Abstract
In the late 19th century, Otto Frank published the first description of a ventricular pressure-volume diagram, thus laid the foundation for modern cardiovascular physiology. Since then, the analysis of the pressure-volume loops became a reference tool for the study of the ventricular pump properties. However, understanding cardiovascular performance requires both the evaluation of ventricular properties and the modulating effects of the arterial system, since the heart and the arterial tree are anatomically and functionally related structures. The study of the coupling between the cardiac function and the properties of the arterial system, or ventriculo-arterial (VA) coupling, provides then a comprehensive characterization of the performance of the cardiovascular system in both health and disease. The assessment of cardiovascular function is an essential element of the hemodynamic evaluation of critically ill patients. Both left and right ventricular dysfunction and arterial system disturbances are frequent in these patients. Since VA coupling ultimately defines de performance and efficiency of the cardiovascular system, the analysis of the interaction between the heart and the arterial system could offer a broader perspective of the hemodynamic disorders associated with common conditions, such as septic shock, heart failure, or right ventricular dysfunction. Moreover, this analysis could also provide valuable information about their pathophysiological mechanisms and may help to determine the best therapeutic strategy to correct them. In this review, we will describe the basic principles of the VA coupling assessment, its limitations, and the most common methods for its estimation at the bedside. Then, we will summarize the current knowledge of the application of VA coupling in critically ill patients and suggest some recommendations for further research.
Keywords: Ventricular-arterial coupling, arterial effective elastance, ventricular elastance, pressure-volume loop, critical care
Introduction
The concept of ventriculo-arterial (VA) coupling has been around for more than 30 years but has recently gained popularity in the critical care setting (1). This concept emerges from the logical notion that the heart and the arterial system are inherently related as they are anatomically and functionally linked structures (2). Coupling refers, therefore, to the pumping action of the heart when connected to the load opposed by the arterial system (3). From this perspective, the concept of VA coupling offers several potential benefits for the analysis of the cardiovascular system. It allows us to define the behavior of the heart and the arterial system as an interconnected system and not as isolated structures, but it also provides a valuable method for assessing cardiovascular performance relating both cardiac and arterial functions (4). The evaluation of the interaction of cardiac contractility with the arterial system would provide a more comprehensive understanding of the cardiovascular function and cardiac energetics, not only on the disease but also in normal physiological states (5). For example, age-dependent increased in vascular stiffening is a known physiological phenomenon and the consequence of the widening of arterial pulse pressure in the elderly (6). This age-related increase in vascular stiffening is associated with a compensatory change in left ventricular (LV) structure at rest, which maintains the normal coupling between the heart and the arterial system but with a decreased cardiovascular reserve during exercise (6,7). Moreover, these changes are further enhanced by comorbidities, such as arterial hypertension, diabetes, and kidney disease (8). On the other hand, the hemodynamic disorders in septic shock are characterized by a loss of peripheral vasomotor tone and cardiac dysfunction. Septic patients exhibit an abnormal ventriculo-arterial interaction mainly because of an impaired LV performance (9), and this situation seems to be related to a poor prognosis (10). Defining how the heart interacts with the arterial system in this condition would allow us to better describe the cardiovascular dysfunction associated with sepsis and, eventually, it may help us to determine the best therapeutic strategy.
In this review, we will first define the basic principles for understanding the VA coupling concept, how to interpret the pressure-volume diagrams for analyzing its main components, and how to estimate it using non-invasive methods. We will also briefly summarize the current evidence on the use of the VA coupling in critically ill patients and try to justify why the assessment of the interaction between the heart and the arterial system may be an essential element to consider in this setting.
General principles of ventriculo-arterial coupling
Assessment of ventriculo-arterial coupling
The evaluation of the interaction between the heart and the arterial system requires first that both ventricular systolic function and systemic arterial properties must be expressed in the same units. The work of Sunagawa and coworkers characterized the ventricular contractile function as the slope end-systolic ventricular pressure-volume relation, and the arterial system properties as the effective arterial elastance (Ea) (11). Both terms have the dimensions of elastance (i.e., the change in pressure for a change in volume). While Ees represents the cardiac contractility, Ea expresses all the extracardiac forces opposing to ventricular ejection or arterial load. Therefore, the interaction between the cardiac contractility and the arterial system can be easily analyzed as the ratio of Ea to Ees (2).
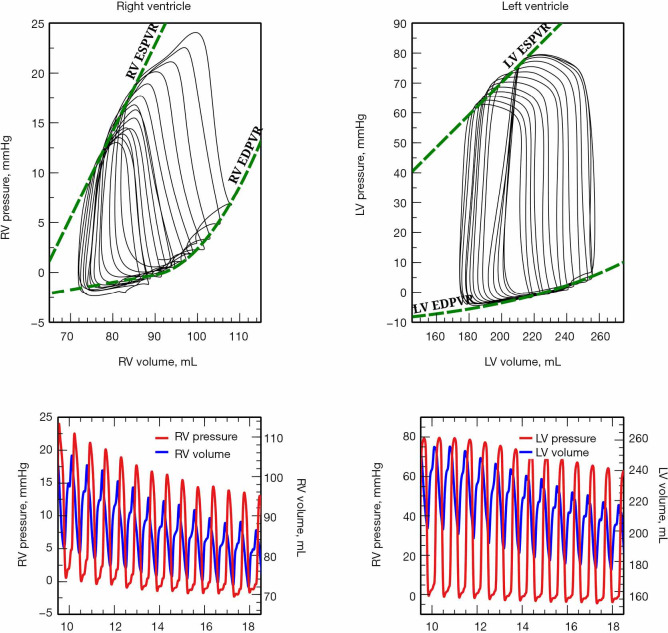
While cardiac function is usually represented in the Frank-Starling curve, the ventricular pressure-volume (PV) relation also provides a complete characterization of cardiac pump performance. This PV diagram, or PV loop, is created combining the simultaneous measurements of the intraventricular pressure and volume obtained during one or several comparable cardiac beats (Figure 1). In this PV loop, it is possible to identify the different stages of the cardiac cycle and derive some essential parameters of the intrinsic ventricular pump properties (12,13). If we were able to obtain the pressure and volume from the right and left ventricles simultaneously, that would allow us to construct right and left PV loops and easily show some of the apparent differences between the two ventricles (Figure 2). For example, in the right ventricle (RV), the pressure generated is significantly lower than in the left ventricle (LV). In the RV, all the ventricular ejection virtually occurs after reaching the systolic pressure because of the low pulmonary afterload, which makes the shape of the RV PV loop less rectangular than on the left side. Moreover, if we now decrease progressively cardiac preload, as during the inflation of a balloon in the inferior vena cava, we can describe different PV loops for various levels of cardiac preload (Figure 3). The straight line connecting the end-systolic PV points or points at maximum elastance (red points in Figure 3) represents the end-systolic PV relationship (ESPVR). Its slope is named the maximal ventricular elastance, or Ees. The ESPVR also has an intercept with the volume axis called V0, which represents the hypothetical unstressed volume of the ventricle. Ees defines the contractile state of the ventricle, and it is relatively insensitive to loading conditions (14). Therefore, when ventricular contractility changes, Ees changes proportionally (Figure 4). The exponential curve fit connecting the end-diastolic pressure-volume points (blue points in Figure 3) also depicts the end-diastolic PV relationship (EDPVR), which characterizes the passive viscoelastic properties of the ventricle in diastole (15).
