogenized in 10 mL phosphate buffer (pH 6.0) and incubated overnight (12 h) in a refrigerator (2 ∼3°C) for hydration of the matrix. Samples (0.5 g) were subjected to enzymatic hydrolysis in duplicate, whereby samples were initially treated with heat stable α-amylase (50 μL) for 30 min in a water bath at 95°C. Reactions were terminated by altering the pH and temperature. Proteolysis was initiated by the addition of 50 μL protease solution after adjusting the pH of the tubes to 7.5 (60°C for 30 min). The reaction was terminated by addition of 0.325 M HCl. After adjusting the pH, amyloglucosidase solution (150 μL) was added and the tubes were incubated at 60°C for 30 min. Reactions were terminated by heat treatment and residues were separated by centrifugation. The liquid portion was transferred into a 100 mL volumetric flask and made up to 100 mL with milli Q water. Diluted samples were used to analyze glucose liberated from digested sample by GOD-POD kits. The optical density was measured at 505 nm and concentrations were calculated using glucose standard. Results were expressed as mean±standard error of the mean.
In vivo GI
Healthy adult volunteers (10 males and 2 females, body mass index: 23.44±0.66 kg/m2) participated in the study (Table 4). Subjects were healthy males or non-pregnant and non-lactating females 21 to 40 years of age. Inclusion criteria included fasting blood sugar levels <110 mg/dL. Exclusion criteria included any chronic disease, any known allergy, or intolerance to wheat and soy, consumption of any medications which interfere with the physiology of glucose tolerance, and a history of liver, kidney, or gastrointestinal disease. Ethical clearance for the study was obtained from the Institutional Ethics Committee of National Institute of Nutrition (ICMR) (IEC-NIN, registration no.: ECR/351/Inst/AP/2013). The protocol and consent form used in the study was approved by number 1/1/2017 dated 13.04.2017. The committee certifies that IEC is in full compliance with guidelines laid out by the ICMR, India. Informed written consent was obtained from each volunteer before the enrolling in the study.
Table 4.
Demographic and clinical characteristics of subjects (n=12)
| Variables | Mean | Range |
|---|---|---|
| Sex (male/female) | 10/2 | − |
| Age (years) | 32.60±0.99 | 28∼40 |
| Weight (kg) | 64.90±1.72 | 55∼74 |
| Height (cm) | 166.30±1.47 | 155∼174 |
| Body mass index (kg/m2) | 23.44±0.66 | 20.0∼26.5 |
| Fasting blood sugar (mmol/L) | 5.25±0.10 | 4.78∼5.67 |
Experimental design
Subjects were served 50 g of glucose portion (dextrose) on three occasions, followed by test sample (rotis) containing 50 g available carbohydrate. Blood glucose levels were monitored in capillary blood using a glucometer during fasting and at intervals for up to 120 min (15, 30, 45, 60, 90, and 120 min) after consumption glucose or test food (FAO/WHO, 1998). Glucose (50 g) was made up to 200 mL with drinking water and the same volume of water was provided with the test foods. The incremental area under the curve (IAUC) for blood glucose was calculated for both the test food and glucose using the trapezoid method, and the mean IAUC for the test foods and glucose were calculated for individual subjects. Then GI was calculated by using the formula:
Sensory evaluation
Sensory attributes were evaluated by a group of semi- trained panelists [both men and women (n=32)] using a 5-point Hedonic scale. The study design and protocol used in this study was approved by the institutional ethical committee. The contents of the multigrain roti were described to individuals; those who were sensitive to any of the ingredients were excluded from the study. The pros and cons of the study were explained to subjects and consent was obtained before the study start. The average age of the panelists was 30±11 years. The multigrain roti was evaluated for its acceptability with reference to appearance, colour, consistency, odour, texture, taste and feel in the mouth, and overall palatability, as described previously (Feldeisen and Tucker, 2007).
Storage studies
Multigrain flour (C1, lower GI) was analyzed for storage stability based on the rate of FFA formation and microbial load accumulation for up to 3.5 months at intervals of 15 days. The flours were stored at room temperature in an airtight aluminium foil. The duration of storage was chosen based on the labelling of the commercial flour products, which have an expiry period of 2 months.
