This cohort study compares the cancer spectrum and frequencies between male BRCA1 and BRCA2 pathogenic variant carriers.
Key Points
Question
Are there cancer phenotype differences between male BRCA1 and BRCA2 pathogenic variant carriers?
Findings
In this cohort study of 6902 men with a BRCA1 or BRCA2 pathogenic variant, being affected by cancer, particularly breast, prostate, and pancreatic cancers and developing multiple primary tumors, was associated with a higher probability for a man of being a BRCA2, rather than a BRCA1, pathogenic variant carrier.
Meaning
Surveillance programs in men with BRCA1 and BRCA2 pathogenic variants should be tailored in light of these gene-specific cancer phenotype differences. These results may inform the design of prospective studies on cancer risks in male BRCA1 and BRCA2 pathogenic variant carriers.
Abstract
Importance
The limited data on cancer phenotypes in men with germline BRCA1 and BRCA2 pathogenic variants (PVs) have hampered the development of evidence-based recommendations for early cancer detection and risk reduction in this population.
Objective
To compare the cancer spectrum and frequencies between male BRCA1 and BRCA2 PV carriers.
Design, Setting, and Participants
Retrospective cohort study of 6902 men, including 3651 BRCA1 and 3251 BRCA2 PV carriers, older than 18 years recruited from cancer genetics clinics from 1966 to 2017 by 53 study groups in 33 countries worldwide collaborating through the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Clinical data and pathologic characteristics were collected.
Main Outcomes and Measures
BRCA1/2 status was the outcome in a logistic regression, and cancer diagnoses were the independent predictors. All odds ratios (ORs) were adjusted for age, country of origin, and calendar year of the first interview.
Results
Among the 6902 men in the study (median [range] age, 51.6 [18-100] years), 1634 cancers were diagnosed in 1376 men (19.9%), the majority (922 of 1,376 [67%]) being BRCA2 PV carriers. Being affected by any cancer was associated with a higher probability of being a BRCA2, rather than a BRCA1, PV carrier (OR, 3.23; 95% CI, 2.81-3.70; P < .001), as well as developing 2 (OR, 7.97; 95% CI, 5.47-11.60; P < .001) and 3 (OR, 19.60; 95% CI, 4.64-82.89; P < .001) primary tumors. A higher frequency of breast (OR, 5.47; 95% CI, 4.06-7.37; P < .001) and prostate (OR, 1.39; 95% CI, 1.09-1.78; P = .008) cancers was associated with a higher probability of being a BRCA2 PV carrier. Among cancers other than breast and prostate, pancreatic cancer was associated with a higher probability (OR, 3.00; 95% CI, 1.55-5.81; P = .001) and colorectal cancer with a lower probability (OR, 0.47; 95% CI, 0.29-0.78; P = .003) of being a BRCA2 PV carrier.
Conclusions and Relevance
Significant differences in the cancer spectrum were observed in male BRCA2, compared with BRCA1, PV carriers. These data may inform future recommendations for surveillance of BRCA1/2-associated cancers and guide future prospective studies for estimating cancer risks in men with BRCA1/2 PVs.
Introduction
While there are a substantial number of studies on cancer risks and the cancer spectrum in female carriers of germline pathogenic variants (PVs) in BRCA1 (OMIM 113705) and BRCA2 (OMIM 600185),1,2,3,4 data on male BRCA1/2 PV carriers are limited and have primarily focused on breast and/or prostate cancers. Population-based studies have shown that BRCA1 and BRCA2 PVs account for up to 2% and 13% of male breast cancer cases, respectively.5 The lifetime risk of male breast cancer has been estimated at 1% to 5% for BRCA1 and 5% to 10% for BRCA2 PV carriers, vs 0.1% in the general male population.2,3,6,7,8 Additionally, BRCA1 and BRCA2 PVs have been estimated to account for less than 1% and approximately 2% of incident prostate cancer diagnoses, respectively.9,10 Estimates of lifetime prostate cancer risk associated with BRCA1 and BRCA2 PVs vary, with some studies reporting higher risk for male BRCA2 PV carriers,10,11,12,13,14,15 while other studies did not find any increased risk.16,17,18 Pathogenic variants in BRCA1 and, more frequently, in BRCA2 have been reported in male patients diagnosed with other cancer types.13,14,19,20,21,22,23,24 However, current risk estimates for cancers other than breast and prostate are based on handfuls of cases in a limited number of families.
BRCA1/2-associated tumors in men exhibit specific pathologic features and poor clinical outcome. A specific BRCA2-associated breast cancer phenotype, hallmarked by high histopathologic grade, a feature suggestive of biological aggressiveness, has been reported in men.25 Compared with age-matched controls, men with BRCA1/2-associated prostate cancer more frequently have early-onset (<65 years) and aggressive disease.15,26 Specifically, BRCA2 PVs were identified as an independent negative prognostic factor in patients with prostate cancer.27 There is also some evidence suggesting that patients with BRCA1/2-associated pancreatic cancer may exhibit worse prognosis compared with noncarriers.28 In the aggregate, these observations highlight the need for large collaborations to improve and expand data on the cancer spectrum in male BRCA1/2 PV carriers to optimize guidelines for cancer risk management in this group.29
The Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) is an international collaboration that has collected data on female and male BRCA1/2 PV carriers.30 Using this series, to our knowledge the largest collected worldwide, we aimed to characterize the spectrum of cancers diagnosed in male BRCA1/2 PV carriers and identify differences between BRCA1 and BRCA2 PV carriers. Such information could form the foundation for future screening and surveillance recommendations regarding BRCA1/2-associated cancers in men and for future studies aimed to estimate lifetime risks of cancers other than breast and prostate in male BRCA1/2 PV carriers.
