Abstract
Background
Cerebral ischemia (CI) can lead to ischemic stroke. The most effective therapy for cerebral ischemic stroke is the early restoration of blood reperfusion. However, reperfusion after CI can result in cerebral ischemia reperfusion (CI/R) injury. This study aimed to detect the effect of eriocitrin on cerebral I/R injury and investigate the underlying mechanism.
Methods
Seventy male Sprague-Dawley (SD) rats were randomly divided into 5 groups: the control group, the cerebral I/R group, the I/R + eriocitrin 8 mg/kg group, the I/R + eriocitrin 16 mg/kg group, and the I/R + eriocitrin 32 mg/kg group. Different doses of eriocitrin or 0.5% carboxymethyl cellulose sodium were administrated to the rats once daily for 7 days before middle cerebral artery occlusion (MCAO). PCR staining was performed to observe cerebral infarction. Hematoxylin and eosin (H&E) staining was carried out to observe the damage to the brain tissue. Terminal-deoxynucleotidyl transferase mediated nick end labeling (TUNEL) was used to detect apoptosis. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect the relative mRNA levels of related molecules. Western blot was used to detect the expression of related proteins. The detection kits were used to detect superoxide dismutase (SOD) and lactic dehydrogenase (LDH) activity, and malondialdehyde (MDA) content respectively. Enzyme-linked immunosorbent assay (ELISA) was used to detect TNF-radiation, interleukin-6 (IL-6), and interleukin-10 (IL-10).
Results
The results showed that Eriocitrin significantly reduced the cerebral infarct volume, cerebral water content, and cerebral indexes. Eriocitrin treatment alleviated pathological injury, promoted cell proliferation, and inhibited cell apoptosis. Eriocitrin upregulated SOD activity and downregulated MDA and LDH content. Eriocitrin also effectively decreased the levels of IL-6 and tumor necrosis factor-α (TNF-α), but increased the content of IL-10 in serum and brain tissues. Furthermore, Eriocitrin increased the phosphorylation of nuclear factor erythroid 2-related factor (Nrf2), as well as the expressions of heme-oxygenase-1 (HO-1) and quinine oxidoreductase 1 (NQO1). Moreover, Eriocitrin decreased the phosphorylation of nuclear factor-κB (NF-κB) p65.
Conclusions
Our results indicated that Eriocitrin attenuated oxidative injury and inflammatory response in rats with CI/R via the Nrf2/HO-1/NQO1/NF-κB signaling pathway.
Keywords: Eriocitrin, cerebral ischemia reperfusion (CI/R), oxidative injury, inflammatory response, nuclear factor erythroid 2-related factor pathway (Nrf2 pathway)
Introduction
Cerebral ischemia (CI) occurs when there is a sudden interruption to the cerebral blood flow. It can result in ischemic stroke with dysfunction in the brain as well as other parts of body controlled by the cerebral ischemic regions (1). With a high incidence, prevalence, and mortality, ischemic stroke continues to be a leading cause of chronic disability (2). The swift restoration of blood reperfusion is the most effective way of treating cerebral ischemic stroke; however, cerebral ischemia reperfusion (CI/R) can also inflict severe injury on the brain (3). Therefore, the development of treatment for CI/R injury has become extremely important in ischemic stroke therapy.
There is growing evidence that some fruits are good for your health. As one of the bioactive molecules found in lemon, the flavonoid eriocitrin (eriodictyol 7-rutinoside) has been shown to have antioxidant properties (4). Previous reports have indicated that eriocitrin can suppress exercise-induced oxidative damage in rat liver (4) and ameliorates diet-induced hepatic steatosis (5). More impressively, eriocitrin suppressed the proliferation in human hepatocellular carcinoma cells (6). After CI/R injury, increased oxidative stress and inflammation play central roles in triggering cellular and subsequent tissue damage (7). Therefore, we speculated that eriocitrin could alleviate oxidative injury nuclear factor erythroid 2-related factor (Nrf2), an inducible transcription factor, has been shown to be associated with endogenous antioxidant systems (8). As one of the most important mechanisms for protecting the brain from oxidative stress, it has served as a target for stroke treatment (9). Pilose antler peptide (10), uric acid (11), and protodioscin (12) have been reported to exert protective effects on CI/R injury through the Nrf2 pathway. Therefore, we predicted that eriocitrin could protect the brain from CI/R injury via the Nrf2 pathway.
In this study, eriocitrin was used to treat rats with CI/R injury. Eriocitrin was shown to alleviate oxidative injury and inflammatory response in rats with CI/R through the Nrf2/heme-oxygenase-1 (HO-1)/quinine oxidoreductase 1 (NQO1)/nuclear factor-κB (NF-κB) signaling pathway.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4258).
Methods
Reagents
Eriocitrin powder (purity ≥98%) was purchased from Tianjin Shilan Technology Co., (China). It was dissolved in 0.5% carboxymethyl cellulose sodium before use. interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and interleukin-10 (IL-10) enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D system Inc (MN, USA). superoxide dismutase (SOD), malondialdehyde (MDA), and lactic dehydrogenase (LDH) kits were purchased from Solarbio (Beijing, China). All of the antibodies (Caspase-3, Caspase-9, Nrf2, p-Nrf2, NQO-1, HO-1, NF-κB p65, p-p65) used were purchased from Abcam (Cambridge, UK).