FFA
Flour samples (10 g) were added into conical flasks containing 50 μL of 95% ethanol for extraction of FFA from the flour. Phenolphthalein indicator (2∼3 drops) was then added, and samples were titrated with 0.1 N NaOH until a faint pink colour appeared. The FFA content and acid value was calculated using the formula below:
where V is volume (mL) of standard sodium hydroxide required for titration, N is normality of standard sodium hydroxide used in titration, and W is weight in gram of sample used (Temba et al., 2017).
Microbiological study
Sample preparation: Sample (1 g) was suspended in 100 mL peptone water and was shaken for 1 h. The 100 μL inoculum was spread on nutrient agar (NA, bacteria) and SDA for moulds to determine the number of colony forming units.
Determination of total bacterial count: Nutrient agar media was used to determinate the total bacterial count. NA plates were dried and inoculated with suitably diluted inoculum by using the spread plate method. Plates were incubated at 37°C for 18∼24 h. The number of colonies formed was counted using a colony counter and results were expressed as the number of colonies forming units per gram of sample.
Determination of yeast and mould colonies: Samples were inoculated onto SDA medium by using the spread plate method and incubated at room temperature for 72 h. The visible colonies were counted and results were expressed as colony-forming unit/g of sample (Sharaf and Sabra, 2012).
Statistical analysis
The results were expressed as mean±standard error (SE) of three separate experiments. Differences between means were determined using t-tests for 2 groups or ANOVA followed by Tukey’s post-hoc honestly significant difference test for three groups. P<0.05 was considered statistically significant.
RESULTS
The proximate and micronutrient composition of multigrain flour
The proximate and micronutrient composition of multigrain flour was calculated based on the sum of the nutritional compositions of the individual components (Table 1). The protein contents of C1 and C2 were 15.18 and 13.58 g/100 g of flour, respectively, in which defatted soya and bengal gram were the major sources of protein (Table 1). The fat, energy, and carbohydrate contents of C1 and C2 were comparable, whereas the total dietary fiber content, contributed to by bengal gram of C2 (14.13) was higher than C1 (11.77). Micronutrients play a major role in the regulation of metabolic homeostasis; therefore, the amounts of key micronutrient in the multigrain flours were calculated. The Ca content of C1 was higher than that of C2, mainly due to finger millet in the ingredients (Table 2). The Se content per 100 g was approximately 40∼50% of the recommended daily amount (RDA) for adults, whereas the other micronutrients were in the range of 5∼25% of the RDA (based on FDA vitamins and minerals chart).
