This systematic review and meta-analysis assesses whether the use of airway checklists is associated with improved outcomes in patients undergoing endotracheal intubation.
Key Points
Question
Is the use of airway checklists associated with improved outcomes in patients undergoing endotracheal intubation?
Findings
This systematic review and meta-analysis of 11 studies with 3261 patients undergoing endotracheal intubation did not find a difference in mortality or most secondary outcomes associated with checklist use.
Meaning
The findings suggest that the use of airway checklists during endotracheal intubation is not associated with improved outcomes.
Abstract
Importance
Endotracheal intubation of critically ill patients is a high-risk procedure. Checklists have been advocated to improve outcomes.
Objective
To assess whether the available evidence supports an association of use of airway checklists with improved clinical outcomes in patients undergoing endotracheal intubation.
Data Sources
For this systematic review and meta-analysis, PubMed (OVID), Embase, Cochrane, CINAHL, and SCOPUS were searched without limitations using the Medical Subject Heading terms and keywords airway; management; airway management; intubation, intratracheal; checklist; and quality improvement to identify studies published between January 1, 1960, and June 1, 2019. A supplementary search of the gray literature was performed, including conference abstracts and clinical trial registries.
Study Selection
Full-text reviews were performed to determine final eligibility for inclusion. Included studies were randomized clinical trials or observational human studies that compared checklist use with any comparator for endotracheal intubation and assessed 1 of the predefined outcomes.
Data Extraction and Synthesis
Data extraction and quality assessment were performed using the Newcastle-Ottawa Scale for observational studies and Cochrane risk of bias tool for randomized clinical trials. Study results were meta-analyzed using a random-effects model. Reporting of this study follows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Main Outcomes and Measures
The primary outcome was mortality. Secondary outcomes included first-pass success and known complications of endotracheal intubation, including esophageal intubation, hypoxia, hypotension, and cardiac arrest.
Results
The search identified 1649 unique citations of which 11 (3261 patients) met the inclusion criteria. One randomized clinical trial and 3 observational studies had a low risk of bias. Checklist use was not associated with decreased mortality (5 studies [2095 patients]; relative risk, 0.97; 95% CI, 0.80-1.18; I2 = 0%). Checklist use was associated with a decrease in hypoxic events (8 studies [3010 patients]; relative risk, 0.75; 95% CI, 0.59-0.95; I2 = 33%) but no other secondary outcomes. Studies with a low risk of bias did not demonstrate decreased hypoxia associated with checklist use.
Conclusions and Relevance
The findings suggest that use of airway checklists is not associated with improved clinical outcomes during and after endotracheal intubation, which may affect practitioners’ decision to use checklists in this setting.
Introduction
Endotracheal intubation (ETI) is a frequently used life-saving procedure. In the US annually, 15 million operating room intubations and 650 000 hospital intubations outside the operating room are performed, including 346 000 emergency department (ED) intubations.1,2 Despite its frequency, ETI is a high-risk procedure, with significant rates of respiratory complications, hemodynamic instability, and cardiac arrest.3,4,5,6,7 Interventions to improve the safety and success of ETI could thus have a substantial effect on public health. Checklists are a form of cognitive strategy intended to force operators to ensure appropriate preparation before a procedure. Checklists have been associated with improved outcomes in multiple aspects of health care8,9,10,11,12,13 and have been endorsed as a means to reduce complications during ETI.6,14,15,16
The theoretical benefits of checklist use must be balanced with potential risks. Checklist adoption often faces numerous barriers and may require a substantial investment of time and resources.17,18 Checklist fatigue may occur with checklist endorsement for multiple different procedures.19 Furthermore, checklists are not universally correlated with improved outcomes18,20 and, in some cases, have even been associated with harm.21 This study evaluated the association between checklist use and clinical outcomes after ETI.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. The protocol has been published on PROSPERO (CRD42019140071).
Data Sources and Search Strategy
PubMed (OVID), Embase, Cochrane, CINAHL, and SCOPUS were searched without limitations to identify studies published between January 1, 1960, and June 1, 2019. The following Medical Subject Heading terms and keywords were identified collaboratively between 2 of us (J.S.T., L.M.M.) (the eAppendix in the Supplement gives the search details): airway; management; airway management; intubation, intratracheal; checklist; and quality improvement. In addition, a supplementary search of the gray literature was performed, including conference abstracts and clinical trial registries, but only peer-reviewed publications were eligible for inclusion. Bibliographies of included studies and relevant reviews were hand searched, and experts in the field were queried to identify additional studies.
Study Eligibility Criteria and Study Selection
Included studies met the following criteria: (1) evaluated an airway checklist regardless of checklist content in patients being intubated in any setting (protocols or procedures that did not use a checklist were not included), (2) included a comparator group without checklist use, and (3) assessed at least 1 of the predefined outcomes. Simulation studies or studies with no comparator group or no assessment of the outcomes of interest were excluded.
After the removal of duplicates, all titles and abstracts identified by the search were screened independently by 2 of us (J.S.T., A.W.B.). Full text was obtained for all articles deemed to be possibly relevant by either screener. Full-text reviews were performed independently by 2 of us (S.L.P., B.R.H.) to determine final eligibility for inclusion in the review. Disagreements about inclusion were resolved through discussion. If additional information was needed to determine eligibility, we attempted to contact the corresponding authors for individual studies.