Animals
Seventy male Sprague-Dawley rats (weight: 200–220 g) were purchased from Junke Bioengineering co. LTD (Nanjing, China). The animals were kept in an air-conditioned room at 23±1 °C with 45–55% humidity, under a 12/12 h light/dark cycle. The rats were kept for 7 days and given free access to water and a standard laboratory feed before the experiments. All experiments involving animals were performed according to the National Institutes of Health (NIH) guidelines for the Care and Use of Animals. All experiments were approved by the ethics committee of West China Hospital (No. 2018-29).
Middle cerebral artery occlusion (MCAO)/reperfusion model
The MCAO model was performed as previously described, with slight modification (13). In brief, the rats were anesthetized with 350 mg/kg chloral hydrate. Then, the internal carotid artery (ICA), right common carotid artery, and external carotid artery (ECA) of each rat were carefully dissected. A 4-0 nylon filament with a rounded tip was inserted into the ICA through the ECA stump and pushed gently towards the middle cerebral artery (MCA) to achieve MCAO. After MCAO for 2 h, the filament was gently removed to initiate reperfusion and the skin was sutured. The rats in control group underwent the same surgical procedure except for the occlusion/reperfusion. The rectal temperature of each rat was maintained at 37±0.5 °C with a heating pad from the start of surgery.
Experimental design
The Seventy rats were randomly divided into 5 groups: the control group, the model group (I/R), and 3 dose groups (the I/R + eriocitrin 8 mg/kg group, the 16 mg/kg group, and the 32 mg/kg group). Different doses of eriocitrin diluted with 0.5% carboxymethyl cellulose sodium were orally administrated to the rats once daily for 7 days before the MCAO. The control group and cerebral I/R group rats were fed the same volume of carboxymethyl cellulose sodium. After 1 h of the final drug treatment, the MCAO surgery was started.
Triphenyltetrazolium chloride (TTC) staining
After 24 h of reperfusion, the rats were sacrificed. Their brains were promptly removed and maintained at −20 °C for 2 min. Then, the brains were cut into 2 mm coronal sections, which were stained with 2% 2, 3, 5-TTC for 30 min at 37 °C, and then fixed with 4% paraformaldehyde overnight. The sections were observed using a high-resolution digital camera. Image processing software (Image-Pro Plus, Version 6.0; MEDIA CYBERNETICS, USA) was used to quantify the rate of cerebral infarction.
Measurements of cerebral edema and cerebral indexes
After 24 h of reperfusion, the brains were swiftly removed. The brain samples and bodies of the rats were weighed immediately and recorded as brain wet weight and body weight. The cerebral index was expressed as brain wet weight/body weight. Then, the brain samples were dried at 100 °C for 24 h and the dry weight was recorded. The cerebral edema was evaluated according to the percentage of water content, using the following formula: cerebral water content = [(brain wet weight − dry weight)/(brain wet weight)] × 100%.
Hematoxylin and eosin (H&E) staining
The brain tissues of the rats were harvested, fixed in 4% paraformaldehyde for 20 h, and then embedded with paraffin. Following that, the brain tissues were cut into 4 µm-thick sections. Subsequently, H&E staining was performed according to the manufacturer’s instructions. Finally, the results were observed using an optical microscope (OLYMPUS, Japan).
Terminal-deoxynucleotidyl transferase mediated nick end labeling (TUNEL) assay
After 24 h of reperfusion, the rats were anesthetized and transcardially perfused with cold saline followed by 10% buffered formalin phosphate. Paraffinized brain sections were used to investigate the histological injury by TUNEL. The TUNEL experiment was carried out as previously described (14).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using an RNA Extraction kit (Qiagen, Germany) according to the manufacturer’s instructions. Total cDNA was synthesized using a one-step cDNA synthesis kit (Takara, Japan). The RT-PCR was performed as previously described (15). The primers of Survivin (Forward: 5'-ATC GAC GCG TTC TTT GAA AGC AGT CGA GGG GGC-3', Reverse: 5'-CCC AAG CTT TCT GGC GGT TAA TGG CGC GCC-3') and Ki67 (forward, 5'-AGAGAGTGTCTATCAGCCGA-3', reverse, 5'-CATTGACCTTTGAGGACCAT-3') were synthesized by Genewiz (Suzhou, China). qPCR was carried out using the ABI SYBR Green PCR Master Mix (Applied Biosystems, USA).
Western blot
The primary antibodies, including anti-caspase-3, anti-caspase-9, anti-Nrf2, anti-NQO1, anti-HO-1, anti-NF-κB p65, and anti-actin, were purchased from Abcam (Cambridge, UK). After 24 h of reperfusion, the rat brain samples were homogenized, washed with phosphate buffered saline (PBS), and then lysed with radio immunoprecipitation assay (RIPA) lysis buffer. The total protein content was determined using a BCA kit (Solarbio, Beijing, China). After centrifugation at 12,000 rpm for 10 min, the supernatants were harvested for the measurement of protein concentration. The protein was isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Solarbio, Beijing, China) and then transferred to polyvinylidene difluoride (PVDF) membranes (Solarbio, Beijing, China). The membranes were blocked with 5% nonfat dry milk and incubated overnight at 4 °C with anti-caspase-3, anti-caspase-9, anti-Nrf2, anti-NQO1, anti-HO-1, anti-NF-κB p65, and anti-actin. Following that, the membranes were washed with tris-buffered saline and Tween 20 (TBST) buffer. The secondary antibody was added to the membranes. The blots were detected with an ECL detection kit (Solarbio, Beijing, China). Actin was served as a loading control.