Glycemic carbohydrate and GIs of multigrain products (Indian bread)
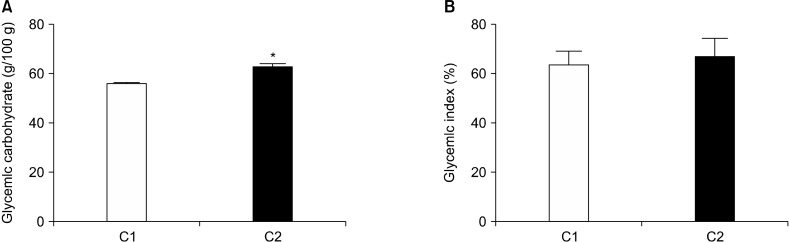
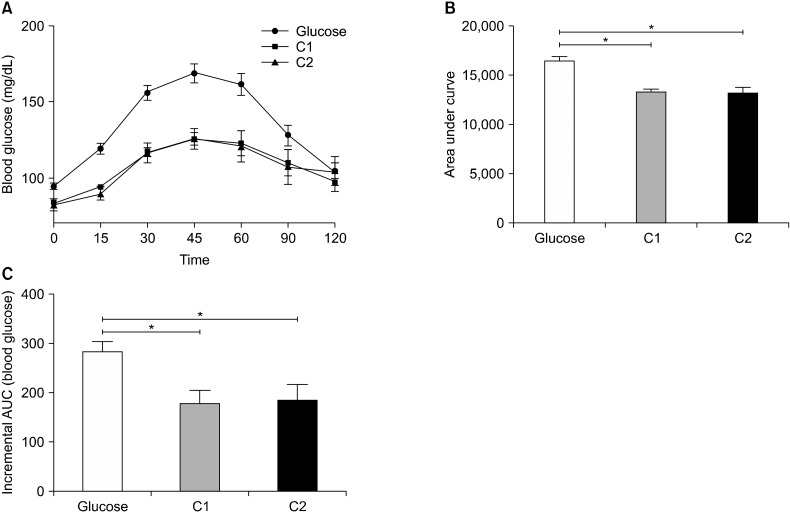
The glycemic carbohydrate content of C1 roti (55.4±0.54 g/100 g) was significantly lower than that of C2 (62.3± 0.89 g/100 g) (P<0.05, Fig. 1A). However, the GI was comparable between both C1 and C2 roti (63.2±5.45 and 66.2±8.08%, respectively) (Fig. 1B). The in vivo GIs were analyzed on healthy volunteers (Table 4). The average blood glucose levels at different time points following consumption of glucose and the roti samples were determined (Fig. 2A). Blood glucose levels peaked at 45 min and showed a trend of normalization after 60 min. After 120 min, initial levels were restored following consumption of both the reference and test products. However, the glycemic responses of the test products were significantly lower than those of the reference food at all time points. Both the area under the curve (Fig. 2B) and IAUC of blood glucose (Fig. 2C) were significantly lower in healthy volunteers following consumption of C1 and C2 compared with for glucose (P<0.05), as measured by the trapezoid rule. The calculated GI of C1 and C2 were 63.2 and 66.2, respectively, therefore the products fell in the middle of the GI category (55∼69).
Fig. 1.
Glycemic carbohydrate contents (A) and glycemic indexes (B) of the multigrain roti. Values show mean±SE. *Significant differences between groups at P<0.05.
Fig. 2.
Glycemic indexes of food products. The impact of the test foods and reference food on blood glucose levels (A), the area under the curve (AUC) for blood glucose levels (B), and the incremental area under the curve (IAUC) for blood glucose levels (C). Values show mean±SE (n=12). *Significant differences between groups at P<0.05.
Sensory evaluation
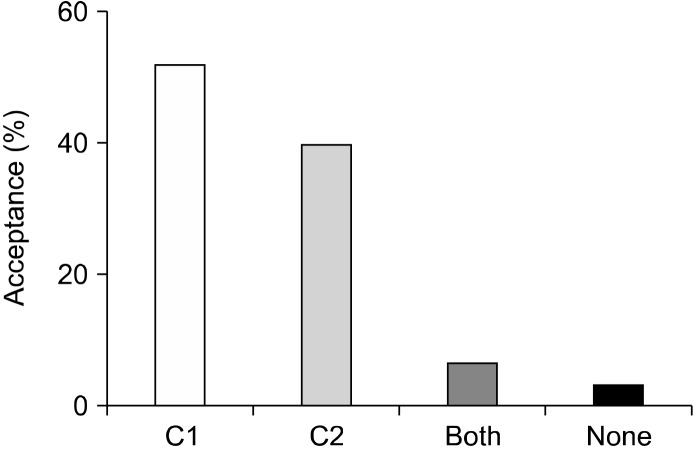
The acceptability of the multigrain products were analyzed using a 5-point Hedonic scale, based on its appearance, color, consistency, odor, texture, taste, and mouthfeel. The average acceptance scores for both C1 and C2 were 3.38 and 3.39, respectively, on the 5-point scale (Table 5). Of the 32 subjects, 51.5% preferred C1, 39.4% preferred C2, and 6.06% accepted both products equally (Fig. 3).
Table 5.
Sensory evaluation of multigrain Indian bread
| Sensory parameters | Composition 1 | Composition 2 |
|---|---|---|
| Apperance | 3.28±0.10 | 3.28±0.12 |
| Colour | 3.59±0.09 | 3.59±0.09 |
| Consistency | 3.34±0.10 | 3.59±0.10 |
| Odour | 3.47±0.09 | 3.56±0.11 |
| Texture | 3.44±0.12 | 3.53±0.12 |
| Taste | 3.25±0.13 | 3.16±0.15 |
| Mouth feel | 3.34±0.18 | 3.19±0.13 |
| Palatablility | 3.39±0.13 | 3.40±0.10 |
| Average | 3.38±0.08 | 3.39±0.08 |
Fig. 3.