Quality Appraisals
Risk of bias of the included studies was assessed using 2 different quality assessment tools. Randomized clinical trials were assessed by the Cochrane risk of bias tool.22 In brief, each study was assigned a high, low, or unclear risk of bias in each of 7 domains: random sequence generation, allocation concealment, blinding of participants and caregivers, blinding of outcome assessors, attrition bias, incomplete outcome bias, and other bias. Observational studies were assessed using the Newcastle-Ottawa Scale.23 This scale assigns up to 4 points for low risk of bias in the domain of selection of patients and comparators, 2 possible points for comparability, and 3 possible points for low risk of bias in determination of exposure. Studies are thus awarded between 0 and 9 points, with higher scores indicating lower risk of bias. Each study underwent quality assessment by 2 of us (S.L.P., B.R.H.) independently, with disagreements resolved through discussion.
Data Extraction
Two of us (J.S.T., A.W.B.) independently extracted data from each study. Data abstracted included year of publication, country, clinical setting, study design, inclusion and exclusion criteria, components of used checklists, number of patients, comparator interventions, and primary outcomes. For each study, the number of patients with and without each of our predefined outcomes was calculated for patients for whom a checklist was used and for the control group. Any discrepancies were resolved through discussion. In cases of missing data or need for clarification, we contacted corresponding authors of the original studies.
Outcomes
The primary outcome was mortality. We chose mortality as a primary outcome because it is the most patient-important outcome, and ETI is often performed on patients with significant risk of death. Mortality was recorded according to how it was reported in individual studies. If multiple measures of mortality were given, hospital mortality was used preferentially. Other outcomes of interest included rates of hypoxia, rates of hypotension, first-pass intubation success, time to successful intubation, peri-intubation arrest, esophageal intubation, and hospital length of stay. First-attempt intubation success was defined as successful ETI before removing the laryngoscope from the patient’s mouth. Peri-intubation arrest was defined as any loss of pulses that required cardiopulmonary resuscitation or defibrillation within 60 minutes after ETI. We allowed hypoxia and hypotension to be defined as described in individual studies because more granular data were not available. We performed preplanned sensitivity analyses of all included outcomes, including only studies with a low risk of bias. Subgroup analyses were performed for pediatric vs adult studies and ED vs intensive care unit (ICU) studies.
Statistical Analysis
Study results were meta-analyzed using a random-effects model to generate the summary relative risk (RRs) with corresponding 95% CIs. Heterogeneity (I2 and P values) were also reported. A 2-sided P < .05 was considered to be statistically significant. Statistical analyses were performed using the metan module of StataMP, version 16 (StataCorp LLC).
Results
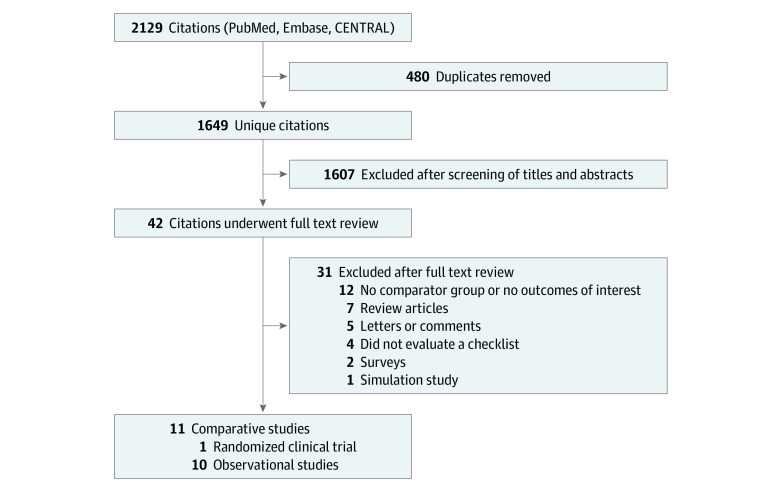
Figure 1 outlines the flow of study identification. The initial database search returned 1649 unique citations. After screening of titles and abstracts, 1607 citations were excluded, and 42 articles underwent full-text review, with 11 meeting inclusion criteria.1,24,25,26,27,28,29,30,31,32,33 Reasons for exclusion are listed in Figure 1. We requested clarification about inclusion criteria from the authors of 2 studies.25,34 Both studies described the use of an intubating protocol without specifying whether a checklist was used. We were able to confirm that a checklist was used in the study by Corl et al,25 but we were unable to confirm the use of a checklist for the other study,34 which was thus excluded.
Figure 1. Study Identification.
Characteristics of Studies and Patients
Across the 11 included studies,1,24,25,26,27,28,29,30,31,32,33 there were a total of 3261 ETIs performed in 13 institutions in 6 countries. Table 1 gives the characteristics of the individual studies. Seven studies1,24,26,29,30,31,32 were conducted in the ED, 3 studies25,27,28 in the ICU, and 1 study33 in both the operating room and ICU. Only 1 (ICU-based) study28 was a randomized clinical trial. Eight studies24,25,26,27,29,30,32,33 used a before-and-after observational design, with 6 studies25,26,29,30,32,33 being prospective and 2 studies24,27 being retrospective. The remaining 2 studies1,31 were prospective case series. None of the observational studies attempted to correct for baseline differences between groups. Five studies25,26,27,29,30 included significant cointerventions (such as health care professional education, equipment changes, health care professional team modeling, and medication changes) in addition to a checklist. Preintubation checklist details were reported in 8 studies24,25,26,27,28,29,32,33 and were heterogeneous. Most checklists included assessment of preoxygenation and medication, but other checklist components were inconsistent (eTable in the Supplement).