Determining the levels of SOD, MDA, and LDH in brain tissues
After 24 h of reperfusion, experimental rats were sacrificed and decapitated. The brain tissues were added to cold normal saline and homogenized with a high-speed homogenizer at 12,000 rpm for 10 min at 4 °C. The supernatant was collected and stored at −80 °C for the subsequent experiments. The levels of SOD, MDA, and LDH were determined with test kits (Solarbio, Beijing, China). All procedures were performed according to the manufacturer’s instructions.
Measurements of cytokines in serum and brain tissues
After 24 h of reperfusion, experimental rats were sacrificed and decapitated. Their blood was collected and centrifuged at 3,000 rpm for 10 min. Then, the serum samples were placed for storage at −80 °C. The serum samples and brain tissue supernatant stored previously were used for the detection of IL-6, TNF-α, and IL-10 with ELISA kits (Biocompare, USA). All of the specific procedures were based on the kit instructions.
Statistical analysis
All the data were processed by GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA), and the results were expressed as the mean ± standard deviation (SD). Difference between groups were analyzed by one-way analysis of variance (ANOVA). A significant difference was considered when P value ≤0.05.
Results
Effects of eriocitrin on cerebral injury in rats with CI/R
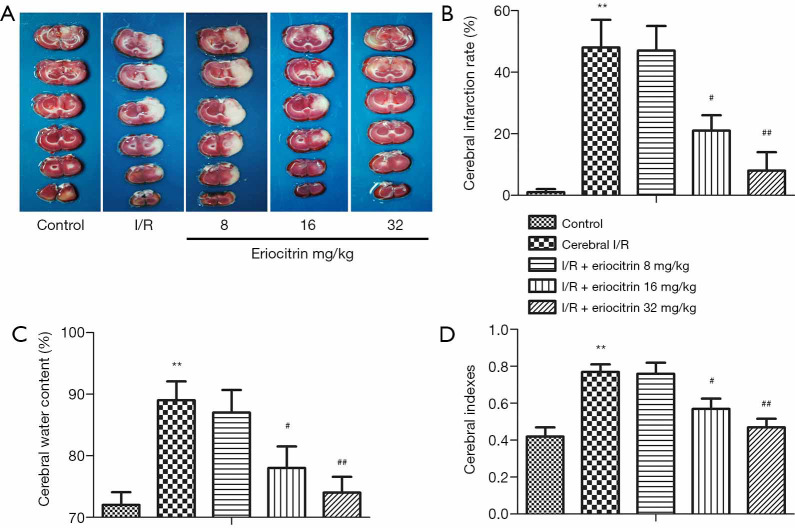
The cerebral infarct size of the rats in this study was determined by TTC staining. The results showed that a significant cerebral infarction appeared in the I/R group compared with that in the control group. In the I/R + eriocitrin groups, the infarction rate was markedly reduced in the I/R + eriocitrin 16 mg/kg and I/R+ Eriocitrin 32 mg/kg groups, while in the I/R+ Eriocitrin 8 mg/kg group, it was not significantly changed (Figure 1A,B). As shown in Figure 1C,D, the cerebral water content and cerebral indexes in the I/R group were highly increased compared to the control group. In the I/R + eriocitrin groups, the cerebral indexes of the high-dose (32 mg/kg) and medium-dose (16 mg/kg) groups were reduced in a dose-dependent manner compared with that of the I/R group, while the low-dose (8 mg/kg) group showed no significant difference. Taken together, a high or medium dose eriocitrin could relieve the damage caused after cerebral I/R injury in rats.
Figure 1.
The effects of eriocitrin on cerebral injury in rats with cerebral I/R. (A) TCC staining was used to assess cerebral infarct volume. (B) The statistical analysis of the cerebral infarction rate. (C) The measurement of the cerebral water content. (D) The statistical analysis of the cerebral indexes. **, P<0.01 vs. control group; #, P<0.05 vs. cerebral I/R group; ##, P<0.01 vs. cerebral I/R group. I/R, ischemia reperfusion.