The acceptability of multigrain roti. Values show mean± SE (n=32).
Storage study
Based on acceptability and available carbohydrate content, the C1 flour was further investigated in a storage study. The microbiological load and FFA content of the multigrain flour stored for different time intervals were determined (Table 6). Since Food and Agriculture Organization (FAO) guidelines are not available for multigrain flour, the results were compared with pure wheat flour only. The FFA content and fat acidity gradually increased after 45 days, and were 50∼55% higher than those of fresh samples at 3.5 months. The FFA content or acid value of fresh multigrain flour is higher than the levels described in the FAO guidelines for pure wheat flour, which might be due to the high-fat content in millets that represent 40% of the total composition. However, the values are well within those of other millet-based flours, as demonstrated by Goyal et al. (2017). The microbial pattern observed was similar to that of the fat acidity; the total bacterial and mould counts gradually increased with the duration of storage. However, the values were well within the safety levels described for wheat flour in the FAO guidelines.
Table 6.
Changes in chemical content and microbial quality of the multigrain flour stored at room temperature for different durations of time
| Duration of storage (days) | Microbiological count (CFU/g) | Free fatty acids (%) | |
|---|---|---|---|
| Bacteria (log10) | Moulds (log10) | ||
| 0 | 2.15±0.2 | 2.48±0.1 | 0.61±0.02 |
| 30 | 2.87±0.1 | 2.95±0.1 | 0.60±0.02 |
| 45 | 2.88±0.1 | 2.84±0.1 | 0.72±0.02* |
| 60 | 3.11±0.1* | 3.08±0.1* | 0.77±0.02* |
| 75 | 3.24±0.1* | 3.18±0.1* | 0.85±0.01* |
| 90 | 3.25±0.1* | 3.20±0.1* | 0.85±0.01* |
| 105 | 3.32±0.1* | 3.20±0.1* | 0.96±0.01* |
The values show mean±SE.
*Statistically significant differences in the same column from days 0 (P<0.05).
CFU, colony-forming unit.
DISCUSSION
The prevalence of metabolic syndrome is increasing significantly worldwide and in India, nearly 40% of the urban population is suffering from various metabolic diseases (NIN, 2017). Several scientific studies have shown that the carbohydrate content of food plays a major role in the prevalence of metabolic syndrome (Liese et al., 2003; Mellen et al., 2008). More importantly, the quality of carbohydrates in terms of the content of readily digestible and resistant starch (prebiotics) and the quantity of the carbohydrate (available carbohydrate) regulates the secretion of insulin, its action, and the counteraction of other hormones which may lead to insulin resistance (Jaeger and Cardello, 2009). The high GI (Atkinson et al., 2008), low cereal fiber, and high refined carbohydrate content (Schulze et al., 2004) are strongly associated with the prevalence of metabolic syndrome. Therefore, in this study whole grain-based multi-grain flour was formulated and analyzed for its glycemic carbohydrate content and GI, and therefore its ability to promote a healthy diet to prevent and/or manage the metabolic syndrome.
Wheat-based flatbreads or rotis are a common staple food for the Indian population. Since millets and whole grains have health benefits, in this study millets like ragi, sorghum, barley, bajra, barnyard, and foxtail were used to prepare the multigrain flour, in addition to bengal gram or soy as a source of protein. The millet content was maintained in the range 40∼45% of the flour to enhance the functional properties of the food via its antioxidant properties, high dietary fiber content, and high protein content containing a balanced amino acid profile (Sarita and Singh, 2016; Singh and Sarita, 2016). Further, scientific studies have shown the anti-diabetic properties of millets, as evidenced by their ability to regulate postprandial glucose levels by inhibiting α-amylase activity (Shobana et al., 2009; Kim et al., 2011) and glucose homeostasis by increasing levels of plasma adiponectin (Pradhan et al., 2010; Thathola et al., 2011).