Methods
CIMBA Study Participants
Investigators collaborating through CIMBA (http://cimba.ccge.medschl.cam.ac.uk/) have collected data on men older than 18 years who carry pathogenic and likely pathogenic BRCA1 or BRCA2 variants, with the majority of carriers identified and recruited via cancer genetics clinics.25 Variant pathogenicity was defined as previously described.31 The present study includes data from 6902 male BRCA1/2 PV carriers collected by 53 study groups in 33 countries from 1966 to 2017 (eTables 1 and 2 in the Supplement).
Data collected for each individual included year of birth, a unique family identifier, ethnicity, age at cancer diagnosis, primary tumor site (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] coding), age at last observation, and clinical data from medical, pathology, or tumor registry records.25 Most individuals (77%) reported herein are self-reported as white Caucasian, with other ethnicities not as equally represented (eTable 3 in the Supplement). Recruited BRCA1 or BRCA2 PV carriers include probands and tested family members (eTable 4 in the Supplement). Data on first-degree and second-degree family history of male breast, prostate, and female breast cancer were also collected and were available for a subset of individuals (eTable 5 in the Supplement). Written informed consent was obtained from all study participants, as part of the protocol approved by the individual ethics committees at the participating centers.
Statistical Methods
The primary objective was to compare cancer diagnoses between male BRCA1 and BRCA2 PV carriers. We used logistic regression to estimate the association between BRCA1/2 PV status (outcome) and cancer diagnosis (independent variable). Individuals with no cancer diagnosis at last follow-up were considered unaffected (reference group), whereas individuals with 1 or more diagnoses of cancer at any site were grouped as affected. This provides an estimate of the odds ratio (OR) comparing the odds of being a BRCA2 PV carrier in the affected group to the odds of being a BRCA2 PV carrier in the unaffected group. In practice, under a univariate analysis, this can be interpreted as the OR of a BRCA2 carrier being affected compared with the odds of a BRCA1 PV carrier being affected. Differences in age at first cancer diagnosis by cancer site (breast, prostate, other sites) between BRCA1 and BRCA2 PV carriers and in intercancer intervals were assessed by the nonparametric Mann-Whitney test.
A separate cancer-only logistic regression was performed (using the same approach described above) restricted to affected individuals in which all tumors arising in affected male carriers were taken into consideration. The independent variables were defined as the cancer site (breast cancer vs all cancers but breast; prostate cancer vs all cancers but prostate; cancers at other sites vs breast and prostate cancers). A further analysis was performed, including only tumors at sites other than breast and prostate to address possible ascertainment bias of breast and prostate cancers. In this analysis, the independent variables were specific cancer sites, namely colorectal cancer, melanoma, and pancreatic cancer (colorectal cancer vs all other cancers; melanoma vs all other cancers; pancreatic cancer vs all other cancers). To assess the potential influence of survival bias, these analyses were also repeated after omitting cancer diagnoses occurring more than 5 years prior to study recruitment.
Confounders included in the logistic regression models were prespecified and were chosen on the basis of previous studies on CIMBA male carrier series25,31 and by considering factors related to the study design. All analyses were adjusted for age at cancer diagnosis (affected individuals) or age at last follow-up (unaffected individuals) and country of origin. In addition, adjustments for calendar year of the first interview were included in all analyses as a surrogate for year of genetic testing, based on the groupings of 2000 or earlier, 2001-2010, and after 2010, to account for ascertainment biases owing to differential genetic testing approaches and inclusion criteria over time. A logistic regression adjusted also for proband status, estimated considering as probands individuals with a cancer diagnosis date preceding interview prior genetic testing date, was performed. To assess the potential influence of family history, analyses were repeated adjusting for family history of male breast cancer, female breast cancer, and prostate cancer, all included as separate covariates, with each variable grouped as positive, negative, or unknown family history. A robust variance approach was used to allow for dependencies between related individuals. P values of .05 or less were considered statistically significant. All analyses were carried out using Stata, version 13 (StataCorp).
Results
The series included 6902 men with PVs in BRCA1 (n = 3651 [52.9%]) or BRCA2 (n = 3251 [47.1%]). Of the 6902 male BRCA1/2 PV carriers, 1376 (19.9%) had at least 1 cancer diagnosis, the majority of whom (67.0%) harbored a BRCA2 PV. Median (range) age in the whole series was 51.6 (18-100) years. Age distribution is reported in the eFigure in the Supplement.