Table 1. Study Characteristics.
| Source | Study type | Country | Setting | Patients, No. | Outcomes assessed |
|---|---|---|---|---|---|
| Conroy et al,24 2014 | Retrospective before and after | US | ED | 187 | Mortality and FPS |
| Corl et al,25 2018 | Prospective before and after | US | ICU | 275 | Mortality, FPS, hypoxia, hypotension, cardiac arrest, EI, and hospital LOS |
| Fogg et al,26 2016 | Prospective before and after | Australia | ED | 655 | FPS, hypoxia, hypotension, cardiac arrest, and EI |
| Hatch et al,27 2016 | Retrospective before and after | US | Neonatal ICU | 509 | Mortality, hypoxia, TTI, hypotension, cardiac arrest, and EI |
| Janz et al,28 2018 | Randomized clinical trial | US | ICU | 262 | Mortality, FPS, hypoxia, TTI, hypotension, cardiac arrest, and EI |
| Kerrey et al,29 2015 | Prospective before and after | US | Pediatric ED | 189 | Hypoxia |
| Lewis et al,1 2018 | Prospective case series | South Africa | ED and prehospital | 41 | FPS |
| Long et al,30 2017 | Prospective before and after | Australia | Pediatric ED | 117 | FPS, hypoxia, and hypotension |
| Powell et al,31 2018 | Prospective case series | New Zealand | ED | 23 | FPS |
| Smith et al,32 2015 | Prospective before and after | US | ED | 141 | FPS, hypoxia, hypotension, TTI, cardiac arrest, and EI |
| Szucs et al,33 2019 | Prospective before and after | Hungary | ICU and OR | 862 | Mortality, FPS, hypoxia, hypotension, and cardiac arrest |
Abbreviations: ED, emergency department; EI, esophageal intubation; FPS, first-pass success; ICU, intensive care unit; LOS, length of stay; OR, operating room; TTI, time to intubation.
Definitions of hypoxia and hypotension varied among the studies. Definitions of hypoxia ranged from less than 60% to less than 93%, with a median of 90%. Two studies26,27 defined hypotension as a decrease in blood pressure that required intervention with fluid bolus or vasopressor. Other studies25,28,30,32,33 used definitions that included a systolic blood pressure from 70 to 90 mm Hg.
The only randomized clinical trial28 had a high risk of bias for blinding because neither practitioners nor outcome assessors were blinded to treatment group. Allocation concealment had an unclear risk of bias. The study28 had a low risk for random sequence generation, attrition bias, incomplete outcomes, and other bias. This study28 was included in the low risk of bias sensitivity analyses.
Table 2 outlines the risk of bias assessments for each domain of the Newcastle-Ottawa Scale across all 10 included observational studies.1,24,25,26,27,29,30,31,32,33 Of 9 possible stars awarded in the Newcastle-Ottawa Scale, scores ranged from 4 to 8 stars.
Table 2. Newcastle-Ottawa Scale Scores for Observational Studies.
| Source | Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposed cohort representative | Selection of unexposed | Exposure ascertainment blind or objective? | Outcome not present pre-exposure? | Controlled for other interventions/important confounders? | Similar baseline demographics or other confounders? | Outcome assessment blind or objective? | Follow-up long enough? | Lost to follow-up | |
| Conroy et al,24 2014 | Low risk of bias | High risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias |
| Corl et al,25 2018 | Low risk of bias | High risk of bias | Low risk of bias | Low risk of bias | High risk of bias | Low risk of bias | High risk of bias | Low risk of bias | Low risk of bias |
| Fogg et al,26 2016 | Low risk of bias | High risk of bias | Low risk of bias | High risk of bias | High risk of bias | High risk of bias | High risk of bias | Low risk of bias | Low risk of bias |
| Hatch et al,27 2016 | Low risk of bias | High risk of bias | Low risk of bias | High risk of bias | High risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | No |
| Kerrey et al,29 2015 | Low risk of bias | High risk of bias | Low risk of bias | High risk of bias | High risk of bias | High risk of bias | Low risk of bias | Low risk of bias | Low risk of bias |
| Lewis et al,1 2018 | Low risk of bias | High risk of bias | Low risk of bias | Low risk of bias | High risk of bias | High risk of bias | High risk of bias | Low risk of bias | Low risk of bias |
| Long et al,30 2017 | Low risk of bias | High risk of bias | Low risk of bias | High risk of bias | High risk of bias | High risk of bias | High risk of bias | Low risk of bias | Low risk of bias |
| Powell et al,31 2018 | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | High risk of bias | High risk of bias | High risk of bias | Low risk of bias | Low risk of bias |
| Smith et al,32 2015 | Low risk of bias | High risk of bias | Low risk of bias | High risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias |
| Szucs et al,33 2019 | Low risk of bias | High risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias | Low risk of bias |
We defined overall low risk of bias for observational studies24,32,33 as at least 7 of 9 possible stars. Three studies24,32,33 met these criteria. The remaining observational studies,1,25,26,27,29,30,31 with 4 to 6 stars, were deemed to have high to moderate risk of bias.
Main Results
Forest plots with summary estimates of RRs and 95% CIs for binary outcomes are displayed in Figure 2. For the primary outcome, mortality was reported in 5 studies24,25,27,28,33 with 2095 patients. The pooled mortality rate was 11.3%. No association was found between mortality and preintubation checklist use (pooled RR, 0.97; 95% CI, 0.80-1.18), with low heterogeneity (I2 = 0%).
Figure 2. Summary Estimates of Relative Risks (RRs) for Binary Outcomes.