In this study, the glycemic carbohydrate content of C1 and C2 were in the range of 55∼62, which correlates with results from our previous work (Korrapati et al., 2018) and others (Radhika et al., 2010; Devindra et al., 2017). The GIs of the products had lowered, which may be attributing to millets having a large amount of slowly digestible carbohydrates compare with refined wheat (Liu et al., 2006). In previous studies, the GIs of multigrain and normal wheat roti ranged from 44∼81. Factors including gluten content, method of processing, preparation (dry roasting/type of oil used), and the food supplements (such as dhal, chutney, and curry) determine the GI of the food (Chaturvedi et al., 1997; Urooj and Puttaraj, 2000; Thondre and Henry, 2009; Radhika et al., 2010; Devindra et al., 2017; Korrapati et al., 2018).
The hypoglycemic responses of C1 and C2 were might be attributed to inhibitory action on α-glucosidase and α-amylase activities of millets including foxtail, sorghum, and ragi (Shobana et al., 2009; Shobana et al., 2010; Kim et al., 2011), and deceleration of digestive process, including delaying gastric emptying and intestinal absorption (Jenkins et al., 1986). Studies have shown that barley α-glucan can reduce the GIs of wheat rotis and is attributed to their ability to delay carbohydrate digestion and absorption from the gut by increasing the viscosity of the stomach and intestinal contents (Thondre and Henry, 2009). Similarly, oat bran rich in α-glucan demonstrates increased hypoglycemic responses in diabetic patients compared with reference food, and may have a role in reducing the glycemic potential of the test food in the current study.
Further, a study from Jali et al. (2012) showed that consumption of a foxtail based diabetic diet for 90 days improves glycemic control and lowers plasma lipid concentrations in patients with type 2 diabetes. Consumption of barley kernel based bread for 3 consecutive days improves appetite regulation and glucose homeostasis in middle-aged subjects, which is relevant for the prevention of obesity and metabolic syndrome (Nilsson et al., 2015). Consumption of non-sweetened soy protein (Azadbakht et al., 2008; Mueller et al., 2012) and sorghum (Park et al., 2012) is negatively correlated with diabetes risk, which may increase the functionality of the developed multigrain products (rotis) for preventing diabetes and obesity. Further, these multigrain flours can be used for producing bread, pizza, noodles, and biscuits to enhance product availability and consumer choice and improve health conditions.
The commercialization of the products is highly dependent on the duration of the stability of the product, therefore we monitored the acidity and microbial loads of multigrain flour samples stored for different durations of time. The quality of the cereal flours deteriorates with time after processing, mainly due to the reaction between the fat and the lipid-degrading enzymes in the cereals (Rose et al., 2008). Further, the high rate of peroxidase activity and the high content of enzyme that alter the phenolic content are known to alter the taste and odor of the product. Since millets contain a high amount of fat and phenolics compared with wheat, they are highly susceptible to rancidity (Ocheme, 2007). Therefore, defatted flours show promising results for enhancing the stability of many millet products. In our study, the rate of FFA accumulation was comparable to that of other millet flour (Goyal et al., 2017) and the millets not alter the texture or odor of the flour for 3.5 months. Moreover, studies have shown a positive correlation between acidity and microbial contamination (Temba et al., 2017), and similar results were noticed in this study. However, the values were found to lie within limits described in the FAO guidelines for wheat flour.
This study concluded that consumption of foods with a higher GI and low dietary fiber content is associated with various components of metabolic syndrome. Since a well-balanced diet can prevent or delay onset of metabolic syndrome, food with a low GI index has health benefits for a large proportion of the population. In this study, we formulated whole grain-based multigrain flour with a moderate GI and a high dietary fiber content, which might have potential health benefits, compared with high glycemic refined flour.
ACKNOWLEDGEMENTS
The authors wish to thank the Director, National Institute of Nutrition, ICMR for her support to the study. The authors gratefully acknowledge the financial support by SERB, DST to conduct this study.