Effects of eriocitrin on histopathological injury in rats with CI/R
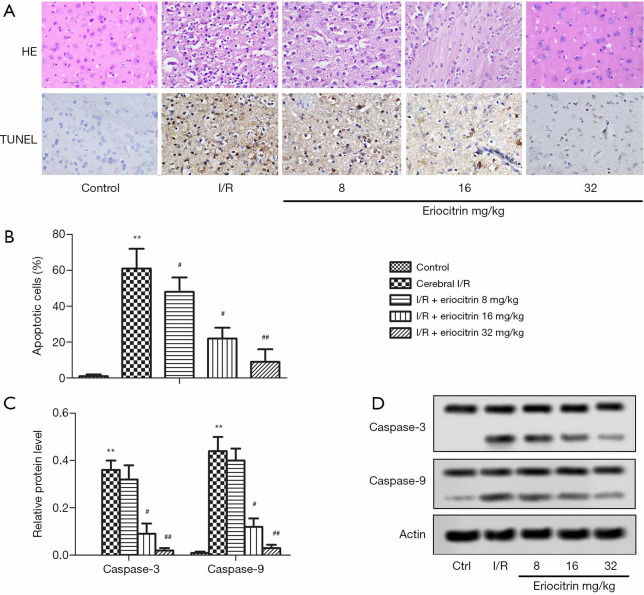
H&E and TUNEL staining revealed that the apoptotic cells were significantly increased in the I/R group compared with the control group (Figure 2A,B). RT-PCR detection of apoptosis-related proteins showed that the relative mRNA levels of caspase-3 and caspase-9 in the I/R group were significantly higher than those in the control group. In the I/R + eriocitrin groups, the mRNA levels of caspase-3 and caspase-9 in the high and middle-dose groups were markedly lower than those in the I/R group, but there was no significantly change in the low-dose group (Figure 2C). Moreover, the protein expressions of caspase-3 and caspase-9 were obviously increased in the I/R group compared with the control group. Meanwhile, in the I/R + eriocitrin groups, the protein expressions of caspase-3 and caspase-9 in the high and middle-dose groups were significantly downregulated in a dose-dependent manner, while there was no prominent change in the low group (Figure 2D). These results suggested that eriocitrin alleviated cell apoptosis in rats with cerebral I/R.
Figure 2.
The effects of eriocitrin on histological injury in rats with cerebral I/R. (A) Representative photomicrographs of H&E staining and TUNEL staining (Scale Bar =50 µm). (B) The statistical analysis of apoptotic cells. (C) The statistical graph of the protein levels of caspase-3 and caspase-9 by Western Blot. (D) Apoptosis-related proteins caspase-3 and caspase-9 were measured by western blotting. **, P<0.01 vs. control group; #, P<0.05 vs. cerebral I/R group; ##, P<0.01 vs. cerebral I/R group. I/R, ischemia reperfusion; H&E, hematoxylin and eosin; TUNEL, terminal-deoxynucleotidyl transferase mediated nick end labeling.
Effects of eriocitrin on oxidative injury in rats with CI/R
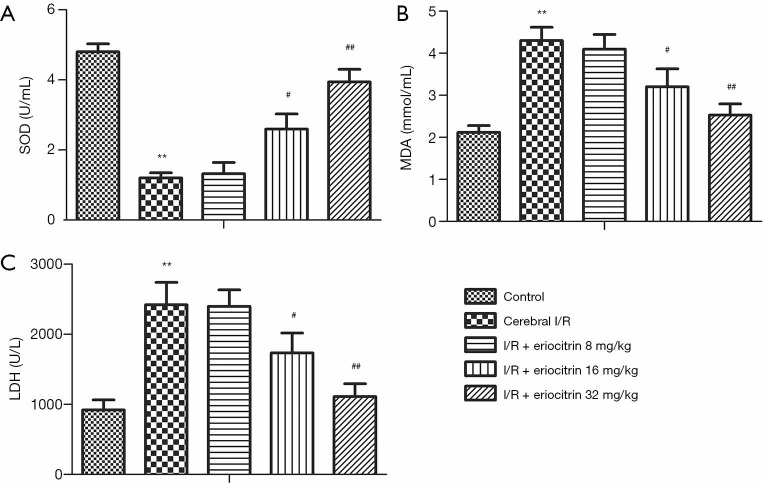
To examine the anti-oxidative property of eriocitrin on CI/R, the concentrations of SOD, MDA, and LDH in the brain tissues of the rats were detected. As shown in Figure 3A, the concentration of SOD in the I/R group was significantly lower than that in control group. Also, the concentrations of SOD in the high and middle-dose groups were obviously increased compared to the I/R group, and there was no prominent change in the low-dose group. The contents of MDA and LDH in the I/R group were markedly higher than those in the control group. Moreover, the contents of MDA and LDH in the high and middle-dose groups were significantly lower in a dose-dependent manner, while there was no significant change in the low-dose group (Figure 3B,C). These results indicated that an adequate dose of eriocitrin (16 mg/kg and 32 mg/kg) alleviated oxidative injury in the rats with cerebral I/R.
Figure 3.
The effects of eriocitrin on oxidative injury in rats with cerebral I/R. (A) Quantification of SOD. (B) Quantification of MDA. (C) Quantification of LDH. **, P<0.01 vs. control group; #, P<0.05 vs. cerebral I/R group; ##, P<0.01 vs. cerebral I/R group. I/R, ischemia reperfusion; SOD, superoxide dismutase; MDA, malondialdehyde; LDH, lactic dehydrogenase.