Figure 1.
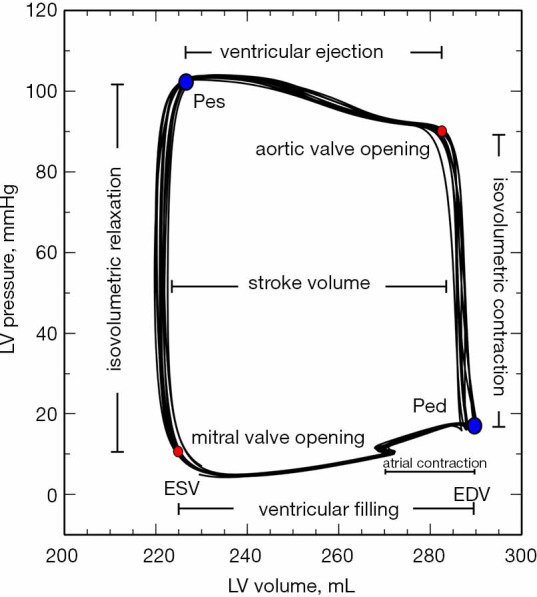
Left ventricular pressure-volume diagram. Pressure-volume loops obtained during an end-expiratory pause. At the lower-left corner (red circle), the mitral valve opens, and the ventricle starts rapidly to fill up during diastole. This stage concludes with the atrial contraction at the end-diastolic pressure (Ped) and volume (EDV) (lower-right corner, blue circle). Then, the mitral valve closes, and the isovolumetric contraction phase starts. When the intraventricular pressure exceeds the aortic pressure, the aortic valve opens (upper-right corner, red circle), and the ventricle ejects decreasing intraventricular volume until end-systolic volume (ESV) and end-systolic pressure (Pes) (upper-left corner, blue circle). When the aortic valve closes, there is an intraventricular pressure decrease without any change in volume (the isovolumetric relaxation phase). When the intraventricular pressure drops below the atrial pressure, the mitral valve opens, and the cardiac cycle starts again.
Figure 2.
Simultaneous right and left ventricular pressure-volume (PV) loops during an inferior vena cava occlusion. Simultaneous recording of right and left ventricular volumes (blue line) and pressures (red line), from which pressure-volume loops were constructed (top, black lines). The dashed green lines represent the end-systolic and end-diastolic pressure-volume relationships (ESPVR and EDPVR), respectively.
Figure 3.
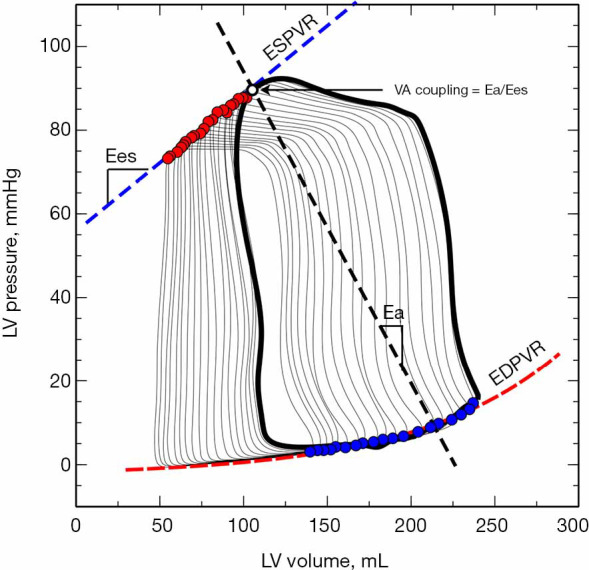
Example of the left ventricular pressure-volume loops during and inferior vena cava occlusion. Left ventricular PV loops during a transient inferior vena cava occlusion (IVC). Red points represent the maximal elastance for each cardiac cycle and the straight line connecting them the end-systolic pressure-volume relationship (ESPVR, blue dashed line). The slope of ESPVR defines the LV end-systolic elastance (Ees), a marker of LV contractility. The exponential curve represented by a dashed red line depicts the end-diastolic pressure-volume relationship (EDVPR), which characterizes the diastolic properties of the ventricle. The dashed black line connecting the end-systolic pressure with the stroke volume (defined by the width of the PV loop: end-diastolic volume minus end-systolic volume) represents the effective arterial elastance (Ea), a net measure of arterial load. The crossing point where the slope of ESPVR (Ees) intersects with the Ea defines the ventriculo-arterial (VA) coupling.
Figure 4.
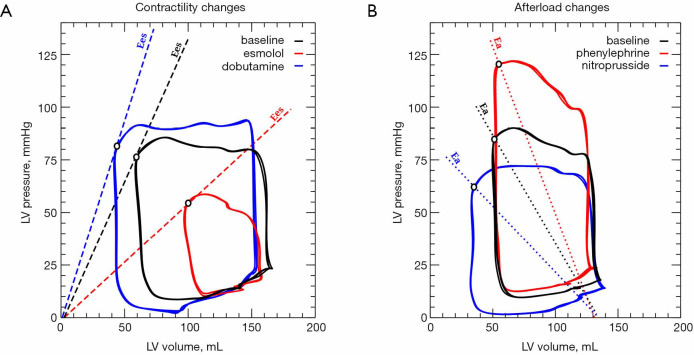
Left ventricular pressure-volume (PV) loops showing the effects of contractility changes on Ees and afterload variations in Ea. (A) Cardiac contractility was increased with dobutamine and decreased with esmolol. The slope of the end-systolic PV relation (ESPVR) defines the end-systolic elastance (Ees, dashed colored lines) and the contractility performance at each stage. (B) Afterload was increased with phenylephrine and decreased with sodium nitroprusside. The lines connecting the end-diastolic volume and end-systolic pressure describes the effective arterial elastance at each stage (Ea, dotted colored lines).
The theoretical framework for ESPVR and EDPVR arises from time-varying elastance model of the ventricle, also named as E(t), which defines the heart as a dynamic chamber that varies its stiffness during the cardiac cycle (16):
| E(t)=P(t)/[V(t)−V0] | [1] |
Where P(t) and V(t) are the instantaneous ventricular pressure and volume, respectively. This model describes the ventricle as an elastic chamber that actively increases its stiffness during systole (with a maximum at the end of the systole) and decreases it with the onset of diastole. E(t) is theoretically insensitive to loading conditions but altered by changes in contractility (17) (Figure 5). The elegance of this model lies in the fact that, if the ventricular PV analysis describes most of the features of the mechanical properties of the ventricle, the ESPVR and EDPVR define its boundaries.
Figure 5.