Footnotes
FUNDING
This work was funded by Science and Engineering Research Board, Department of Science and Technology, Government of India.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
References
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31:2281–2283. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: a longitudinal randomized clinical trial. Diabetes Care. 2008;31:648–654. doi: 10.2337/dc07-2065. [DOI] [PubMed] [Google Scholar]
- Brand-Miller JC, Holt SH, Pawlak DB, McMillan J. Glycemic index and obesity. Am J Clin Nutr. 2002;76:281S–285S. doi: 10.1093/ajcn/76.1.281S. [DOI] [PubMed] [Google Scholar]
- Chaturvedi A, Sarojini G, Nirmala G, Nirmalamma N, Satyanarayana D. Glycemic index of grain amaranth, wheat and rice in NIDDM subjects. Plant Foods Hum Nutr. 1997;50:171–178. doi: 10.1007/BF02436036. [DOI] [PubMed] [Google Scholar]
- Chauhan S, Sonawane SK, Arya SS. Nutritional evaluation of multigrain Khakra. Food Biosci. 2017;19:80–84. doi: 10.1016/j.fbio.2017.06.003. [DOI] [Google Scholar]
- Devindra S, Chouhan S, Katare C, Talari A, Prasad GBKS. Estimation of glycemic carbohydrate and glycemic index/load of commonly consumed cereals, legumes and mixture of cereals and legumes. Int J Diabetes Dev Ctries. 2017;37:426–431. doi: 10.1007/s13410-016-0526-1. [DOI] [Google Scholar]
- FAO. Sorghum and millets in human nutrition (FAO food and nutrition series, no. 27) Food and Agriculture Organization; Rome, Italy: 1995. Available from: http://www.fao.org/3/t0818e/T0818E01.htm#Sorghum. [Google Scholar]
- FAO/WHO. Carbohydrates in human nutrition: report of a joint expert consultation. World Health Organization; Rome, Italy: 1998. p. 140. [Google Scholar]
- Feldeisen SE, Tucker KL. Nutritional strategies in the prevention and treatment of metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:46–60. doi: 10.1139/h06-101. [DOI] [PubMed] [Google Scholar]
- Giacco R, Lappi J, Costabile G, Kolehmainen M, Schwab U, Landberg R, et al. Effects of rye and whole wheat versus refined cereal foods on metabolic risk factors: a randomised controlled two-centre intervention study. Clin Nutr. 2013;32:941–949. doi: 10.1016/j.clnu.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Goyal P, Chugh LK, Berwal MK. Storage effects on flour quality of commonly consumed cereals. J Appl Nat Sci. 2017;9:551–555. doi: 10.31018/jans.v9i1.1228. [DOI] [Google Scholar]
- Jacobs DR, Jr, Marquart L, Slavin J, Kushi LH. Whole-grain intake and cancer: an expanded review and meta-analysis. Nutr Cancer. 1998;30:85–96. doi: 10.1080/01635589809514647. [DOI] [PubMed] [Google Scholar]
- Jaeger SR, Cardello AV. Direct and indirect hedonic scaling methods: a comparison of the labeled affective magnitude (LAM) scale and best-worst scaling. Food Qual Prefer. 2009;20:249–258. doi: 10.1016/j.foodqual.2008.10.005. [DOI] [Google Scholar]
- Jali MV, Kamatar MY, Jali SM, Hiremath MB, Naik RK. Efficacy of value added foxtail millet therapeutic food in the management of diabetes and dyslipidamea in type 2 diabetic patients. Recent Res Sci Technol. 2012;4:3–4. [Google Scholar]
- Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Jenkins MA, Wolever TMS, Taylor RH, Ghafari H. Slow release carbohydrate: mechanism of action of viscous fibers. J Clin Nutr Gastroenterol. 1986;1:237–241. [Google Scholar]
- Kim JS, Hyun TK, Kim MJ. The inhibitory effects of ethanol extracts from sorghum, foxtail millet and proso millet on α-glucosidase and α-amylase activities. Food Chem. 2011;124:1647–1651. doi: 10.1016/j.foodchem.2010.08.020. [DOI] [Google Scholar]