Effects of eriocitrin on inflammatory response in serum and brain tissue of rats with CI/R
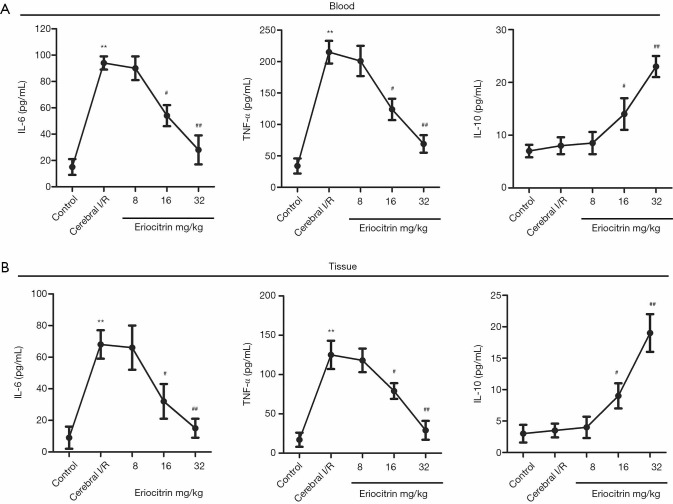
The effects of eriocitrin on inflammatory reactions were investigated by detecting the content of inflammatory cytokines in the blood and tissues. The results from both the blood and tissues showed that the levels of proinflammatory factors IL-6 and TNF-α in the I/R group were significantly increased compared with the control group. In the I/R + eriocitrin groups, the levels of IL-6 and TNF-α were markedly decreased in the high and middle-dose groups compared with the I/R group, while there was no obvious change in the low-dose group. Meanwhile, the level of anti-inflammatory factor IL-10 in the I/R group saw no prominent change compared with the control group. However, in the I/R + eriocitrin groups, compared with the I/R group, the level of IL-10 in the high and middle-dose groups was significantly increased in a dose-dependent manner, although there was no significant change in the low-dose group (Figure 4A,B). These results showed that eriocitrin influenced inflammatory response in rats with CI/R.
Figure 4.
The effects of eriocitrin on inflammatory response in the serum and brain tissue of rats with cerebral I/R. The levels of IL-6, TNF-α, and IL-10 in serum (A) and brain tissue (B) were analyzed by ELISA. **, P<0.01 vs. control group; #, P<0.05 vs. cerebral I/R group; ##, P<0.01 vs. cerebral I/R group. I/R, ischemia reperfusion; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; IL-10, interleukin-10.
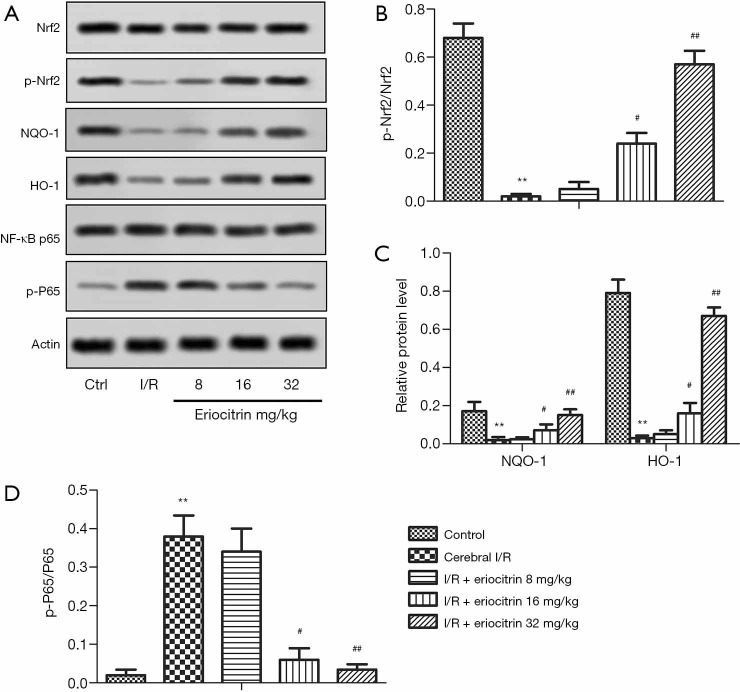
Effects of eriocitrin on Nrf2/HO-1 /NQO1/NF-κB signaling pathway in rats with cerebral I/R
Nrf2 is highly important in anti-oxidative stress (16) and anti-inflammatory response (17). The expressions of Nrf2 and its downstream proteins NQO1, HO-1, and NF-κB p65 were tested by ELISA. Compared with the control group, the levels of phosphorylation of Nrf2 (p-Nrf2)/Nrf2, NQO1 and HO-1 were significantly downregulated in the I/R group. In the I/R + eriocitrin groups, the levels of p-Nrf2/Nrf2, NQO1, and HO-1 in the high and middle-dose groups were obviously increased in a dose-dependent manner compared with the I/R group, while there was no significant change in the low-dose group (Figure 5A,B,C). Compared with the control group the level of phosphorylation of NF-κBp65 (p-P65)/P65 in the I/R group was obviously upregulated. In the I/R + eriocitrin groups, the level of p-P65/P65 in the high and middle-dose groups was significantly decreased in a dose-dependent manner compared with the I/R group (Figure 5A,D). These results showed that eriocitrin modulated the Nrf2/HO-1/NQO1/NF-κB signaling pathway through the regulation of related proteins.
Figure 5.