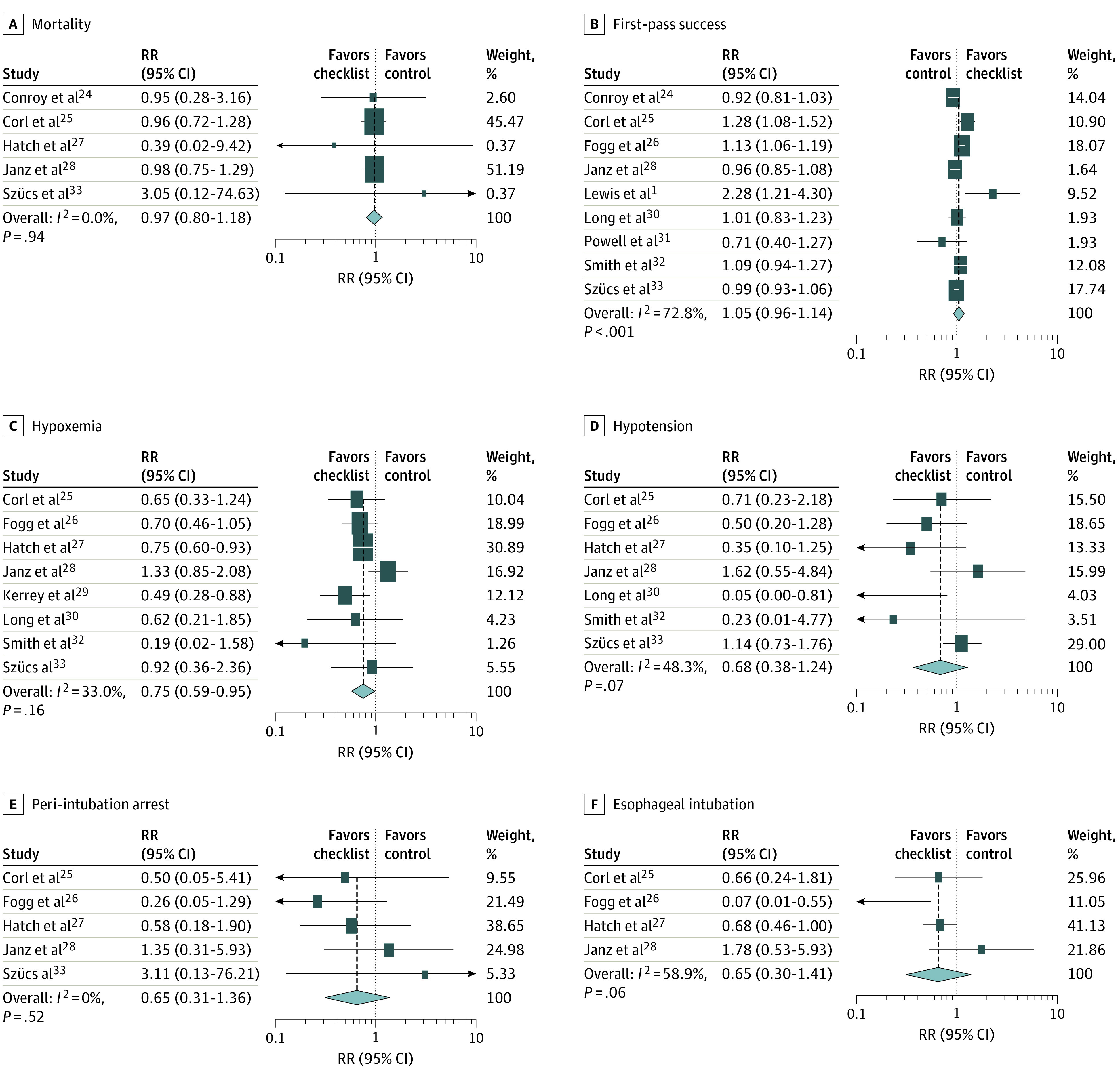
Squares indicate RR estimates, with horizonal lines representing 95% CIs. Diamonds represent pooled estimates, with points indicating 95% CIs. Shaded boxes represent the contribution weight of each study to the meta-analysis. Vertical dashed lines represent the relationship of the 95% CIs around each individual study result with the pooled mean. Weights are from random-effects analysis. A, E, and F, The study by Smith et al32 was not included in the analysis.
Among secondary outcomes, checklist use was not associated with a statistically significant difference in the rate of most adverse events, including esophageal intubation (4 studies25,26,27,28 [1701 patients]; RR, 0.65; 95% CI, 0.30-1.41; I2 = 58.9%), hypotension (7 studies25,26,27,28,30,32,33 [2821 patients]; RR, 0.68; 95% CI, 0.38-1.24; I2 = 48.3%), or peri-intubation cardiac arrest (5 studies25,26,27,28,32 [2563 patients]; RR, 0.65; 95% CI, 0.31-1.36; I2 = 0%). However, checklist use was associated with a decrease in hypoxic events (8 studies25,26,27,28,29,30,32,33 [3010 patients]; RR, 0.75; 95% CI, 0.59-0.95; I2 = 33%). This association was more pronounced in studies26,29,30,32,33 with cutoffs for hypoxia of 90% to 93% (eFigure 1 in the Supplement). Checklist use was also not associated with increased first-pass intubation success (9 studies1,24,25,26,28,30,31,32,33 [2563 patients]; RR, 1.05 with checklist; 95% CI, 0.96-1.14; I2 = 73%) (Figure 2). Time to successful intubation results were not pooled because definitions differed among the studies. Smith et al32 reported decreased time from paralysis to intubation associated with checklist use (82 vs 94 seconds, P = .02). Janz et al28 reported no difference in time from induction to intubation (120 seconds with checklist and 118 seconds without). Lastly, Hatch et al27 reported an increase in time from decision to intubate to successful intubation associated with checklist use (33 vs 27 minutes, P = .01). Hospital length of stay was only reported in 1 study25 and was not different between groups (11 days for checklist group and 12 days for control group, P = .55).