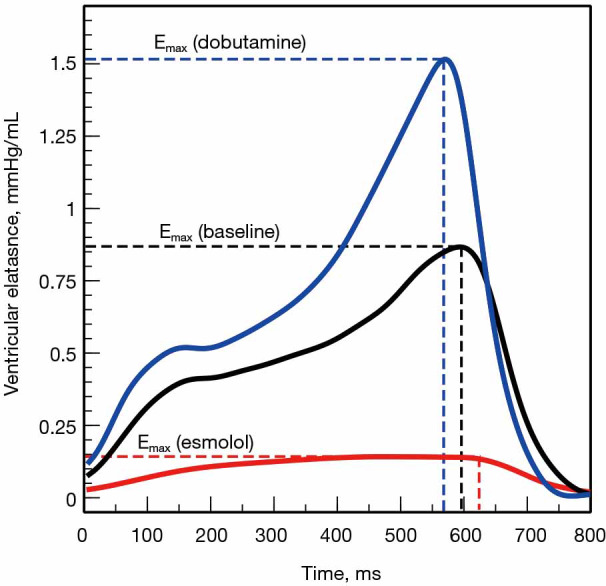
Time-varying elastance model. Time-varying elastance or E(t) curves obtained from the simultaneous measurements of left ventricular pressure and volume in an experimental animal submitted to changes in contractility with dobutamine (blue curve) and esmolol (red curve). Elastance increases during systole until reaching a maximal value (Emax) at the end of the systole. Changes in contractility affect not only the amplitude but also the time to reach Emax: the better the contractility, the higher the magnitude and the sooner the Emax.
While Ees represents the ventricular contractility, Ea is an integrative measure of the arterial system properties (11). Ea, however, should not be considered as a direct measure of the arterial stiffness, but rather a variable integrating all the extracardiac factors impeding ventricular ejection. Ea, therefore, is a net measure of ventricular afterload or arterial load (18), and it was derived from the known 3-element Windkessel model:
| [2] |
Where ts and td are systolic and diastolic periods, respectively, RT the total mean vascular resistance (peripheral resistance plus characteristic impedance), C the net arterial compliance, and τ the diastolic time constant (11,19). However, Ea can be approximated as the slope of the arterial end-systolic pressure (Pes)-stroke volume (SV) relationship on the PV loop (Figure 3) (19). Therefore, Ea can also be calculated as (19):
| [3] |
Changing ventricular afterload will cause Ea to change proportionally. If ventricular afterload increases, the Pes will rise while decreasing SV, and the ventricle will require to generate more pressure before opening the aortic valve (Figure 4).
Being Ea, therefore, a description of the afterload and Ees a measure of the ventricular systolic function, the Ea/Ees ratio becomes then the analytic expression of the interaction between the heart and the systemic vasculature or the VA coupling (Figure 3).
Coupling ventriculo-arterial interaction with cardiac energetics
Ventricular PV loop analysis also provides valuable information about cardiac energetics and insights about how VA coupling defines ventricular performance in physiological and pathophysiological states (20). If we integrate the area delimited by a PV loop, we can calculate the stroke work (SW) produced during a cardiac cycle (light blue area in Figure 6). The SW is the useful fraction of ventricular energy that is delivered to the arterial system for maintaining forward blood flow and providing adequate transport of oxygen and nutrients to peripheral organs. On the other hand, the triangular area defined by the ESPVR, EDPVR and the isovolumetric relaxation phase of the PV loop defines an area called potential energy (PE), which represents the ventricular energy that is dissipated as heat during isovolumetric relaxation (dark blue area in Figure 6). The total mechanical energy generated during systole for a given contractility and loading conditions is, therefore, the sum of the SW and PE, which is referred as the pressure-volume area (PVA). This PVA has been demonstrated to correlate linearly with myocardial oxygen consumption (MVO2) (21). Therefore, if in any pump that uses energy the ratio of the useful work to the total energy consumed defines the efficiency of this pump, the efficiency of the ventricle can be expressed as the ratio between the useful ventricular mechanical work (SW) and the O2 consumed as estimated by PVA:
Figure 6.
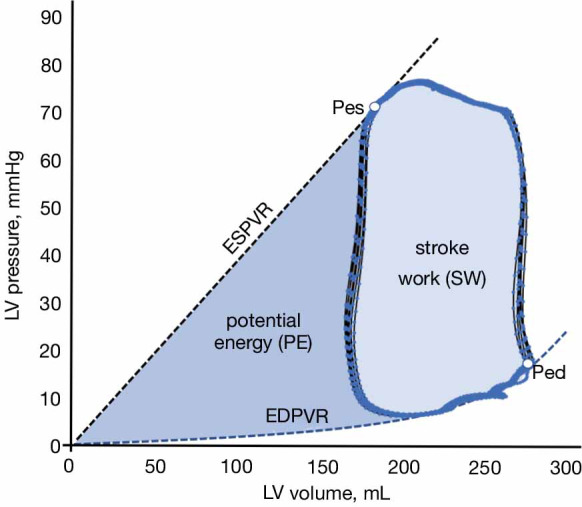
Cardiac energetics analysis from the pressure-volume analysis. The external or stroke work performed by the ventricle is represented by the area within the PV loop (light blue area). The potential energy (PE) represents the elastic energy stored in the ventricle at the end of the systole (dark blue area). The total pressure-volume area (PVA) is defined as the sum of SW and PE. ESPVR and EDPVR indicate the end-systolic and end-diastolic pressure-volume relationships, respectively. Pes and Ped are the end-systolic and end-diastolic pressures.
| [4] |
The main goal of the cardiovascular system is to provide adequate cardiac output to meet the metabolic requirements and generate sufficient blood pressure for allowing normal autoregulation of organ perfusion. Because of the interaction of the arterial system with the heart, a certain arterial pressure and cardiac output that maintain normal physiological functions can be achieved by different combinations of cardiac contractility and loading conditions (22). Theoretical analysis and experimental data have predicted that, under rest conditions, the cardiac function and arterial load are matched to maximize ventricular efficiency rather than SW (2,20). The VA coupling value associated with the optimum ventricular efficiency is usually close to 0.5 (20,23), which implies that the afterload related to the maximum efficiency for a given SW is lower than Ees (Ea =1/2 Ees). In non-physiological or stressful conditions, the ventricle sacrifices its efficiency to maximize SW, which makes equal the Ea to Ees (Ea/Ees =1). Therefore, the optimal combination of cardiac function and vascular system state that delivers the maximal mechanical energy transfer from the ventricle to the arterial tree is found when the ventricle and the arterial tree are matched (11,20,24). In other words, in normal physiological circumstances, the ventricle operates towards a metabolic optimization criterion by minimizing oxygen consumption and enhancing ventricular efficiency. Only when the cardiovascular system is under stressful conditions, the stroke work-maximization criterion prevailed, and both ventricular and arterial elastance are matched. Moreover, if the value of V0 is neglected, a relationship between VA coupling and LV ejection fraction (LVEF) can be mathematically described (25):
| LVEF=Ees/(Ees+Ea) | [5] |
This expression of LVEF as a VA coupling parameter explains why the average LVEF value in healthy people is generally around 65%. As, in physiological conditions, the cardiovascular system works close to maximum LV efficiency, then the VA coupling should be equal to 0.5 (Ea =1, Ees =2), and LVEF =67% (25).
Practically speaking, VA uncoupling occurs when Ea exceeds the value of Ees (Ea/Ees >1), which results in a compromised myocardial SW and efficiency. If the primary mechanism leading to this condition is an Ea increase, then VA coupling will eventually depend on the basal myocardial contractility and its ability to compensate for the afterload mismatch. If the ventricular pump performance is able to cope with the increased afterload, VA coupling will be maintained at expenses of an impaired mechanical efficiency. This scenario characterizes the compensatory increase in Ees in response to the augmented Ea associated with normal aging and arterial hypertension (7). Changes in the structural and biochemical ventricular properties allow to increase Ees and preserve VA coupling despite a progressive increase in Ea, but at expenses of limited exercise capacity. On the other hand, if the first event if a decreased Ees, as in systolic heart failure or cardiogenic shock, the reduced myocardial performance will make the failing heart very sensitive to changes in Ea. Any increase in Ea will significantly impair VA coupling and, any therapy aimed at reduced Ea, such as the use of vasodilators, will substantially improve VA coupling and ventricular efficiency.