The effects of eriocitrin on the Nrf2/HO-1 /NQO1/NF-κB signaling pathway in rats with cerebral I/R. (A) The results of ELISA to examine protein expression. (B) The ratio of p-Nrf2 to Nrf2. (C) The ratio of p-P65 to P65. (D) The expression levels of NQO1 and HO-1. **, P<0.01 vs. control group; #, P<0.05 vs. cerebral I/R group; ##, P<0.01 vs. cerebral I/R group. Nrf2, nuclear factor erythroid 2-related factor; HO-1, heme-oxygenase-1; NQO1, quinine oxidoreductase; NF-κB, nuclear factor-κB; I/R, ischemia reperfusion; ELISA, enzyme-linked immunosorbent assay; p-Nrf2, phosphorylation of Nrf2.
Discussion
Ischemic stroke is a major cause of chronic disability and carries a high mortality rate globally (18). The most effective treatment for ischemic stroke is restoration of the blood supply to the brain tissue; however, restoring the blood supply after minutes or hours of blockage can cause cerebral I/R injury. Cerebral I/R increases the risk of brain hemorrhage, cerebral edema, and further damage to the blood-brain barrier (19). Therefore, an effective treatment for cerebral I/R injury is urgently called for.
In this study, an MCAO rat model was used to determine the effects of eriocitrin on cerebral I/R injury. The cerebral indexes reflected the extent of brain edema. Brain edema was closely related to the ischemic brain damage because it induced an increase in cranial pressure and further inhibited the blood flow to the ischemic area (20). In previous studies on treatment for cerebral I/R injury, the changes of cerebral edema and cerebral obstruction have been consistent (10,11,21). In our study, the cerebral infarct volume and cerebral water content were reduced by adequate doses of eriocitrin. These results indicate that eriocitrin could exert a protective effect on cerebral I/R injury in rats.
The effect of eriocitrin on cell apoptosis has been investigated in previous studies. In human hepatocellular carcinoma cells, it was shown to suppress proliferation by arresting cell cycle in the S phase and induce apoptosis by activating the mitochondria-involved intrinsic signaling pathway (6). Eriocitrin and its metabolites also induce apoptosis in HL-60 cells (22). There are two main pathways for cell apoptosis: the intrinsic pathway and the extrinsic pathway (23). In this study, eriocitrin reduced the levels of caspase-3 and caspase-9, which are related to apoptosis, suggesting that eriocitrin could inhibit the apoptosis and alleviate histopathological injury in rats with CI/R.
As mentioned above, oxidative stress plays a pivotal role in the process of cerebral I/R injury. Oxidative stress, which results from an imbalance between the antioxidant defense system and the production rate of reactive oxygen species (24), can destroy cell macromolecules, leading to protein modification, lipid peroxidation, and DNA damage (25). SOD is an antioxidant enzyme involved in the defense mechanism for reducing oxidative stress (26), and MDA, a product of lipid peroxidation, serves as a useful biomarker for measuring and monitoring oxidative stress (24). SOD and MDA have often been used to assess oxidative stress (27). LDH has been used to evaluate the cytopathic effect of pathogens (28), and it is increased after cerebral I/R injury (21). The results of the current study show that eriocitrin significantly lowered the content of MDA and LDH while restoring the content of SOD. These results indicated that the protective effect of eriocitrin on CI/R injury might be attributed to its antioxidative properties.
CI/R injury is associated with inflammatory cytokines including IL-6, TNF-α, and IL-10. Inflammatory cytokines are regulating factors in the mechanism of inflammation induced by I/R (29). IL-6 is involved in neuronal apoptosis and the mediation of inflammatory cytokines in the pathogenesis of CI (30). TNF-α is a proinflammatory cytokine that can cause brain function injury (31). Meanwhile, as an anti-inflammatory cytokine, IL-10 performs a key role in controlling inflammatory response. The expressions of IL-6, TNF-α, and IL-10 can regulate inflammatory response (32). In this study, eriocitrin decreased the levels of IL-6 and TNF-α, but increased the expression of IL-10. We demonstrated that eriocitrin alleviated inflammatory response in rats with CI/R.
The Nrf2 pathway is an important cellular system which protects tissues from oxidative stress. Nrf2 is a master regulator of antioxidative defense responses (16). Under basal conditions, Nrf2 exists in an inactive form in the cytoplasm, maintaining a low level of Nrf2-regulated gene expression. Under stress conditions, Nrf2 transfers from the cytoplasm to the nucleus, activating the transcription of a variety of antioxidant and detoxification genes (33). The p-Nrf2 mediates its transcriptional activation, thereby regulating the downstream antioxidant enzymes against oxidative stress. Nrf2 is also related to the regulation of the expression of inflammatory genes and, as an anti-inflammatory transcription factor, it inhibits inflammatory response (17). Research has proved that Nrf2 activation has become a potential therapeutic target for neuroprotection in I/R injury (34,35). HO-1 and NQO1 are downstream antioxidant proteins of Nrf2. NF-κB is a key regulator of inflammation which promotes the production of proinflammatory cytokines (36), while NF-κBp65 is a subunit of NF-κB. The expression of p-NF-κBp65 is a sign of NF-κBp65 activation (37). HO-1 and NQO1 can inhibit the transcription of proinflammatory cytokines by blocking NF-κB activation (16). In the present study, the expressions of Nrf2, p-Nrf2, NF-κBp65, p-P65, HO-1, and HQO1 were measured. Our results demonstrated that eriocitrin alleviated oxidative injury and inflammatory response through the promotion of Nrf2 activation and the suppression of NF-κBp65 activation.