Figure 3 displays the results of the pooled analysis of the 4 studies at low risk of bias. In low-risk analyses, airway checklist use was not associated with improvement in any outcome. The nominal but nonstatistically significant suggestion of benefit seen in several primary analyses was absent or reversed in analyses of low risk of bias.
Figure 3. Low Risk of Bias Sensitivity Analysis.
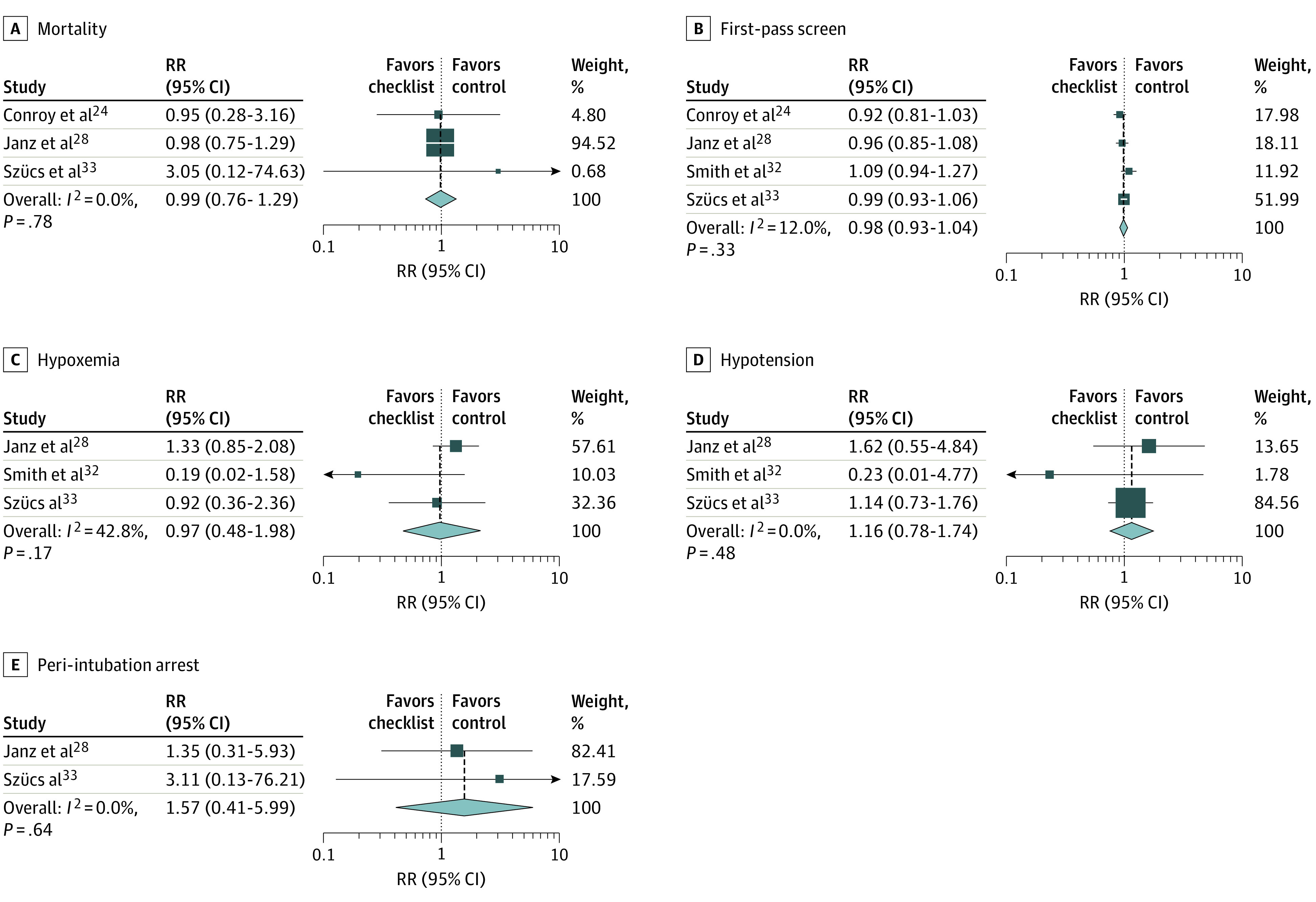
Squares indicate relative risk (RR) estimates, with horizonal lines representing 95% CIs. Diamonds represent pooled estimates, with points indicating 95% CIs. Vertical dashed lines represent the relationship of the 95% CIs around each individual study result with the pooled mean. Weights are from random-effects analysis. A, E, and F, The study by Smith et al32 was not included in the analysis.
Subgroup analyses of ED vs ICU studies are presented in eFigure 2 in the Supplement, representing data from 7 ED studies1,24,26,29,30,31,32 and 3 ICU studies.25,27,28 No association with survival was found in either setting. For other adverse events, nominal estimates that suggested benefit were more marked in ED-based studies1,24,26,29,30,31,32 than ICU-based studies.25,27,28 In the ED studies,1,24,26,29,30,31,32 checklist use was associated with a decrease in hypoxia (RR, 0.61; 95% CI, 0.44-0.83) and esophageal intubation (RR, 0.07; 95% CI, 0.01-0.55). No individual outcomes were statistically significantly different between groups in the ICU studies.25,27,28
Three studies27,29,30 were performed in pediatric settings, and 8 studies1,24,25,26,28,31,32,33 contributed data from primarily adult settings.No differences in any adverse events were found in analyses limited to adult studies. In pediatric studies, checklist use was associated with decreased hypoxia (RR, 0.70; 95% CI, 0.57-0.86) but no other outcomes (eFigure 3 in the Supplement).