Later studies in humans have demonstrated the validity of these assumptions (5,26,27). However, considering that the usual VA coupling in healthy humans ranges from 0.6 to 1.2 (5,23,27,28), it is likely that the healthy cardiovascular system usually works close to both optimal SW and efficiency (28) and defends a constant VA coupling against physiological perturbations (23,29). Whatever the criteria adopted by the cardiovascular system, optimizing metabolic efficiency or maximizing SW, it is clear that VA coupling represents a central determinant of cardiovascular performance and efficiency (5,11) and a powerful predictor of mortality (10,30,31).
Limitations of the VA coupling assessment
Although the assessment of VA coupling with the Ea/Ees framework has proved its usefulness for the understanding of several physiological and pathophysiological processes, it also has a few potential limitations. First, the use of Ea as an index for characterizing the arterial system is a simplification of the aortic input impedance. Aortic impedance represents the best description of the ventricular afterload and defines the pulsatile pressure-flow relationship in the frequency domain (32). Since the evaluation of the VA coupling requires the expression of aortic impedance in a way that allows the direct comparison with the Ees, Ea was derived from the well-known three-element Windkessel model into easily interpretable surrogates. Therefore, Ea is a simplified surrogate of the aortic impedance that uses the three-element Windkessel model for allowing the comparison with Ees and the analysis of VA coupling. However, although this model of the arterial system characterizes many of the main features of the arterial impedance, it fails to reproduce the influence of arterial wave reflections and the arterial wave propagation phenomenon (33). Moreover, since Ea gathers all the components of the Windkessel model into one single number, it does not inform about their relative contribution (34). Therefore, Ea cannot replace the arterial input impedance, and it should only be used as an integrative measure of the arterial load for assessing VA coupling.
Another known limitation of the VA coupling assessment relies on the assumption that the slope of the ESPVR can be approximated by a linear relation that characterizes the myocardial contractile performance (35). This relation was usually described in isolated heart preparations but, in the intact cardiovascular system, the influence of the contractile state and loading conditions could affect the linearity of ESPVR. Therefore, even if this assumption seems to be valid over a broad physiological range of contractile and afterload states, ESPVR may be curvilinear on extreme conditions (36,37). The contractility-dependent curvilinearity of ESPVR seems to be related to changes in the length-dependent activation of the myofilament (36). Furthermore, Ees is also known to be influenced by the geometric and biochemical properties of the ventricle. So, changes in the ventricular mass and geometry, as seen during aging or with the ventricular hypertrophy associated with arterial hypertension, may be related to changes in Ees independently to the ventricular contractility (8). Therefore, although acute alterations in Ees are likely to reflect variations in ventricular contractility, chronic modifications in Ees may represent the combined effects of contractility and changes on the geometric and structural properties of the ventricle (28).
Assessment of ventriculo-arterial coupling at the bedside: single-beat Ees and peripheral Ea estimation
Single-beat methods for estimating Ees
Considering the inherent difficulties related to measuring intraventricular pressure and volumes at the patient’s bedside, different methods for estimating Ees and Ea have been proposed (38). These single-beat methods have been developed to solve the need for cardiac catheterization and the requirement of altering loading conditions for calculating the load-independent slope of ESPVR. These methods assume that the slope of ESPVR can be obtained from a single cardiac cycle without changing loading conditions, as required for the standard calculation of Ees. However, these techniques also have limitations.
The most straightforward approach for estimating Ees is the ratio of Pes to end-systolic volume (ESV) (39,40). This single-beat method simplifies the complexity of the conventional multiple-beat-derived Ees to a single pressure-volume relation, assuming that the V0 intercept is negligible compared with ESV:
| [6] |
However, this single-beat method should be considered as a crude surrogate for Ees, since assuming a constant volume-axis intercept will make the slope of the Pes-ESV relationship load-dependent (41) (Figure 7).
Figure 7.
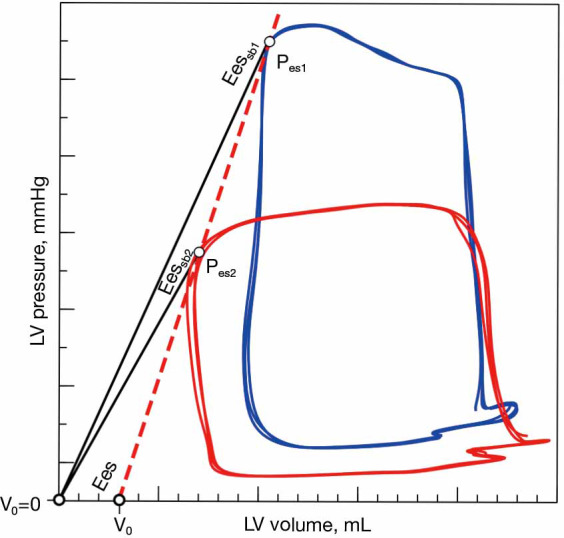
Load-dependency of single-beat Ees estimation. Comparison of end-systolic elastance (Ees) obtained as the slope of end-systolic pressure-volume relation (red dashed line, ESPVR) and single-beat estimation (solid black lines, Eessb1 and Eessb2). When V0 =0 was assumed, the evaluation of Ees by the single-beat method (Eessb = end-systolic pressure or Pes/end-systolic volume) at different levels of afterload results in different slopes, while the actual Ees remains unchanged.
Another well-known method for estimating Ees is the non-invasive single-beat method described by Chen et al. (42), which is based on a previous empirical estimation proposed by Senzaki et al. of a normalized population-averaged elastance [END(avg)]. This time and amplitude-normalized END(avg) was shown to be relatively constant for a wide range of physiological conditions (43). However, in Chen’s method, individual variations from this END(avg) are considered using the information from non-invasive hemodynamic monitoring tools, such as standard echocardiography and peripheral blood pressure measurements.
Ignoring the potential inaccuracies introduced by the noninvasive pressure and echocardiographic measurements (41,44), the main limitation of this method relies on the assumption that END(td) is nearly constant over a wide range of physiological conditions and unaffected by cardiac diseases. However, further experimental and clinical studies have failed to demonstrate the validity of this assumption (41,45,46) and raised doubts about the precision of this estimation (38,45). Despite these drawbacks, Chen’s method is still recommended for the evaluation of Ees in clinical practice (47). Fortunately, further improvements in this technique have been made, although they are still pending validation in critical care patients (46,48).