In summary, eriocitrin alleviated oxidative injury and inflammatory response in rats with CI/R via the Nrf2/HO-1 /NQO1/NF-κB signaling pathway. While eriocitrin has previously been applied as an antioxidant, to our knowledge, this is the first study to use it in the treatment of cerebral I/R injury. This study presents a promising novel treatment for cerebral I/R injury. However, the protective effects of eriocitrin in clinical application demand further investigation.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All experiments involving animals were performed according to the National Institutes of Health (NIH) guidelines for the Care and Use of Animals. All experiments were approved by the ethics committee of the West China Hospital (No. 2018-29).
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4258
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4258
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4258). The authors have no conflicts of interest to declare.
(English Language Editor: J. Reynolds)
References
- 1.Turley KR, Toledo-Pereyra LH, Kothari RU. Molecular mechanisms in the pathogenesis and treatment of acute ischemic stroke. J Invest Surg 2005;18:207-18. 10.1080/08941930591004449 [DOI] [PubMed] [Google Scholar]
- 2.Kong Z, Shen Q, Jiang J, et al. Wogonin improves functional neuroprotection for acute cerebral ischemia in rats by promoting angiogenesis via TGF-β1. Ann Transl Med 2019;7:639. 10.21037/atm.2019.10.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Z, Xin Z, Di W, et al. Melatonin and mitochondrial function during ischemia/reperfusion injury. Cell Mol Life Sci 2017;74:3989-98. 10.1007/s00018-017-2618-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minato K-i, Miyake Y, Fukumoto S, et al. Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sciences 2003;72:1609-16. 10.1016/S0024-3205(02)02443-8 [DOI] [PubMed] [Google Scholar]
- 5.Hiramitsu M, Shimada Y, Kuroyanagi J, et al. Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci Rep 2014;4:3708. 10.1038/srep03708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Zhang H, Zhou J, et al. Eriocitrin from lemon suppresses the proliferation of human hepatocellular carcinoma cells through inducing apoptosis and arresting cell cycle. Cancer Chemotherapy and Pharmacology 2016;78:1143-50. 10.1007/s00280-016-3171-y [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Zhu H, Gattoni-Celli S, et al. Dietary supplementation of GrandFusion(®) mitigates cerebral ischemia-induced neuronal damage and attenuates inflammation. Nutr Neurosci 2016;19:290-300. 10.1179/1476830515Y.0000000021 [DOI] [PubMed] [Google Scholar]
- 8.Chang X, Zhang K, Zhou R, et al. Cardioprotective effects of salidroside on myocardial ischemia-reperfusion injury in coronary artery occlusion-induced rats and Langendorff-perfused rat hearts. Int J Cardiol 2016;215:532-44. 10.1016/j.ijcard.2016.04.108 [DOI] [PubMed] [Google Scholar]
- 9.Wu G, Zhu L, Yuan X, et al. Britanin Ameliorates Cerebral Ischemia-Reperfusion Injury by Inducing the Nrf2 Protective Pathway. Antioxid Redox Signal 2017;27:754-68. 10.1089/ars.2016.6885 [DOI] [PubMed] [Google Scholar]
- 10.Bai L, Shi W, Liu J, et al. Protective effect of pilose antler peptide on cerebral ischemia/reperfusion (I/R) injury through Nrf-2/OH-1/NF-kappaB pathway. Int J Biol Macromol 2017;102:741-8. 10.1016/j.ijbiomac.2017.04.091 [DOI] [PubMed] [Google Scholar]
- 11.Ya BL, Liu Q, Li HF, et al. Uric Acid Protects against Focal Cerebral Ischemia/Reperfusion-Induced Oxidative Stress via Activating Nrf2 and Regulating Neurotrophic Factor Expression. Oxid Med Cell Longev 2018;2018:6069150. [DOI] [PMC free article] [PubMed]
- 12.Shu K, Zhang Y. Protodioscin protects PC12 cells against oxygen and glucose deprivation-induced injury through miR-124/AKT/Nrf2 pathway. Cell Stress Chaperones 2019;24:1091-9. 10.1007/s12192-019-01031-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Chua CC, Gao J, et al. Humanin Is a Novel Neuroprotective Agent Against Stroke. Stroke 2006;37:2613-9. 10.1161/01.STR.0000242772.94277.1f [DOI] [PubMed] [Google Scholar]
- 14.Crowley LC, Marfell BJ, Waterhouse NJ. Detection of DNA Fragmentation in Apoptotic Cells by TUNEL. Cold Spring Harb Protoc 2016. doi: . 10.1101/pdb.prot087221 [DOI] [PubMed] [Google Scholar]