Discussion
We identified 1 randomized clinical trial28 and 10 observational studies1,24,25,26,27,29,30,31,32,33 that compared clinical outcomes in ETI associated with and without an airway checklist. Summary estimates found no association between checklist use and mortality or most secondary outcomes, with the exception of decreased hypoxia. However, this association was not present in the sensitivity analysis of only studies with low risk of bias.28,32,33 Similarly, nominal but nonstatistically significant estimates that suggest benefit in several secondary outcomes were not apparent in sensitivity analyses of low risk of bias. Subgroup analyses suggested that checklist use may be more likely to be associated with decreased adverse events in pediatric settings and EDs compared with adult and ICU settings.
ETI is a high-risk procedure.3,4,5,6,7,8 Given that checklists have been associated with improved outcomes in other areas of health care,8,9,10,11,12,13,35 some have endorsed them for use with ETI.6,14,15,16,36 However, limited evidence supports such recommendations. Cabrini et al37 performed a systemic review of randomized clinical trials that evaluated any drug, technique, or device aimed at improving ETI. Similar to our review, the only randomized clinical trial that they identified that evaluated checklist use was the study by Janz et al,28 which found no benefit in any clinical outcomes. Hardy and Horner38 completed a “short-cut review” and concluded that checklists were likely beneficial, but further evidence was needed. That review, which was completed before several of the studies included in our review were published, included a conference abstract39 and 3 observational studies,24,29,32 129 of which had high risk of bias. Despite this lack of evidence, checklists for ETI are widely recommended.6,14,15,16
After pooling results from 11 different studies1,24,25,26,27,28,29,30,31,32,33 with more than 3000 patients, the only benefit statistically associated with checklist use in our systematic review was decreased hypoxic events. Because of the heterogeneity of hypoxia cutoffs in the included studies,1,24,25,26,27,28,29,30,31,32,33 hypoxia subgroups were defined based on the study definition of hypoxia. Results from studies26,29,30,32,33 using a cutoff of 90% or higher found an association with decreased hypoxia in patients intubated with use of a checklist, whereas this association was not observed in studies25,27,28 that examined more severe hypoxia (cutoffs of 60%-80%). More importantly, the finding of decreased hypoxia appeared to be primarily reported in studies with high risk of bias25,26,27,28,29,30 because the low risk of bias sensitivity analysis found no suggestion of decreased hypoxia. Most included studies25,26,27,29,30,32,33 used a before-and-after observational design. Such studies have high risk of bias for several reasons, including the Hawthorne effect, temporal improvement in care, and selection bias, and are prone to overestimation of effect sizes in favor of interventions.40 We found no suggestion of benefit for any outcomes when considering only studies with low risk of bias.32,33
Given the heterogeneity of patient populations and settings of the included studies,1,24,25,26,27,28,29,30,31,32,33 we performed subgroup analyses for adult and pediatric patients as well as ED and ICU settings. Subgroup analyses necessarily decrease the power to detect associations in each subgroup compared with the overall meta-analysis, and accordingly we found no statistically significant difference in most outcomes in subgroup analyses. Although only a few subgroup associations were apparent, some nominal suggestion of benefit was seen in several outcomes, with the estimates of effect generally more positive in pediatric and ED studies compared with adult and ICU studies. We propose that these differences are more likely related to the risk of bias in studies included in respective subgroups than to a true difference in effect size.
The current absence of evidence of benefit does not equate to a proven lack of benefit. It may be that checklists for ETI are associated with a decreased rate of rare, catastrophic events, such as peri-intubation cardiac arrest or cricothyrotomy. The number of patients required to define the effect of checklists on such rare events would be enormous. Although large, high-quality studies are needed to investigate checklist use further, randomized clinical trials of the size required to define precise effect estimates may not be feasible.
Limitations
This study has limitations. No studies contained data for all the predefined outcomes, and no outcome was reported in more than 9 studies, with only 5 studies providing results for our primary outcome of mortality. This limitation contributed to wide 95% CIs around the effect estimates for many of our results. In some cases, the 95% CIs included the possibility of substantial benefit. Large sample sizes would be needed to have sufficient power to detect checklist benefit for rare events. One before-and-after observational study34 was not included in our analysis. This study34 used an intubating bundle protocol. We were unable to confirm whether a checklist was used during the intervention phase of the study; thus, it was excluded. The results of this excluded study34 were consistent with those of the meta-analysis, with a decrease in severe hypoxemia noted in the intervention period but no difference in mortality, esophageal intubation, or length-of-stay measures. Most contributing studies1,24,25,26,27,29,30,31,32,33 were observational and frequently included multiple cointerventions, further obscuring what associations could be attributed to checklist implementation. All the observational studies1,24,25,26,27,29,30,31,32,33 were case series or had before-and-after cohort designs, which are particularly prone to bias. Only 424,28,32,33 of 11 included studies had low risk of bias. Estimates of effect in studies with low risk of bias were consistently less positive than in analyses that included all studies, suggesting that bias may have played a role in the results of higher-risk studies. Lastly, checklists may be more beneficial in settings that have low performance before implementation than in settings where the checklist items are already performed regularly at baseline. This information was not consistently available in the included studies. It is possible that academic centers, where studies are more likely to be performed, are already high performing, and checklist implementation would be more valuable in other settings.