Peripheral estimation of Ea
The calculation of Ea requires obtaining SV and Pes measurements. While SV can be easily estimated at the bedside using standard hemodynamic monitors or echocardiography, the Pes still requires cardiac catheterization. As arterial pressure measurement is part of the standard monitoring in critically ill patients, several attempts have been made to estimate Ea using peripheral arterial pressure. These estimates are mostly based on mean aortic pressure (11,22), 90% of the systolic aortic pressure (19,49-51), or the aortic dicrotic notch pressure (52-54). In clinical practice, 90% of the radial systolic pressure has been extensively used as a surrogate for Pes (1). However, considering how the arterial wave reflections may influence on peripheral measurements and the constancy of mean arterial pressure (MAP) across the arterial system, the Ea estimate based on MAP/SV seems to offer a better surrogate over different hemodynamic conditions and interchangeably when measured in any peripheral arterial site (55).
VA coupling in critically ill patients
After laying the basis for understanding the basic principles of assessing VA coupling, we can now describe how these concepts could be applied and what their relevance could be in critically ill patients. Bearing in mind that many of the interventions frequently performed in these patients can directly or indirectly affect the arterial load and the ventricular function, questions such as “Is the ventricle ready to face an increase in arterial pressure?” or “Will the arterial system change after the introduction of an inotropic drug?” are essentially related to the evaluation of VA coupling. Physiologically speaking, it is reasonable to argue that the assessment of VA coupling should be of interest in critically ill patients (1). However, because of the need for invasive measurements, the evaluation of VA coupling in critical care has been scarce until the introduction of single-beat methods and non-invasive technologies, such as echocardiography. Nevertheless, although increasing, the number of clinical studies performed in critically ill patients remains still limited.
Below, we summarize some of the current evidence about the use of the VA coupling assessment in the critically ill patient.
VA coupling and pharmacological interventions
One of the most common interventions in critical care is the use of norepinephrine for restoring arterial blood pressure. The complex effects of this vasopressor on contractility and loading conditions, affecting both arterial and venous vessels, make the prediction of its hemodynamic effects a challenge. In this regard, Guinot et al. recently studied 28 patients with arterial hypotension in the postcardiac surgery period and demonstrated that the Ea/Ees ratio predicted the increase in the SV in response to a norepinephrine infusion (56). Although all patients corrected arterial hypotension, only those with an altered VA coupling also increased SV. Therefore, the cardiovascular response to norepinephrine depends on the effects on the vascular system and cardiac contractility, but also on their interactions.
Another typical therapeutic intervention is the use of inotropes. Guarracino et al. evaluated the effect of a single intravenous bolus of levosimendan on VA coupling in 15 ischemic cardiomyopathy patients (57). Ees was calculated as the slope of the line from two Pes/ESV measurements obtained at baseline and after a metaraminol infusion. Pes was estimated as the arterial dicrotic notch pressure, and ESV was measured using transesophageal echocardiography. In these patients, levosimendan decreased Ea and increased Ees, which decreased VA coupling (from 1.76±1 to 0.83±0.2, P=0.002). These authors demonstrated that levosimendan improves VA coupling and cardiovascular performance by enhancing myocardial contractility and reducing afterload.
VA coupling in sepsis and septic shock
Sepsis and septic shock represent a characteristic entity of critical care and a challenge for hemodynamic management. Hemodynamic disorders in sepsis frequently involve all components of the cardiovascular function. They are characterized by a variable degree of cardiac dysfunction and a loss of peripheral vasomotor tone (58). Both phenomena may affect the interaction between myocardial performance and peripheral vascular function. So, it is easy to understand the potential interest in evaluating VA coupling in these conditions. Guarracino et al. compared hemodynamic data from 25 septic shock and 25 non-septic patients upon ICU admission (9). These patients were studied after initial fluid resuscitation but before starting vasoactive therapy. VA coupling was studied by combining the estimation of Ees by the single-beat method and the peripheral Ea. VA decoupling was defined as an Ea/Ees >1.36. Septic shock patients had a lower Ea [1.4 (1.1–1.48) vs. 2.3 (2.02–2.45) mmHg/mL, P<0.0001], but also a proportionally lower Ees [0.7 (0.59–1.1) vs. 2.1 (1.57–2.3) mmHg/mL, P<0.0001]. So, VA coupling was significantly higher in septic patients when compared with non-septic group [1.81 (1.49–2.03) vs. 1.07 (0.95–1.18), P=0.01]. This clinical study was the first demonstrating that septic shock was associated with a VA decoupling, mainly related to an impaired LV performance.
Similarly, in a prospective cohort of 63 elderly patients with septic shock, Yan et at. demonstrated that VA coupling (Ees = Chen’s method and Ea =90% systolic arterial pressure/SV) was independently associated with 28-day mortality (10). Despite similar levels of cardiac output and MAP, VA coupling was lower in survivors than in non-survivors (1.61±0.56 vs. 2.09±055, P=0.001), mostly because of a higher Ees.
Guarracino et al. have recently performed a comprehensive hemodynamic evaluation, including VA coupling assessment, in 50 patients at the early stages of sepsis and after sequential hemodynamic interventions (volume expansion, norepinephrine, and dobutamine infusion) (59). They found that VA decoupling was again present during early sepsis, confirming their previous results, with no significant differences between survivors and non-survivors. Volume administration restores VA coupling, while norepinephrine worsened it because of an increase in Ea without a concomitant rise in Ees. Dobutamine has the opposite effect than norepinephrine, increasing Ees without affecting Ea, which resulted in an improvement in VA coupling. This study not only corroborates the relevance of VA coupling on septic patients but also highlights how the usual therapy could affect VA interactions.
Regarding the use of norepinephrine in septic patients, we have recently confirmed the decrease in LV efficiency using a different methodology (60). Using instantaneous carotid pressure and aortic blood flow analysis, we have demonstrated that the increase in norepinephrine was associated with an augmented systolic LV workload and worsened LV efficiency. These effects were mediated by a higher pulse wave velocity and a more significant influence of arterial wave reflections during ventricular ejection.
Another new field of sepsis research is the heart rate control using beta-blockers. Although there is some evidence that such intervention may improve patient outcomes (61), it is still a matter of debate and controversy (62). One of the physiological mechanisms proposed behind this intervention is a reduction in Ea and the potential improvement in VA coupling. Morelli et al. demonstrated that the rate reduction with esmolol resulted in a significant decrease in Ea while improving SV (63). Unfortunately, these authors did not measure Ees, which precludes any definitive conclusion about the eventual impact of this therapy on VA coupling.
Effects of anesthesia on VA coupling
Although the studies focused on the impact of anesthetics on VA coupling have been mainly performed in the operating room, they could have relevant implications in the management of critical care patients at the time of choosing sedative drugs. Pittarello et al. prospectively evaluated the effects of a slow-bolus of remifentanil on VA coupling and LV mechanical efficiency in 14 patients undergoing elective coronary artery bypass graft (64). Using transesophageal echocardiography for ventricular volumes and radial dicrotic notch pressure for estimating Pes, they calculated Ees by creating two end-systolic PV points (at baseline and after an afterload increase with a metaraminol infusion). They found that a slow-bolus of remifentanil decreased both Ea and the Ees while keeping constant VA coupling and close to the values reported for healthy adults (0.64±0.26 before and 0.68±0.19 after remifentanil, P: N.S). These authors also suggested that, even if a slow-bolus of remifentanil decreases afterload, higher doses or a rapid infusion of this drug could be associated with VA uncoupling due to a marked decrease in myocardial contractility.