- 15.Deng R, Yang D, Radke A, et al. The hypolipidemic agent guggulsterone regulates the expression of human bile salt export pump: dominance of transactivation over farsenoid X receptor-mediated antagonism. J Pharmacol Exp Ther 2007;320:1153-62. 10.1124/jpet.106.113837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vomund S, Schafer A, Parnham MJ, et al. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int J Mol Sci 2017;18:2772. 10.3390/ijms18122772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills EL, Ryan DG, Prag HA, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018;556:113-7. 10.1038/nature25986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling X, Zheng Y, Tao J, et al. Association study of polymorphisms in the ABO gene with ischemic stroke in the Chinese population. BMC Neurol 2016;16:146. 10.1186/s12883-016-0671-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Husseini N, Laskowitz DT. The role of neuroendocrine pathways in prognosis after stroke. Expert Rev Neurother 2014;14:217-32. 10.1586/14737175.2014.877841 [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Ling J, Wang X, et al. Discovery of a potential anti-ischemic stroke agent: 3-pentylbenzo[c]thiophen-1(3H)-one. J Med Chem 2012;55:7173-81. 10.1021/jm300681r [DOI] [PubMed] [Google Scholar]
- 21.Yang S, Ning F, Li J, et al. Therapeutic Effect Analysis of Sinomenine on Rat Cerebral Ischemia-Reperfusion Injury. J Stroke Cerebrovasc Dis 2016;25:1263-9. 10.1016/j.jstrokecerebrovasdis.2016.02.023 [DOI] [PubMed] [Google Scholar]
- 22.Ogata S, Miyake Y, Yamamoto K, et al. Apoptosis induced by the flavonoid from lemon fruit (Citrus limon BURM. f.) and its metabolites in HL-60 cells. Biosci Biotechnol Biochem 2000;64:1075-8. 10.1271/bbb.64.1075 [DOI] [PubMed] [Google Scholar]
- 23.Walther U, Emmrich K, Ramer R, et al. Lovastatin lactone elicits human lung cancer cell apoptosis via a COX-2/PPARgamma-dependent pathway. Oncotarget 2016;7:10345-62. 10.18632/oncotarget.7213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakrabarti SK, Ghosh S, Banerjee S, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab 2016;20:674-8. 10.4103/2230-8210.190555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang W, Luo F, Lu Q, et al. The protective effect of Trillin LPS-induced acute lung injury by the regulations of inflammation and oxidative state. Chem Biol Interact 2016;243:127-34. 10.1016/j.cbi.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 26.Mosa KA, El-Naggar M, Ramamoorthy K, et al. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front Plant Sci 2018;9:872. 10.3389/fpls.2018.00872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He J, Li H, Li G, et al. Hyperoside protects against cerebral ischemia-reperfusion injury by alleviating oxidative stress, inflammation and apoptosis in rats. Biotechnol Biotechnol Equip 2019;33:798-806. 10.1080/13102818.2019.1620633 [DOI] [Google Scholar]
- 28.Viguier C, Arora S, Gilmartin N, et al. Mastitis detection: current trends and future perspectives. Trends Biotechnol 2009;27:486-93. 10.1016/j.tibtech.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 29.Shinohara N, Nakamura T, Abe Y, et al. d-Allose Attenuates Overexpression of Inflammatory Cytokines after Cerebral Ischemia/Reperfusion Injury in Gerbil. J Stroke Cerebrovasc Dis 2016;25:2184-8. 10.1016/j.jstrokecerebrovasdis.2016.01.030 [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Li Y, Fu Q, et al. Perillaldehyde attenuates cerebral ischemia-reperfusion injury-triggered overexpression of inflammatory cytokines via modulating Akt/JNK pathway in the rat brain cortex. Biochem Biophys Res Commun 2014;454:65-70. 10.1016/j.bbrc.2014.10.025 [DOI] [PubMed] [Google Scholar]
- 31.Zhu L, Chen T, Chang X, et al. Salidroside ameliorates arthritis-induced brain cognition deficits by regulating Rho/ROCK/NF-kappaB pathway. Neuropharmacology 2016;103:134-42. 10.1016/j.neuropharm.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 32.Ip WKE, Hoshi N, Shouval DS, et al. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017;356:513-9. 10.1126/science.aal3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakka VP, Prakash-Babu P, Vemuganti R. Crosstalk Between Endoplasmic Reticulum Stress, Oxidative Stress, and Autophagy: Potential Therapeutic Targets for Acute CNS Injuries. Mol Neurobiol 2016;53:532-44. 10.1007/s12035-014-9029-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang R, Xu M, Wang Y, et al. Nrf2-a Promising Therapeutic Target for Defensing Against Oxidative Stress in Stroke. Mol Neurobiol 2017;54:6006-17. 10.1007/s12035-016-0111-0 [DOI] [PubMed] [Google Scholar]
- 35.Wu C, Li T, Zhu B, et al. Scoparone protects neuronal cells from oxygen glucose deprivation/reoxygenation injury. RSC Advances 2019;9:2302-8. 10.1039/C8RA09867K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen T, Mou Y, Tan J, et al. The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol 2015;25:55-64. 10.1016/j.intimp.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 37.Bai C, Yang X, Zou K, et al. Anti-proliferative effect of RCE-4 from Reineckia carnea on human cervical cancer HeLa cells by inhibiting the PI3K/Akt/mTOR signaling pathway and NF-kappaB activation. Naunyn Schmiedebergs Arch Pharmacol 2016;389:573-84. 10.1007/s00210-016-1217-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as