Conclusions
We found no association between survival and checklist use in patients undergoing ETI in this systematic review and meta-analysis. Checklist use was associated with a decrease in hypoxic events but no other secondary outcomes, although point estimates favored checklist use. Analyses of studies with low risk of bias found no association with decreased hypoxia, and point estimates did not suggest benefit. Additional high-quality studies in this area are needed, but current evidence does not support checklist use to improve clinical outcomes in patients undergoing ETI.
eAppendix. Search Strategy
eFigure 1. Hypoxia Rates by Cutoff
eFigure 2. Subgroup Analysis, ICU vs ED
eFigure 3. Subgroup Analysis, Adult vs Pediatric Studies
eTable. Commonly Used Elements of Airway Checklists
References
- 1.Lewis CT, Brown J, Inglis AC, Naumann DN, Crombie N. Emergency intubation in trauma in KwaZulu-Natal Province, South Africa. S Afr Med J. 2018;108(8):660-666. doi: 10.7196/SAMJ.2018.v108i8.12670 [DOI] [PubMed] [Google Scholar]
- 2.Durbin CG Jr, Bell CT, Shilling AM. Elective intubation. Respir Care. 2014;59(6):825-846. doi: 10.4187/respcare.02802 [DOI] [PubMed] [Google Scholar]
- 3.Bowles TM, Freshwater-Turner DA, Janssen DJ, Peden CJ; RTIC Severn Group . Out-of-theatre tracheal intubation: prospective multicentre study of clinical practice and adverse events. Br J Anaesth. 2011;107(5):687-692. doi: 10.1093/bja/aer251 [DOI] [PubMed] [Google Scholar]
- 4.Cook TM, Woodall N, Harper J, Benger J; Fourth National Audit Project . Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society, part 2: intensive care and emergency departments. Br J Anaesth. 2011;106(5):632-642. doi: 10.1093/bja/aer059 [DOI] [PubMed] [Google Scholar]
- 5.Kim WY, Kwak MK, Ko BS, et al. Factors associated with the occurrence of cardiac arrest after emergency tracheal intubation in the emergency department. PLoS One. 2014;9(11):e112779. doi: 10.1371/journal.pone.0112779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Löllgen RMC, Pontin J, Gow M, McCaskill ME. Adverse events and risk factors during emergency intubation in a tertiary paediatric emergency department. Eur J Emerg Med. 2018;25(3):209-215. doi: 10.1097/MEJ.0000000000000439 [DOI] [PubMed] [Google Scholar]
- 7.Manthous CA. Avoiding circulatory complications during endotracheal intubation and initiation of positive pressure ventilation. J Emerg Med. 2010;38(5):622-631. doi: 10.1016/j.jemermed.2009.01.018 [DOI] [PubMed] [Google Scholar]
- 8.Carlos WG, Patel DG, Vannostrand KM, Gupta S, Cucci AR, Bosslet GT. Intensive care unit rounding checklist implementation: effect of accountability measures on physician compliance. Ann Am Thorac Soc. 2015;12(4):533-538. doi: 10.1513/AnnalsATS.201410-494OC [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Kan T, Li S, Qiu C, Gui L. Use and implementation of standard operating procedures and checklists in prehospital emergency medicine: a literature review. Am J Emerg Med. 2016;34(12):2432-2439. doi: 10.1016/j.ajem.2016.09.057 [DOI] [PubMed] [Google Scholar]
- 10.Haugen AS, Søfteland E, Almeland SK, et al. Effect of the World Health Organization checklist on patient outcomes: a stepped wedge cluster randomized controlled trial. Ann Surg. 2015;261(5):821-828. doi: 10.1097/SLA.0000000000000716 [DOI] [PubMed] [Google Scholar]
- 11.Hazelton JP, Orfe EC, Colacino AM, et al. The impact of a multidisciplinary safety checklist on adverse procedural events during bedside bronchoscopy-guided percutaneous tracheostomy. J Trauma Acute Care Surg. 2015;79(1):111-115. doi: 10.1097/TA.0000000000000700 [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Peters MJ; PICU/NICU Risk Action Group . ‘Safety by DEFAULT’: introduction and impact of a paediatric ward round checklist. Crit Care. 2013;17(5):R232. doi: 10.1186/cc13055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howie WO, Dutton RP. Implementation of an evidence-based extubation checklist to reduce extubation failure in patients with trauma: a pilot study. AANA J. 2012;80(3):179-184. [PubMed] [Google Scholar]
- 14.Lockey DJ, Crewdson K, Davies G, et al. AAGBI: safer pre-hospital anaesthesia 2017: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia. 2017;72(3):379-390. doi: 10.1111/anae.13779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neily J, Silla ES, Sum-Ping SJT, et al. Anesthesia adverse events voluntarily reported in the Veterans Health Administration and lessons learned. Anesth Analg. 2018;126(2):471-477. doi: 10.1213/ANE.0000000000002149 [DOI] [PubMed] [Google Scholar]
- 16.Sherren PB, Tricklebank S, Glover G. Development of a standard operating procedure and checklist for rapid sequence induction in the critically ill. Scand J Trauma Resusc Emerg Med. 2014;22:41. doi: 10.1186/s13049-014-0041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis KF, Napolitano N, Li S, et al. ; National Airway Emergency for Children (NEAR4KIDS) and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network . Promoters and barriers to implementation of tracheal intubation airway safety bundle: a mixed-method analysis. Pediatr Crit Care Med. 2017;18(10):965-972. doi: 10.1097/PCC.0000000000001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leape LL. The checklist conundrum. N Engl J Med. 2014;370(11):1063-1064. doi: 10.1056/NEJMe1315851 [DOI] [PubMed] [Google Scholar]