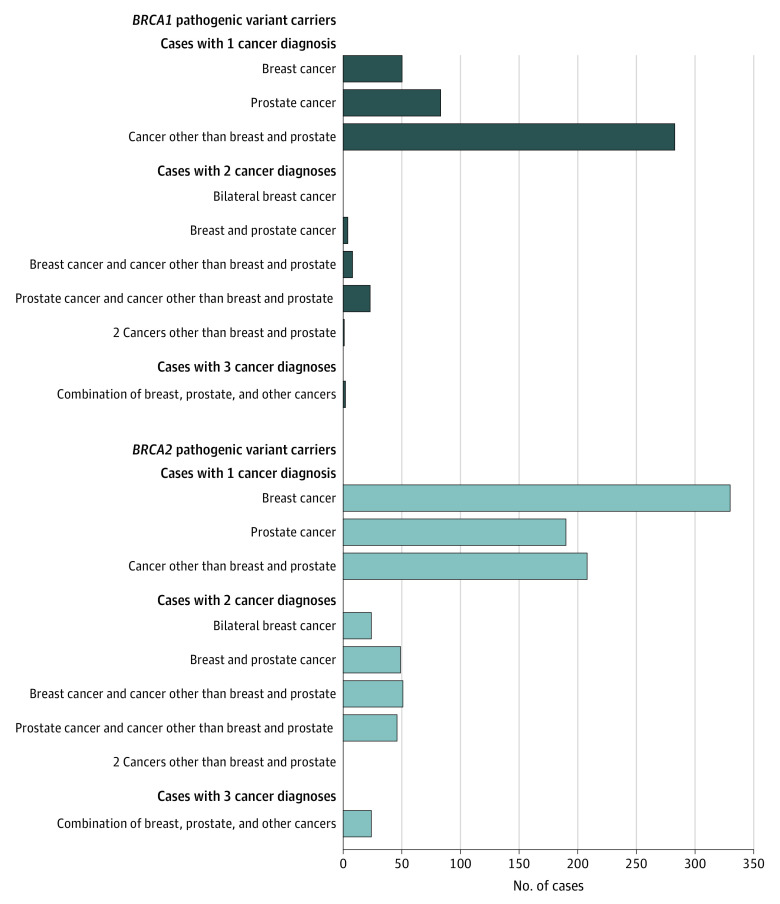
Of the 1376 carriers with cancer, 1144 (83.1%) were diagnosed with 1 cancer, 206 (15.0%) had 2 cancers, and 26 (1.9%) had 3 independent cancer diagnoses (Table 1). The number and type of cancer diagnoses varied greatly depending on which gene was mutated (Table 1 and Figure 1). Notably, all individuals diagnosed with 2 independent breast cancers had a BRCA2 PV. Overall, being affected by any cancer was associated with a higher probability of being a BRCA2, rather than a BRCA1, PV carrier (OR, 3.23; 95% CI, 2.81-3.70; P < .001). Similarly, developing multiple cancers, particularly 2 (OR, 7.97; 95% CI, 5.47-11.60; P < .001) and 3 (OR, 19.60; 95% CI, 4.64-82.89; P < .001) primary tumors, was associated with a higher probability of being a BRCA2 PV carrier in analyses adjusted for age, country of origin, and calendar year of interview (Table 1). Analyses adjusted also for family history of male breast cancer, female breast cancer, and prostate cancer gave similar results (eTable 6 in the Supplement).
Table 1. Cancer Diagnosis in Male BRCA1/2 Pathogenic Variant (PV) Carriers Within CIMBA Data Set and Odds Ratios (ORs) in Predicting BRCA2 PV Carrier Status.
| No. (%) | Adjusted OR (95% CI)a | P value | |||
|---|---|---|---|---|---|
| Total (N = 6902) | BRCA1 (n = 3651) | BRCA2 (n = 3251) | |||
| Unaffected | 5526 (80.1) | 3197 (87.6) | 2329 (71.6) | 1.00 [Reference] | |
| Affected | 1376 (19.9) | 454 (12.4) | 922 (28.4) | 3.23 (2.81-3.70) | <.001 |
| Cases with 1 cancer diagnosis | 1144 (83.1) | 416 (91.6) | 728 (79.0) | 2.77 (2.40-3.20) | <.001 |
| Breast cancer | 380 (33.2) | 50 (12.0) | 330 (45.3) | ||
| Prostate cancer | 273 (23.9) | 83 (20.0) | 190 (26.1) | ||
| Cancer other than breast and prostate | 491 (42.9) | 283 (68.0) | 208 (28.6) | ||
| Cases with 2 cancer diagnoses | 206 (15.0) | 36 (8.0) | 170 (18.4) | 7.97 (5.47-11.60) | <.001 |
| Bilateral breast cancer | 24 (11.7) | 0 | 24 (14.1) | ||
| Breast and prostate cancer | 53 (25.7) | 4 (11.1) | 49 (28.8) | ||
| Breast cancer and cancer other than breast and prostate | 59 (28.6) | 8 (22.2) | 51 (30.0) | ||
| Prostate cancer and cancer other than breast and prostate | 69 (33.5) | 23 (63.9) | 46 (27.1) | ||
| Two cancers other than breast and prostate | 1 (0.5) | 1 (2.8) | 0 | ||
| Cases with 3 cancer diagnoses | 26 (1.9) | 2 (0.4) | 24 (2.6) | 19.60 (4.64-82.89) | <.001 |
| Bilateral breast and prostate cancer | 5 (19.2) | 0 | 5 (20.8) | ||
| Bilateral breast and cancer other than breast and prostate | 7 (26.9) | 0 | 7 (29.2) | ||
| Prostate cancer and 2 cancers other than breast and prostate | 1 (3.8) | 1 (50.0) | 0 | ||
| Breast, prostate, and cancer other than breast and prostate | 13 (50.0) | 1 (50.0) | 12 (50.0) | ||
Abbreviation: CIMBA, Consortium of Investigators of Modifiers of BRCA1/2.