- Korrapati D, Jeyakumar SM, Katragadda S, Ponday LR, Acharya V, Epparapalli S, et al. Development of low glycemic index foods and their glucose response in young healthy non-diabetic subjects. Prev Nutr Food Sci. 2018;23:181–188. doi: 10.3746/pnf.2018.23.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese AD, Roach AK, Sparks KC, Marquart L, D'Agostino RB, Jr, Mayer-Davis EJ. Whole-grain intake and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Am J Clin Nutr. 2003;78:965–971. doi: 10.1093/ajcn/78.5.965. [DOI] [PubMed] [Google Scholar]
- Liu Q, Donner E, Yin Y, Huang RL, Fan MZ. The physicochemical properties and in vitro digestibility of selected cereals, tubers and legumes grown in China. Food Chem. 2006;99:470–477. doi: 10.1016/j.foodchem.2005.08.008. [DOI] [Google Scholar]
- Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K. Indian food composition tables. National Institute of Nutrition (Indian Council of Medical Research); Hyderabad, Telangana, India: 2017. p. 578. [Google Scholar]
- Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103:E26. doi: 10.1542/peds.103.3.e26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis. 2008;18:283–290. doi: 10.1016/j.numecd.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Mohan V, Radhika G, Vijayalakshmi P, Sudha V. Can the diabetes/cardiovascular disease epidemic in India be explained, at least in part, by excess refined grain (rice) intake? Indian J Med Res. 2010;131:369–372. [PubMed] [Google Scholar]
- Mueller NT, Odegaard AO, Gross MD, Koh WP, Yu MC, Yuan JM, et al. Soy intake and risk of type 2 diabetes in Chinese Singaporeans. Eur J Nutr. 2012;51:1033–1040. doi: 10.1007/s00394-011-0276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson AC, Johansson-Boll EV, Björck IM. Increased gut hormones and insulin sensitivity index following a 3-d intervention with a barley kernel-based product: a randomised cross- over study in healthy middle-aged subjects. Br J Nutr. 2015;114:899–907. doi: 10.1017/S0007114515002524. [DOI] [PubMed] [Google Scholar]
- NIN. Diet and nutritional status of urban population in India and prevalence of obesity, hypertension, diabetes and hyperlipidemia in urban men and women. National Institute of Nutrition (Indian Council of Medical Research); Hyderabad, Telangana, India: 2017. p. 32. [Google Scholar]
- Ocheme OB. Effect of storage of millet flour on the quality and acceptability of millet flour porridge (Enyiokwolla) J Food Technol. 2007;5:215–219. [Google Scholar]
- Okarter N, Liu RH. Health benefits of whole grain phytochemicals. Crit Rev Food Sci Nutr. 2010;50:193–208. doi: 10.1080/10408390802248734. [DOI] [PubMed] [Google Scholar]
- Park JH, Lee SH, Chung IM, Park Y. Sorghum extract exerts an anti-diabetic effect by improving insulin sensitivity via PPAR-γ in mice fed a high-fat diet. Nutr Res Pract. 2012;6:322–327. doi: 10.4162/nrp.2012.6.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak P, Srivastava S, Grover S. Development of food products based on millets, legumes and fenugreek seeds and their suitability in the diabetic diet. Int J Food Sci Nutr. 2000;51:409–414. doi: 10.1080/096374800427019. [DOI] [PubMed] [Google Scholar]
- Pradhan A, Nag SK, Patil SK. Dietary management of finger millet (Eleusine coracana L. Gaerth) controls diabetes. Curr Sci. 2010;98:763–765. [Google Scholar]
- Radhika G, Sumathi C, Ganesan A, Sudha V, Jeya Kumar Henry C, Mohan V. Glycaemic index of Indian flatbreads (rotis) prepared using whole wheat flour and 'atta mix'-added whole wheat flour. Br J Nutr. 2010;103:1642–1647. doi: 10.1017/S0007114509993680. [DOI] [PubMed] [Google Scholar]
- Rose DJ, Ogden LV, Dunn ML, Pike OA. Enhanced lipid stability in whole wheat flour by lipase inactivation and antioxidant retention. Cereal Chem. 2008;85:218–223. doi: 10.1094/CCHEM-85-2-0218. [DOI] [Google Scholar]