Right VA coupling
The application of the VA coupling concept on the interactions between the RV and pulmonary circulation relies on the validity of the same assumptions made on the left side. In the early eighties, Piene was the first to describe the interaction between the RV and the pulmonary load (65). He proposed a new approach that translated pulmonary vascular input impedance into the time domain to be related to ventricular performance indices. This innovative approach set the preliminary basis for the development of new strategies for the analysis of the right VA coupling.
Although the study of the interaction between the RV and the pulmonary circulation has gained significant interest in chronic pulmonary hypertension (66,67), the right VA uncoupling is not an unusual situation in critical care. This right VA uncoupling may be originated by a primary problem on RV systolic function or due to a pulmonary load mismatch. Primary RV dysfunction is a common situation on septic patients, RV myocardial infarct, or cardiac surgery (68). On the other hand, increased pulmonary load has been typically associated with acute respiratory distress syndrome (ARDS), pulmonary embolism, or even sepsis (69,70). The balance between RV function and pulmonary afterload will eventually determine the right VA coupling and efficiency. In this regard, pulmonary vascular dysfunction (PVD) associated with ARDS may represent a paradigm of how increased pulmonary afterload may define the evolution of RV dysfunction. This PVD refers to a complex combination of pulmonary vascular changes associated with ARDS, involving modifications in arterial vascular mechanics and increased pulmonary wave reflections (70). These changes increase RV afterload and eventually may affect RV ejection performance. The compensatory increase in RV contractility required to overcome the increased RV afterload would probably maintain VA coupling but at expenses of sacrificing RV efficiency. When the right VA coupling is lost, then the transfer of the mechanical energy from the RV to the pulmonary circulation becomes compromised (71). Noteworthy, strategies aimed to prevent or minimize the impact of PVD on ARDS patients are not only pharmacological. Lung protective ventilation or even prone positioning may have a role in the unloading of the RV and improving right VA coupling (70).
Future directions
Bearing in mind the potential usefulness of VA coupling, the authors of this review consider the following areas of particular interest for future research in the critical care setting:
Reliable methods to evaluate VA coupling. In this way, we need accurate and precise methods for assessing VA coupling or, preferably, Ea and Ees independently. These methods should be ideally developed using routine hemodynamic monitoring tools. Preferably, they should also allow the continuous tracking of VA coupling changes. Although non-invasive assessment of right VA coupling still seems a far shore (67,72,73), considering the significant impact of RV dysfunction and acute pulmonary hypertension in critically ill patients, the interest in this field should be encouraged.
Description of the VA coupling in relevant situations and interventions. As stated above, the current knowledge about the VA coupling in critically ill patients is still scarce. More information is needed about the potential role of VA coupling in severely ill patients and how our therapeutic interventions could affect it.
Closely related to the previous question, we should also answer the question: is it possible to improve the patient’s outcome with interventions focused on optimizing VA coupling? Although we are still far from answer this question, we should first consider that such optimization is not guaranteed or even defined in critically ill patients. Furthermore, there is still little information about how to proceed or if an intervention aimed to optimize VA coupling will improve prognosis. However, determining the role of VA coupling as a potential therapeutic target, and the best way to achieve an optimal level of coupling seems to be a reasonable priority.
Conclusions
VA coupling characterizes the interaction between the myocardial contractile function and the load opposed by the arterial circulation. This interaction defines the cardiovascular performance and efficiency and can be analytically defined by relating ventricular and arterial elastances. Although the calculation of VA coupling requires invasive measures of ventricular pressure and volumes, non-invasive single-beat methods for Ees and peripheral estimates of Ea have been developed. These non-invasive surrogates, even if limited, have already demonstrated their potential application in critically ill patients.
Although VA coupling is a well-established and valuable tool for understanding how the cardiovascular system works, its acceptance into the intensive care culture is still taking its first but promising steps.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors want to thank Cristhian Potes and Kevin Moses from Edwards Lifesciences for providing the data for constructing the right and left pressure-volume loops.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Glenn Hernández and Guo-wei Tu) for the series “Hemodynamic Monitoring in Critically Ill Patients” published in Annals of Translational Medicine. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm.2020.04.10). The series “Hemodynamic Monitoring in Critically Ill Patients” was commissioned by the editorial office without any funding or sponsorship. MIMG reports personal fees from Edwards Lifesciencies, outside the submitted work. AS has no other conflicts to disclose.
References
- 1.Guarracino F, Baldassarri R, Pinsky MR. Ventriculo-arterial decoupling in acutely altered hemodynamic states. Crit Care 2013;17:213. 10.1186/cc12522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sunagawa K, Sagawa K, Maughan WL. Ventricular interaction with the loading system. Ann Biomed Eng 1984;12:163-89. 10.1007/BF02584229 [DOI] [PubMed] [Google Scholar]
- 3.Yin FCP, Avolio AP. Ventricular/vascular coupling: clinical, physiological, and engineering aspects. New York: Springer-Verlag; 1987. [Google Scholar]
- 4.Kass DA, Kelly RP. Ventriculo-arterial coupling: concepts, assumptions, and applications. Ann Biomed Eng 1992;20:41-62. 10.1007/BF02368505 [DOI] [PubMed] [Google Scholar]
- 5.Starling MR. Left ventricular-arterial coupling relations in the normal human heart. Am Heart J 1993;125:1659-66. 10.1016/0002-8703(93)90756-Y [DOI] [PubMed] [Google Scholar]
- 6.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension 2005;46:185-93. 10.1161/01.HYP.0000168053.34306.d4 [DOI] [PubMed] [Google Scholar]