Analyses adjusted for age at cancer diagnosis/last follow-up, country of origin, and calendar year of interview.
Figure 1. Cancer Diagnoses in Male BRCA1 and BRCA2 Pathogenic Variant Carriers.
Type of cancer diagnoses reported in the 1376 affected male BRCA1 (n = 454) and BRCA2 (n = 922) pathogenic variant carriers in the Consortium of Investigators of Modifiers of BRCA1/2 data set.
Among male BRCA2 PV carriers with more than 1 cancer diagnosis, significantly shorter median intercancer intervals were observed for cases with a first diagnosis of breast (5.0 years) or prostate (3.4 years) cancers compared with cases with a first diagnosis of other cancers (7 years; Mann-Whitney test P = .03 and P = .005, respectively). Focusing on the first cancer diagnosed, breast (n = 485 [35.3%]) and prostate (n = 337 [24.5%]) cancers represented the majority of all first diagnoses (Table 2). Both breast and prostate cancers occurred more frequently in BRCA2 PV carriers (46.4% and 25.6%, respectively) compared with BRCA1 PV carriers (12.5% and 22.3%) (Table 2; eTable 7 in the Supplement). Median age at first cancer diagnosis was 61.5 years for breast cancer and 63.2 years for prostate cancer and were similar for BRCA1 and BRCA2 PV carriers (Table 2). Nonbreast and nonprostate cancers combined (n = 554) represented 40.2% of all first cancer diagnoses, with a median age at diagnosis of 59.2 years (Table 2). The proportion of cancers other than breast and prostate taken together is larger in BRCA1 PV carriers (65.2%) compared with BRCA2 PV carriers (28.0%), while mean age at first diagnosis was statistically significantly older in BRCA1 (61.8 years) compared with BRCA2 PV carriers (56.5 years; Mann-Whitney test P = .003).
Table 2. Age at First Cancer Diagnosis According to Cancer Site and BRCA1/2 Pathogenic Variant (PV) Status in the 1376 Affected Male Carriers Within CIMBA Data Set.
| Cancer diagnosis | Total carriers | BRCA1 PV carriers | BRCA2 PV carriers | P valuea | |||
|---|---|---|---|---|---|---|---|
| No. (%) | Age at diagnosis, median (IQR) | No. (%) | Age at diagnosis, median (IQR) | No. (%) | Age at diagnosis, median (IQR) | ||
| Male breast cancer | 485 (35.3) | 61.5 (16.0) | 57 (12.5) | 61.0 (20.0) | 428 (46.4) | 61.5 (15.3) | .87 |
| Prostate cancer | 337 (24.5) | 63.2 (12.5) | 101 (22.3) | 65.0 (12.0) | 236 (25.6) | 63.1 (12.2) | .09 |
| Cancers other than breast and prostate | 554 (40.2) | 59.2 (19.6) | 296 (65.2) | 61.8 (20.0) | 258 (28.0) | 56.5 (20.3) | .003 |
Abbreviations: CIMBA, Consortium of Investigators of Modifiers of BRCA1/2; IQR, interquartile range.
Mann-Whitney test for the comparison of median age at first cancer diagnosis between male BRCA1 and BRCA2 PV carriers.
A total of 1634 cancers were reported in the 1376 affected individuals, of which 494 (30.2%) were in BRCA1 PV carriers and 1140 (69.8%) were in BRCA2 PV carriers (Table 3). The analysis restricted to affected individuals and adjusted for age, country of origin, and calendar year of interview showed that a higher frequency of breast (OR, 5.47; 95% CI, 4.06-7.37; P < .001) and prostate (OR, 1.39; 95% CI, 1.09-1.78; P = .008) cancers, and a lower frequency of cancers other than breast and prostate combined (OR, 0.22; 95% CI, 0.18-0.28; P < .001) were associated with a higher probability of being a BRCA2, rather than a BRCA1, PV carrier. Specifically, 643 of 1634 tumors (39.4%) were in sites other than breast and prostate, of which 319 (64.6%) were diagnosed in BRCA1 PV carriers and 324 (28.4%) were diagnosed in BRCA2 PV carriers (Table 3).
Table 3. Analysis Restricted to the Total Tumors Reported in the 1376 Affected Male BRCA1/2 Pathogenic Variant (PV) Carriers Within CIMBA Data Set and Odds Ratios (ORs) in Predicting BRCA2 PV Carrier Status.
| Cancer diagnosis | No. (%) | Adjusted OR (95% CI)a | P value | ||
|---|---|---|---|---|---|
| Total | BRCA1 | BRCA2 | |||
| All cancers | 1634 | 494 | 1140 | 1.00 [Reference] | |
| Male breast cancer | 577 (35.3) | 63 (12.7) | 514 (45.1) | 5.47 (4.06-7.37) | <.001 |
| Prostate cancer | 414 (25.3) | 112 (22.7) | 302 (26.5) | 1.39 (1.09-1.78) | .008 |
| Cancers other than breast and prostate | 643 (39.4) | 319 (64.6) | 324 (28.4) | 0.22 (0.18-0.28) | <.001 |
| Colorectal cancer | 84 (13.1) | 55 (17.2) | 29 (9.0) | 0.47 (0.29-0.78) | .003 |
| Melanoma | 62 (9.6) | 33 (10.3) | 29 (9.0) | 0.76 (0.43-1.34) | .35 |
| Pancreatic cancer | 48 (7.5) | 13 (4.1) | 35 (10.8) | 3.00 (1.55-5.81) | .001 |
Abbreviation: CIMBA, Consortium of Investigators of Modifiers of BRCA1/2.