- 19.Stock CT, Sundt T. Timeout for checklists? Ann Surg. 2015;261(5):841-842. doi: 10.1097/SLA.0000000000001141 [DOI] [PubMed] [Google Scholar]
- 20.Williams AK, Cotter RA, Bompadre V, Goldberg MJ, Steinman SS. Patient safety checklists: do they improve patient safety for supracondylar humerus fractures? J Pediatr Orthop. 2019;39(5):232-236. doi: 10.1097/BPO.0000000000000928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al Ashry HS, Abuzaid AS, Walters RW, Modrykamien AM. Effect of compliance with a nurse-led intensive care unit checklist on clinical outcomes in mechanically and nonmechanically ventilated patients. J Intensive Care Med. 2016;31(4):252-257. doi: 10.1177/0885066614533910 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 24.Conroy MJ, Weingart GS, Carlson JN. Impact of checklists on peri-intubation care in ED trauma patients. Am J Emerg Med. 2014;32(6):541-544. doi: 10.1016/j.ajem.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 25.Corl KA, Dado C, Agarwal A, et al. A modified Montpellier protocol for intubating intensive care unit patients is associated with an increase in first-pass intubation success and fewer complications. J Crit Care. 2018;44:191-195. doi: 10.1016/j.jcrc.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fogg T, Alkhouri H, Vassiliadis J. The Royal North Shore Hospital Emergency Department airway registry: closing the audit loop. Emerg Med Australas. 2016;28(1):27-33. doi: 10.1111/1742-6723.12496 [DOI] [PubMed] [Google Scholar]
- 27.Hatch LD, Grubb PH, Lea AS, et al. Interventions to improve patient safety during intubation in the neonatal intensive care unit. Pediatrics. 2016;138(4):e20160069. doi: 10.1542/peds.2016-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janz DR, Semler MW, Joffe AM, et al. ; Check-UP Investigators*; Pragmatic Critical Care Research Group . A multicenter randomized trial of a checklist for endotracheal intubation of critically ill adults. Chest. 2018;153(4):816-824. doi: 10.1016/j.chest.2017.08.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerrey BT, Mittiga MR, Rinderknecht AS, et al. Reducing the incidence of oxyhaemoglobin desaturation during rapid sequence intubation in a paediatric emergency department. BMJ Qual Saf. 2015;24(11):709-717. doi: 10.1136/bmjqs-2014-003713 [DOI] [PubMed] [Google Scholar]
- 30.Long E, Cincotta DR, Grindlay J, et al. A quality improvement initiative to increase the safety of pediatric emergency airway management. Paediatr Anaesth. 2017;27(12):1271-1277. doi: 10.1111/pan.13275 [DOI] [PubMed] [Google Scholar]
- 31.Powell E, Alkhouri H, McCarthy S, et al. A sequential case series of 23 intubations in a rural emergency department in New Zealand. Aust J Rural Health. 2018;26(1):48-55. doi: 10.1111/ajr.12366 [DOI] [PubMed] [Google Scholar]
- 32.Smith KA, High K, Collins SP, Self WH. A preprocedural checklist improves the safety of emergency department intubation of trauma patients. Acad Emerg Med. 2015;22(8):989-992. doi: 10.1111/acem.12717 [DOI] [PubMed] [Google Scholar]
- 33.Szűcs ZP, Farkas J, Schimert P, Baranyai Z, Dinya E. The impact of a checklist on the short-term complications of airway management in adults [in Hungarian]. Orv Hetil. 2019;160(26):1025-1035. [DOI] [PubMed] [Google Scholar]
- 34.Jaber S, Jung B, Corne P, et al. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Intensive Care Med. 2010;36(2):248-255. doi: 10.1007/s00134-009-1717-8 [DOI] [PubMed] [Google Scholar]
- 35.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. doi: 10.1056/NEJMoa061115 [DOI] [PubMed] [Google Scholar]
- 36.Weingart S, Hua A. An Intubation Checklist for Emergency Department Physicians. Vol 33. ACEP Now; 2014. [Google Scholar]
- 37.Cabrini L, Landoni G, Baiardo Redaelli M, et al. Tracheal intubation in critically ill patients: a comprehensive systematic review of randomized trials. Crit Care. 2018;22(1):6. doi: 10.1186/s13054-017-1927-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardy G, Horner D. BET 2: should real resuscitationists use airway checklists? Emerg Med J. 2016;33(6):439-441. doi: 10.1136/emermed-2016-205871.2 [DOI] [PubMed] [Google Scholar]
- 39.Shanmugasundaram P, Wilson G, Parke T. Improving intubation safety in critically ill patients. J Intensive Care Soc. 2014;15(1):1751-437. [Google Scholar]
- 40.Goodacre S. Uncontrolled before-after studies: discouraged by Cochrane and the EMJ. Emerg Med J. 2015;32(7):507-508. doi: 10.1136/emermed-2015-204761 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Search Strategy
eFigure 1. Hypoxia Rates by Cutoff
eFigure 2. Subgroup Analysis, ICU vs ED
eFigure 3. Subgroup Analysis, Adult vs Pediatric Studies
eTable. Commonly Used Elements of Airway Checklists