- 7.Chantler PD, Lakatta EG. Arterial-ventricular coupling with aging and disease. Front Physiol 2012;3:90. 10.3389/fphys.2012.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Cardiol Clin 2011;29:447-59. 10.1016/j.ccl.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 9.Guarracino F, Ferro B, Morelli A, et al. Ventriculoarterial decoupling in human septic shock. Crit Care 2014;18:R80. 10.1186/cc13842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan J, Zhou X, Hu B, et al. Prognostic value of left ventricular-arterial coupling in elderly patients with septic shock. J Crit Care 2017;42:289-93. 10.1016/j.jcrc.2017.08.017 [DOI] [PubMed] [Google Scholar]
- 11.Sunagawa K, Maughan WL, Burkhoff D, et al. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 1983;245:H773-80. [DOI] [PubMed] [Google Scholar]
- 12.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 2005;289:H501-12. 10.1152/ajpheart.00138.2005 [DOI] [PubMed] [Google Scholar]
- 13.Bastos MB, Burkhoff D, Maly J, et al. Invasive left ventricle pressure-volume analysis: overview and practical clinical implications. Eur Heart J 2020;41:1286-97. 10.1093/eurheartj/ehz552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kass DA, Maughan WL. From 'Emax' to pressure-volume relations: a broader view. Circulation 1988;77:1203-12. 10.1161/01.CIR.77.6.1203 [DOI] [PubMed] [Google Scholar]
- 15.Mirsky I. Assessment of diastolic function: suggested methods and future considerations. Circulation 1984;69:836-41. 10.1161/01.CIR.69.4.836 [DOI] [PubMed] [Google Scholar]
- 16.Suga H, Sagawa K. Mathematical interrelationship between instantaneous ventricular pressure-volume ratio and myocardial force-velocity relation. Ann Biomed Eng 1972;1:160-81. 10.1007/BF02584205 [DOI] [PubMed] [Google Scholar]
- 17.Maughan WL, Sunagawa K, Burkhoff D, et al. Effect of arterial impedance changes on the end-systolic pressure-volume relation. Circ Res 1984;54:595-602. 10.1161/01.RES.54.5.595 [DOI] [PubMed] [Google Scholar]
- 18.Monge Garcia MI, Saludes Orduna P, Cecconi M. Understanding arterial load. Intensive Care Med 2016;42:1625-7. 10.1007/s00134-016-4212-z [DOI] [PubMed] [Google Scholar]
- 19.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation 1992;86:513-21. 10.1161/01.CIR.86.2.513 [DOI] [PubMed] [Google Scholar]
- 20.Burkhoff D, Sagawa K. Ventricular efficiency predicted by an analytical model. Am J Physiol 1986;250:R1021-7. [DOI] [PubMed] [Google Scholar]
- 21.Suga H, Hayashi T, Shirahata M. Ventricular systolic pressure-volume area as predictor of cardiac oxygen consumption. Am J Physiol 1981;240:H39-44. [DOI] [PubMed] [Google Scholar]
- 22.Sunagawa K, Maughan WL, Sagawa K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res 1985;56:586-95. 10.1161/01.RES.56.4.586 [DOI] [PubMed] [Google Scholar]
- 23.De Tombe PP, Jones S, Burkhoff D, et al. Ventricular stroke work and efficiency both remain nearly optimal despite altered vascular loading. Am J Physiol 1993;264:H1817-24. [DOI] [PubMed] [Google Scholar]
- 24.Elzinga G, Westerhof N. Matching between ventricle and arterial load. An evolutionary process. Circ Res 1991;68:1495-500. 10.1161/01.RES.68.6.1495 [DOI] [PubMed] [Google Scholar]
- 25.Robotham JL, Takata M, Berman M, et al. Ejection fraction revisited. Anesthesiology 1991;74:172-83. 10.1097/00000542-199101000-00026 [DOI] [PubMed] [Google Scholar]
- 26.Asanoi H, Sasayama S, Kameyama T. Ventriculoarterial coupling in normal and failing heart in humans. Circ Res 1989;65:483-93. 10.1161/01.RES.65.2.483 [DOI] [PubMed] [Google Scholar]
- 27.Sasayama S, Asanoi H. Coupling between the heart and arterial system in heart failure. Am J Med 1991;90:14S-8S. 10.1016/0002-9343(91)90267-2 [DOI] [PubMed] [Google Scholar]
- 28.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol (1985) 2008;105:1342-51. 10.1152/japplphysiol.90600.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubota T, Alexander J, Jr, Itaya R, et al. Dynamic effects of carotid sinus baroreflex on ventriculoarterial coupling studied in anesthetized dogs. Circ Res 1992;70:1044-53. 10.1161/01.RES.70.5.1044 [DOI] [PubMed] [Google Scholar]
- 30.Ky B, French B, May Khan A, et al. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol 2013;62:1165-72. 10.1016/j.jacc.2013.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonini-Canterin F, Enache R, Popescu BA, et al. Prognostic value of ventricular-arterial coupling and B-type natriuretic peptide in patients after myocardial infarction: a five-year follow-up study. J Am Soc Echocardiogr 2009;22:1239-45. 10.1016/j.echo.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 32.Milnor WR. Arterial impedance as ventricular afterload. Circ Res 1975;36:565-70. 10.1161/01.RES.36.5.565 [DOI] [PubMed] [Google Scholar]
- 33.Burkhoff D, Alexander J, Jr, Schipke J. Assessment of Windkessel as a model of aortic input impedance. Am J Physiol 1988;255:H742-53. [DOI] [PubMed] [Google Scholar]
- 34.Segers P, Stergiopulos N, Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol 2002;282:H1041-6. 10.1152/ajpheart.00764.2001 [DOI] [PubMed] [Google Scholar]
- 35.Sagawa K, Suga H, Shoukas AA, et al. End-systolic pressure/volume ratio: a new index of ventricular contractility. Am J Cardiol 1977;40:748-53. 10.1016/0002-9149(77)90192-8 [DOI] [PubMed] [Google Scholar]
- 36.Kass DA, Beyar R, Lankford E, et al. Influence of contractile state on curvilinearity of in situ end-systolic pressure-volume relations. Circulation 1989;79:167-78. 10.1161/01.CIR.79.1.167 [DOI] [PubMed] [Google Scholar]
- 37.van der Velde ET, Burkhoff D, Steendijk P, et al. Nonlinearity and load sensitivity of end-systolic pressure-volume relation of canine left ventricle in vivo. Circulation 1991;83:315-27. 10.1161/01.CIR.83.1.315 [DOI] [PubMed] [Google Scholar]
- 38.Wo N, Rajagopal V, Cheung MMH, et al. Assessment of single beat end-systolic elastance methods for quantifying ventricular contractility. Heart Vessels 2019;34:716-23. 10.1007/s00380-018-1303-5 [DOI] [PubMed] [Google Scholar]
- 39.Bombardini T, Costantino MF, Sicari R, et al. End-systolic elastance and ventricular-arterial coupling reserve predict cardiac events in patients with negative stress echocardiography. Biomed Res Int 2013;2013:235194. [DOI] [PMC free article] [PubMed]
- 40.Maurer MS, Sackner-Bernstein JD, El-Khoury Rumbarger L, et al. Mechanisms underlying improvements in ejection fraction with carvedilol in heart failure. Circ Heart Fail 2009;2:189-96. 10.1161/CIRCHEARTFAILURE.108.806240 [DOI] [PubMed] [Google Scholar]
- 41.Yotti R, Bermejo J, Benito Y, et al. Validation of noninvasive indices of global systolic function in patients with normal and abnormal loading conditions: a simultaneous echocardiography pressure-volume catheterization study. Circ Cardiovasc Imaging 2014;7:164-72. 10.1161/CIRCIMAGING.113.000722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 2001;38:2028-34. 10.1016/S0735-1097(01)01651-5 [DOI] [PubMed] [Google Scholar]
- 43.Senzaki H, Chen CH, Kass DA. Single-beat estimation of end-systolic pressure-volume relation in humans. A new method with the potential for noninvasive application. Circulation 1996;94:2497-506. 10.1161/01.CIR.94.10.2497 [DOI] [PubMed] [Google Scholar]