Analyses adjusted for age at cancer diagnosis/last follow-up, country of origin and calendar year of interview.
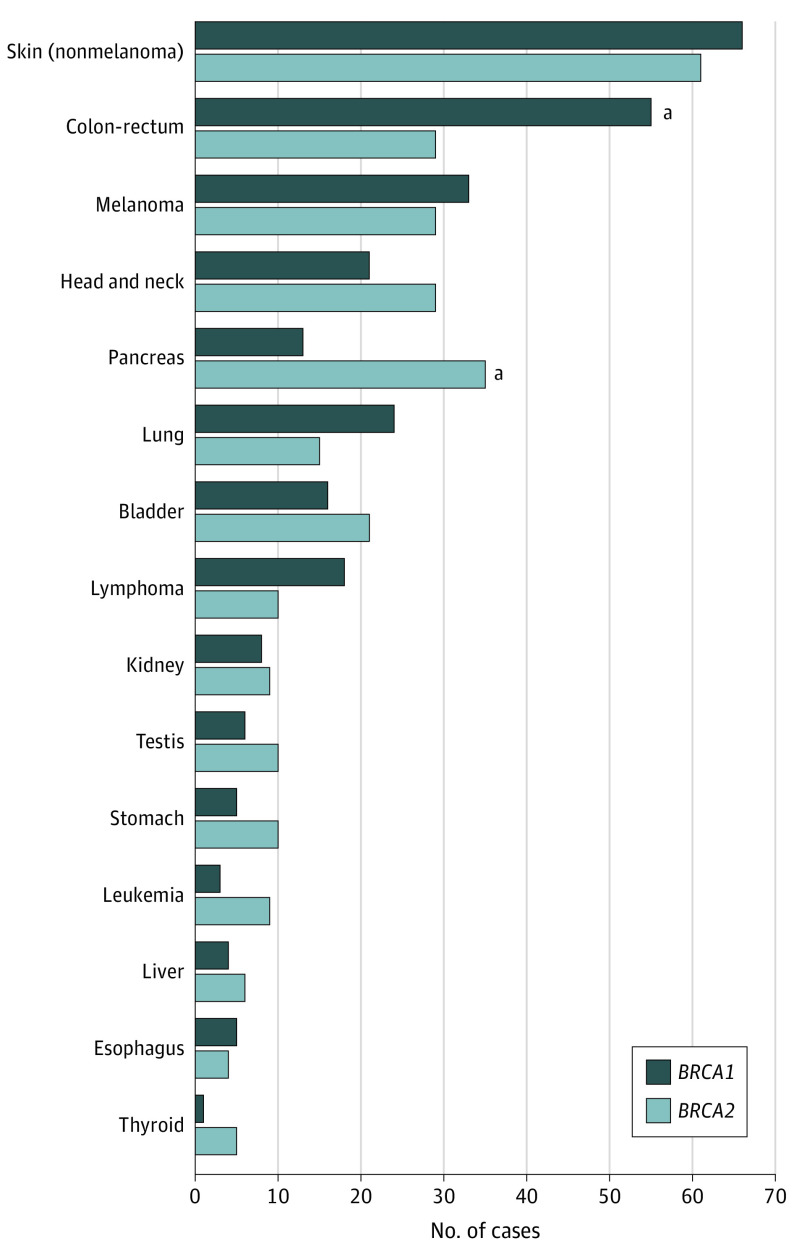
Considering cancers other than breast and prostate, more than 60 different cancer sites were reported (eTable 8 in the Supplement). The most common nonbreast and nonprostate cancer types (>60 diagnoses each) were nonmelanoma skin cancer, colorectal cancer and melanoma; other frequently reported cancer types (>30 diagnoses each) were head and neck, pancreatic, lung, and bladder cancers (Figure 2; eTable 8 in the Supplement). Cancer phenotype varied between BRCA1 and BRCA2 PV carriers (Figure 2 and Table 3). In particular, among the nonbreast and nonprostate cancers, pancreatic cancer was associated with a higher probability of being a BRCA2 carrier (OR, 3.00; 95% CI, 1.55-5.81; P = .001), and colorectal cancer was associated with a lower probability of being a BRCA2 PV carrier (OR, 0.47; 95% CI, 0.29-0.78; P = .003), in analyses adjusted for age, country of origin, and calendar year of interview (Table 3). No statistically significant differences in the frequencies of other cancer diagnoses between BRCA1 and BRCA2 PV carriers were found. Analyses adjusted also for family history of male breast cancer, female breast cancer, and prostate cancer gave similar results (eTable 9 in the Supplement). Similar findings were also obtained in analyses omitting cancer diagnoses occurring more than 5 years prior to study recruitment (eTable 10 in the Supplement).
Figure 2. Spectrum of Cancers Other Than Breast and Prostate in Male BRCA1 and BRCA2 Pathogenic Variant (PV) Carriers.