- Sarita, Singh E. Potential of millets: nutrients composition and health benefits. J Sci Innov Res. 2016;5:46–50. [Google Scholar]
- Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80:348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- Shaheen N, Rahim ATM, Mohiduzzaman M, Banu CP, Bari ML, Tukun AB, et al. Food composition table for Bangladesh. Intergraphic Ltd; Dhaka, Bangladesh: 2013. pp. 187–259. [Google Scholar]
- Shalini A, Sonali G. Optimization of ingredients and process formulations on functional, nutritional, sensory and textural properties of Thalipeeth: Indian multigrain pancake. J Food Process Preserv. 2017;41:e12993. doi: 10.1111/jfpp.12993. doi: 10.1111/jfpp.12993. [DOI] [Google Scholar]
- Sharaf EM, Sabra SM. Microbiological loads for some types of cooked chicken meat products at Al-Taif Governorate, KSA. World Appl Sci J. 2012;17:593–597. [Google Scholar]
- Shobana S, Harsha MR, Platel K, Srinivasan K, Malleshi NG. Amelioration of hyperglycaemia and its associated complications by finger millet (Eleusine coracana L.) seed coat matter in streptozotocin-induced diabetic rats. Br J Nutr. 2010;104:1787–1795. doi: 10.1017/S0007114510002977. [DOI] [PubMed] [Google Scholar]
- Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009;115:1268–1273. doi: 10.1016/j.foodchem.2009.01.042. [DOI] [Google Scholar]
- Singh E, Sarita Potential functional implications of finger millet (Eleusine coracana) in nutritional benefits, processing, health and diseases: a review. Int J Home Sci. 2016;2:151–155. [Google Scholar]
- Tahilramani LM, Sengupta R. Multigrain healthy cookies for diabetes mellitus. Int J Sci Res. 2016;5:1360–1364. [Google Scholar]
- Temba MC, Njobeh PB, Kayitesi E. Storage stability of maize-groundnut composite flours and an assessment of aflatoxin B1 and ochratoxin A contamination in flours and porridges. Food Control. 2017;71:178–186. doi: 10.1016/j.foodcont.2016.06.033. [DOI] [Google Scholar]
- Thathola A, Srivastava S, Singh G. Effect of foxtail millet (Setaria italica) supplementation on serum glucose, serum lipids and glycosylated hemoglobin in type 2 diabetics. Diabetol Croat. 2011;40:23–28. [Google Scholar]
- Thondre PS, Henry CJ. High-molecular-weight barley beta-glucan in chapatis (unleavened Indian flatbread) lowers glycemic index. Nutr Res. 2009;29:480–486. doi: 10.1016/j.nutres.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Tomer V, Kaur A, Kaur A, Kumar A. Glycaemic index of Indian flatbreads (rotis) prepared using multigrain flour and whole wheat flour. Ann Biol. 2018;34:143–147. [Google Scholar]
- Ugare R, Chimmad B, Naik R, Bharati P, Itagi S. Glycemic index and significance of barnyard millet (Echinochloa frumentacae) in type II diabetics. J Food Sci Technol. 2014;51:392–395. doi: 10.1007/s13197-011-0516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urooj A, Puttaraj S. Glycaemic responses to cereal-based Indian food preparations in patients with non-insulin-dependent diabetes mellitus and normal subjects. Br J Nutr. 2000;83:483–488. doi: 10.1017/S0007114505000611. [DOI] [PubMed] [Google Scholar]
- USDA. FoodData Central. Agricultural Research Service, U.S. Department of Agriculture; Washington, DC, USA: 199. Available from: https://fdc.nal.usda.gov/ [Google Scholar]
- Vali Pasha K, Ratnavathi CV, Ajani J, Raju D, Manoj Kumar S, Beedu SR. Proximate, mineral composition and antioxidant activity of traditional small millets cultivated and consumed in Rayalaseema region of south India. J Sci Food Agric. 2018;98:652–660. doi: 10.1002/jsfa.8510. [DOI] [PubMed] [Google Scholar]
- Venn BJ, Green TJ. Glycemic index and glycemic load: measurement issues and their effect on diet-disease relationships. Eur J Clin Nutr. 2007;61:S122–S131. doi: 10.1038/sj.ejcn.1602942. [DOI] [PubMed] [Google Scholar]