- 44.Pagoulatou SZ, Stergiopulos N. Estimating Left Ventricular Elastance from Aortic Flow Waveform, Ventricular Ejection Fraction, and Brachial Pressure: An In Silico Study. Ann Biomed Eng 2018;46:1722-35. 10.1007/s10439-018-2072-0 [DOI] [PubMed] [Google Scholar]
- 45.Kjorstad KE, Korvald C, Myrmel T. Pressure-volume-based single-beat estimations cannot predict left ventricular contractility in vivo. Am J Physiol Heart Circ Physiol 2002;282:H1739-50. 10.1152/ajpheart.00638.2001 [DOI] [PubMed] [Google Scholar]
- 46.Shishido T, Hayashi K, Shigemi K, et al. Single-beat estimation of end-systolic elastance using bilinearly approximated time-varying elastance curve. Circulation 2000;102:1983-9. 10.1161/01.CIR.102.16.1983 [DOI] [PubMed] [Google Scholar]
- 47.Ikonomidis I, Aboyans V, Blacher J, et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail 2019;21:402-24. 10.1002/ejhf.1436 [DOI] [PubMed] [Google Scholar]
- 48.Shigemi K, Fuke S, Une D, et al. Physiological insights of recent clinical diagnostic and therapeutic technologies for cardiovascular diseases. J Physiol Sci 2017;67:655-72. 10.1007/s12576-017-0554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pauca AL, Wallenhaupt SL, Kon ND, et al. Does radial artery pressure accurately reflect aortic pressure? Chest 1992;102:1193-8. 10.1378/chest.102.4.1193 [DOI] [PubMed] [Google Scholar]
- 50.Chen CH, Nakayama M, Talbot M, et al. Verapamil acutely reduces ventricular-vascular stiffening and improves aerobic exercise performance in elderly individuals. J Am Coll Cardiol 1999;33:1602-9. 10.1016/S0735-1097(99)00052-2 [DOI] [PubMed] [Google Scholar]
- 51.Chemla D, Antony I, Lecarpentier Y, et al. Contribution of systemic vascular resistance and total arterial compliance to effective arterial elastance in humans. Am J Physiol Heart Circ Physiol 2003;285:H614-20. 10.1152/ajpheart.00823.2002 [DOI] [PubMed] [Google Scholar]
- 52.Colin P, Slama M, Vahanian A, et al. Hemodynamic correlates of effective arterial elastance in mitral stenosis before and after balloon valvotomy. J Appl Physiol (1985) 1997;83:1083-9. 10.1152/jappl.1997.83.4.1083 [DOI] [PubMed] [Google Scholar]
- 53.Dahlgren G, Veintemilla F, Settergren G, et al. Left ventricular end-systolic pressure estimated from measurements in a peripheral artery. J Cardiothorac Vasc Anesth 1991;5:551-3. 10.1016/1053-0770(91)90004-D [DOI] [PubMed] [Google Scholar]
- 54.Grossman W, Braunwald E, Mann T, et al. Contractile state of the left ventricle in man as evaluated from end-systolic pressure-volume relations. Circulation 1977;56:845-52. 10.1161/01.CIR.56.5.845 [DOI] [PubMed] [Google Scholar]
- 55.Monge Garcia MI, Jian Z, Settels JJ, et al. Reliability of effective arterial elastance using peripheral arterial pressure as surrogate for left ventricular end-systolic pressure. J Clin Monit Comput 2019;33:803-13. 10.1007/s10877-018-0236-y [DOI] [PubMed] [Google Scholar]
- 56.Guinot PG, Longrois D, Kamel S, et al. Ventriculo-Arterial Coupling Analysis Predicts the Hemodynamic Response to Norepinephrine in Hypotensive Postoperative Patients: A Prospective Observational Study. Crit Care Med 2018;46:e17-25. 10.1097/CCM.0000000000002772 [DOI] [PubMed] [Google Scholar]
- 57.Guarracino F, Cariello C, Danella A, et al. Effect of levosimendan on ventriculo-arterial coupling in patients with ischemic cardiomyopathy. Acta Anaesthesiol Scand 2007;51:1217-24. 10.1111/j.1399-6576.2007.01428.x [DOI] [PubMed] [Google Scholar]
- 58.Vieillard-Baron A, Cecconi M. Understanding cardiac failure in sepsis. Intensive Care Med 2014;40:1560-3. 10.1007/s00134-014-3367-8 [DOI] [PubMed] [Google Scholar]
- 59.Guarracino F, Bertini P, Pinsky MR. Cardiovascular determinants of resuscitation from sepsis and septic shock. Crit Care 2019;23:118. 10.1186/s13054-019-2414-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monge Garcia MI, Santos A, Diez Del Corral B, et al. Noradrenaline modifies arterial reflection phenomena and left ventricular efficiency in septic shock patients: A prospective observational study. J Crit Care 2018;47:280-6. 10.1016/j.jcrc.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 61.Morelli A, Ertmer C, Westphal M, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA 2013;310:1683-91. 10.1001/jama.2013.278477 [DOI] [PubMed] [Google Scholar]
- 62.Sanfilippo F, Santonocito C, Morelli A, et al. Beta-blocker use in severe sepsis and septic shock: a systematic review. Curr Med Res Opin 2015;31:1817-25. 10.1185/03007995.2015.1062357 [DOI] [PubMed] [Google Scholar]
- 63.Morelli A, Singer M, Ranieri VM, et al. Heart rate reduction with esmolol is associated with improved arterial elastance in patients with septic shock: a prospective observational study. Intensive Care Med 2016;42:1528-34. 10.1007/s00134-016-4351-2 [DOI] [PubMed] [Google Scholar]
- 64.Pittarello D, Bonato R, Marcassa A, et al. Ventriculo-arterial coupling and mechanical efficiency with remifentanil in patients with coronary artery disease. Acta Anaesthesiol Scand 2004;48:61-8. 10.1111/j.1399-6576.2004.00274.x [DOI] [PubMed] [Google Scholar]
- 65.Piene H. Interaction between the rigt heart ventricle and its arterial load: a quantitative solution. Am J Physiol 1980;238:H932-7. [DOI] [PubMed] [Google Scholar]
- 66.Tello K, Dalmer A, Axmann J, et al. Reserve of Right Ventricular-Arterial Coupling in the Setting of Chronic Overload. Circ Heart Fail 2019;12:e005512. 10.1161/CIRCHEARTFAILURE.118.005512 [DOI] [PubMed] [Google Scholar]
- 67.Sanz J, Garcia-Alvarez A, Fernandez-Friera L, et al. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart 2012;98:238-43. 10.1136/heartjnl-2011-300462 [DOI] [PubMed] [Google Scholar]
- 68.Grignola JC, Domingo E. Acute Right Ventricular Dysfunction in Intensive Care Unit. Biomed Res Int 2017;2017:8217105. [DOI] [PMC free article] [PubMed]
- 69.Vallabhajosyula S, Geske JB, Kumar M, et al. Doppler-defined pulmonary hypertension in sepsis and septic shock. J Crit Care 2019;50:201-6. 10.1016/j.jcrc.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 70.Sipmann FS, Santos A, Tusman G. Heart-lung interactions in acute respiratory distress syndrome: pathophysiology, detection and management strategies. Ann Transl Med 2018;6:27. 10.21037/atm.2017.12.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santos A, Lucchetta L, Monge-Garcia MI, et al. The Open Lung Approach Improves Pulmonary Vascular Mechanics in an Experimental Model of Acute Respiratory Distress Syndrome. Crit Care Med 2017;45:e298-305. 10.1097/CCM.0000000000002082 [DOI] [PubMed] [Google Scholar]
- 72.Inuzuka R, Hsu S, Tedford RJ, et al. Single-Beat Estimation of Right Ventricular Contractility and Its Coupling to Pulmonary Arterial Load in Patients With Pulmonary Hypertension. J Am Heart Assoc 2018. doi: . 10.1161/JAHA.117.007929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bellofiore A, Vanderpool R, Brewis MJ, et al. A novel single-beat approach to assess right ventricular systolic function. J Appl Physiol (1985) 2018;124:283-90. 10.1152/japplphysiol.00258.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as