Cancer sites other than breast and prostate with >5 reported diagnoses in the whole series of male BRCA1/2 PV carriers within the Consortium of Investigators of Modifiers of BRCA1/2 data set.
aSignificant differences between BRCA1 and BRCA2.
Discussion
Men with BRCA1/2 PVs represent an underinvestigated group that poses clinical challenges. The paucity of data on cancers arising in male BRCA1/2 PV carriers has limited the development of evidence-based clinical guidelines for surveillance and prevention in men harboring BRCA1/2 PVs.29
By taking advantage of data collected through CIMBA, we characterized the cancer spectrum in male BRCA1/2 PV carriers and compared BRCA1 with BRCA2 PV carriers in terms of number, site, and age of cancer diagnoses. We believe this study comprises the largest series of male BRCA1/2 PV carriers collected worldwide to date.
Our results highlight specific, unique differences in the cancer spectrum of male BRCA2 vs BRCA1 PV carriers. Being affected with cancer and developing multiple cancer types at younger ages was associated with a higher probability of being a BRCA2 PV carrier.
Intercancer intervals were shorter in male BRCA2 PV carriers with a first diagnosis of breast or prostate cancers, compared with other cancers, thus suggesting that BRCA2-associated breast and prostate cancers may have a worse prognosis. However, age difference at first diagnosis, being older for breast or prostate cancer compared with the other cancers, may affect intercancer intervals.
While recommended guidelines for early detection and cancer risk reduction for female BRCA1/2 PV carriers are evidence based,32 only limited recommendations, based on low-level evidence or expert opinion, are available for male BRCA1/2 PV carriers.29 Current National Comprehensive Cancer Network (NCCN),32 European Society for Medical Oncology (August 2016),33 and American Society of Clinical Oncology (2017)34 guidelines recommend annual clinical breast examination starting at age 30 to 35 years, and clinical prostate cancer screening, particularly for BRCA2 PV carriers, starting at age 40 to 45 years. American Society of Clinical Oncology recommendations also suggest consideration of baseline mammograms on an individual basis.34
Recent studies have shown that mammography can detect clinically occult breast cancer when screening high-risk men, including BRCA1/2 PV carriers.35,36,37 Moreover, interim results from the International Prospective Prostate Cancer Screening (IMPACT) study have shown that the use of systematic prostate-specific antigen screening can detect clinically significant prostate cancers in male BRCA2 PV carriers.38 Based on those findings and on our data demonstrating that male BRCA2 PV carriers more frequently develop breast and prostate cancers as a first or second tumor, future guidelines should consider recommending mammography and systematic prostate-specific antigen testing for male BRCA2 PV carriers, although formal evaluation of these screening strategies is warranted in this set.
Our data also show that among the nonbreast and nonprostate cancers, pancreatic cancer was associated with a higher probability of being a BRCA2 PV carrier. This observation reinforces the evidence of a sex-independent association between BRCA2 PVs and pancreatic cancer.14,19,20 Our findings are consistent with those from previous studies of families with BRCA2 PVs showing that the spectrum of cancers for male carriers is largely attributable to the excess of breast, prostate, and pancreatic cancers.19
A prospective study on screening protocols for male BRCA1/2 PV carriers suggested a role for screening for pancreatic cancer in addition to prostate and breast cancer.24 Both National Comprehensive Cancer Network and European Society for Medical Oncology guidelines suggest individualizing screening for pancreatic cancer based on family specific-cancer history.32,33,34 Our results provide further evidence to consider screening for pancreatic cancer in male BRCA2 PV carriers. However, given the lack of data regarding the effectiveness of any pancreatic cancer screening program, male BRCA2 PV carriers should be strongly encouraged to participate in clinical trials evaluating such screening strategies.33
In our study, most of the commonly reported cancers in male BRCA1/2 PV carriers are also common in the general population and are possibly associated with environmental or lifestyle risk factors, such as smoking, although a role of gene-environment interactions in increasing cancer risks may be suggested.39,40,41,42 However, country-specific environmental influences and lifestyle factors cannot be excluded. The absence of reliable risk estimates in BRCA1/2 PV carriers for these cancers, especially for colorectal cancer,43 leads to uncertainty about appropriate screening protocols. Nevertheless, education and awareness regarding signs and symptoms of these cancer types and strict adherence to population screening guidelines are highly warranted for male BRCA1/2 PV carriers.
Limitations
There are some limitations to the current study. First, this study was largely retrospective, and data may not have been systematically collected. Second, cases were mostly recruited from high-risk clinics and/or high-risk families, and hence a selection bias toward having more affected individuals seems likely. However, the proportions of BRCA1 and BRCA2 PV carriers, as well as affected to unaffected ratios, are consistent with previously reported series of male BRCA1/2 PV carriers.2,7,8,9,14 Furthermore, the series included male carriers, both family probands and members, collected by different centers, and ascertainment bias may have occurred.
We assumed similar biases for BRCA1/2; thus, the study was designed to compare BRCA1 with BRCA2 PV carriers. However, BRCA1/2 genetic testing might have been performed based on cancer types or cancer family history, and genetic testing approaches and inclusion criteria might have changed over time. To account for such biases, different models, adjusted for cancer family history, proband status, and calendar year of the first interview, were performed. To assess the potential influence of survival bias, key analyses were repeated considering only cancer diagnoses within 5 years from study recruitment.
A high number of BRCA1/2 mutations is reported in our series. Recently, an association between specific regions of BRCA2 and prostate cancer risk was demonstrated.31 Genotype-phenotype associations deserve to be further investigated for other cancers, particularly breast and pancreatic, arising in male BRCA2 PV carriers.
The present study design does not allow for inference on the associations of specific cancer types in men with BRCA1 or BRCA2 PVs owing to the lack of a similar comparison group without PVs. Thus, associations between the observed cancer types and BRCA1 or BRCA2 PVs could not be analyzed, and age-specific cancer risks for male carriers could not be estimated. Further research, ideally large prospective studies, to obtain reliable cancer risk estimates in male BRCA1/2 PV carriers is urgently needed to refine clinical management strategies.
Conclusions
Our results, derived from analyses of the largest available (to our knowledge) male BRCA1/2 PV carrier data set, provide reliable data on the cancer spectrum in male BRCA1 and BRCA2 PV carriers. Being affected by any cancer and developing multiple cancers, particularly breast, prostate, and pancreatic cancers, was associated with a higher probability of being a BRCA2, rather than a BRCA1, PV carrier. These data may represent a step toward evidence-based guidelines and may help to refine existing recommendations in specifying distinct surveillance guidelines for men with either BRCA1 or BRCA2 PVs.
eTable 1. Male BRCA1 and BRCA2 PV carriers by study group/country.
eTable 2. BRCA1 and BRCA2 PV spectrum of male carriers included in this study.
eTable 3. Self-reported ethnicity in male BRCA1/2 PV carriers (regardless of cancer diagnosis) within the CIMBA dataset.
eTable 4. Proband/Family member status in breast, prostate and other cancers in male BRCA1/2 PV carriers within the CIMBA dataset.
eTable 5. Family history (FH) of male breast, female breast and prostate cancers in male BRCA1/2 PV carriers (regardless of cancer diagnosis) within the CIMBA dataset.
eTable 6. Cancer diagnosis in male BRCA1/2 PV carriers within the CIMBA dataset and ORs in predicting BRCA2 PV carrier status.
eTable 7. Analysis restricted to the first cancer diagnosis according to cancer site and BRCA1/2 PV in the 1,376 affected male carriers within CIMBA dataset and ORs in predicting BRCA2 PV carrier status.
eTable 8. Cancer sites other than breast and prostate according to BRCA1/2 PV status and mean age at diagnosis.
eTable 9. Analysis restricted to the total cancers reported in the 1,376 affected male BRCA1/2 PV carriers within the CIMBA dataset and ORs in predicting BRCA2 PV carrier status.
eTable 10. Analyses restricted to affected individuals after omitting cancer diagnoses occurring more than 5 years prior to study recruitment.
eFigure 1. Age distribution graphs in the whole series of 6,902 male BRCA1 and BRCA2 PV carriers (A) and in the 1,376 affected male BRCA1 and BRCA2 PV carriers (B). Age is considered at cancer diagnosis for affected individuals and at last follow-up for unaffected individuals.
References
- 1.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. ; BRCA1 and BRCA2 Cohort Consortium . Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402-2416. doi: 10.1001/jama.2017.7112 [DOI] [PubMed] [Google Scholar]
- 2.Breast Cancer Linkage Consortium Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91(15):1310-1316. doi: 10.1093/jnci/91.15.1310 [DOI] [PubMed] [Google Scholar]
- 3.Thompson D, Easton DF; Breast Cancer Linkage Consortium . Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94(18):1358-1365. doi: 10.1093/jnci/94.18.1358 [DOI] [PubMed] [Google Scholar]
- 4.Iqbal J, Ragone A, Lubinski J, et al. ; Hereditary Breast Cancer Study Group . The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer. 2012;107(12):2005-2009. doi: 10.1038/bjc.2012.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzolo P, Silvestri V, Tommasi S, et al. Male breast cancer: genetics, epigenetics, and ethical aspects. Ann Oncol. 2013;24(suppl 8):i75–, i82.. doi: 10.1093/annonc/mdt316 [DOI] [PubMed] [Google Scholar]
- 6.van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, et al. ; Netherlands Collaborative Group on Hereditary Breast Cancer (HEBON) . Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. J Med Genet. 2005;42(9):711-719. doi: 10.1136/jmg.2004.028829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tai YC, Domchek S, Parmigiani G, Chen S. Breast cancer risk among male BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(23):1811-1814. doi: 10.1093/jnci/djm203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans DG, Susnerwala I, Dawson J, Woodward E, Maher ER, Lalloo F. Risk of breast cancer in male BRCA2 carriers. J Med Genet. 2010;47(10):710-711. doi: 10.1136/jmg.2009.075176 [DOI] [PubMed] [Google Scholar]
- 9.Kote-Jarai Z, Leongamornlert D, Saunders E, et al. ; UKGPCS Collaborators . BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105(8):1230-1234. doi: 10.1038/bjc.2011.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leongamornlert D, Mahmud N, Tymrakiewicz M, et al. ; UKGPCS Collaborators . Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106(10):1697-1701. doi: 10.1038/bjc.2012.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards SM, Kote-Jarai Z, Meitz J, et al. ; Cancer Research UK/British Prostate Group UK Familial Prostate Cancer Study Collaborators; British Association of Urological Surgeons Section of Oncology . Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene. Am J Hum Genet. 2003;72(1):1-12. doi: 10.1086/345310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchhoff T, Kauff ND, Mitra N, et al. BRCA mutations and risk of prostate cancer in Ashkenazi Jews. Clin Cancer Res. 2004;10(9):2918-2921. doi: 10.1158/1078-0432.CCR-03-0604 [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim M, Yadav S, Ogunleye F, Zakalik D. Male BRCA mutation carriers: clinical characteristics and cancer spectrum. BMC Cancer. 2018;18(1):179. doi: 10.1186/s12885-018-4098-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mersch J, Jackson MA, Park M, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer. 2015;121(2):269-275. doi: 10.1002/cncr.29041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyberg T, Frost D, Barrowdale D, et al. Prostate cancer risks for male BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Eur Urol. 2020;77(1):24-35. doi: 10.1016/j.eururo.2019.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agalliu I, Kwon EM, Zadory D, et al. Germline mutations in the BRCA2 gene and susceptibility to hereditary prostate cancer. Clin Cancer Res. 2007;13(3):839-843. doi: 10.1158/1078-0432.CCR-06-2164 [DOI] [PubMed] [Google Scholar]
- 17.Fachal L, Gómez-Caamaño A, Celeiro-Muñoz C, et al. BRCA1 mutations do not increase prostate cancer risk: results from a meta-analysis including new data. Prostate. 2011;71(16):1768-1779. doi: 10.1002/pros.21394 [DOI] [PubMed] [Google Scholar]
- 18.Laitman Y, Keinan Boker L, Liphsitz I, et al. Cancer risks in Jewish male BRCA1 and BRCA2 mutation carriers. Breast Cancer Res Treat. 2015;150(3):631-635. doi: 10.1007/s10549-015-3340-4 [DOI] [PubMed] [Google Scholar]
- 19.Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22(4):735-742. doi: 10.1200/JCO.2004.05.055 [DOI] [PubMed] [Google Scholar]
- 20.Risch HA, McLaughlin JR, Cole DE, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98(23):1694-1706. doi: 10.1093/jnci/djj465 [DOI] [PubMed] [Google Scholar]
- 21.Mohamad HB, Apffelstaedt JP. Counseling for male BRCA mutation carriers: a review. Breast. 2008;17(5):441-450. doi: 10.1016/j.breast.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 22.Cavanagh H, Rogers KMA. The role of BRCA1 and BRCA2 mutations in prostate, pancreatic and stomach cancers. Hered Cancer Clin Pract. 2015;13(1):16. doi: 10.1186/s13053-015-0038-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streff H, Profato J, Ye Y, et al. Cancer incidence in first- and second-degree relatives of BRCA1 and BRCA2 mutation carriers. Oncologist. 2016;21(7):869-874. doi: 10.1634/theoncologist.2015-0354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mano R, Tamir S, Kedar I, et al. Malignant abnormalities in male BRCA mutation carriers: results from a prospectively screened cohort. JAMA Oncol. 2018;4(6):872-874. doi: 10.1001/jamaoncol.2018.0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silvestri V, Barrowdale D, Mulligan AM, et al. ; kConFab Investigators; Hereditary Breast and Ovarian Cancer Research Group Netherlands (HEBON); EMBRACE . Male breast cancer in BRCA1 and BRCA2 mutation carriers: pathology data from the Consortium of Investigators of Modifiers of BRCA1/2. Breast Cancer Res. 2016;18(1):15. doi: 10.1186/s13058-016-0671-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leão RRN, Price AJ, James Hamilton R. Germline BRCA mutation in male carriers-ripe for precision oncology? Prostate Cancer Prostatic Dis. 2018;21(1):48-56. doi: 10.1038/s41391-017-0018-5 [DOI] [PubMed] [Google Scholar]
- 27.Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol. 2015;68(2):186-193. doi: 10.1016/j.eururo.2014.10.022 [DOI] [PubMed] [Google Scholar]
- 28.Blair AB, Groot VP, Gemenetzis G, et al. BRCA1/BRCA2 germline mutation carriers and sporadic pancreatic ductal adenocarcinoma. J Am Coll Surg. 2018;226(4):630-637.e1. doi: 10.1016/j.jamcollsurg.2017.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forbes C, Fayter D, de Kock S, Quek RGW. A systematic review of international guidelines and recommendations for the genetic screening, diagnosis, genetic counseling, and treatment of BRCA-mutated breast cancer. Cancer Manag Res. 2019;11:2321-2337. doi: 10.2147/CMAR.S189627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chenevix-Trench G, Milne RL, Antoniou AC, Couch FJ, Easton DF, Goldgar DE; CIMBA . An international initiative to identify genetic modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: the Consortium of Investigators of Modifiers of BRCA1 and BRCA2 (CIMBA). Breast Cancer Res. 2007;9(2):104. doi: 10.1186/bcr1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel VL, Busch EL, Friebel TM, et al. Association of genomic domains in BRCA1 and BRCA2 with prostate cancer risk and aggressiveness. Cancer Res. 2020;80(3):624-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Comprehensive Cancer Network NCCN guidelines genetic/familial high-risk assessment: breast and ovarian. Version 3.2019. Accessed May 14, 2019. https://www.nccn.org/
- 33.Paluch-Shimon S, Cardoso F, Sessa C, et al. ; ESMO Guidelines Committee . Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO clinical practice guidelines for cancer prevention and screening. Ann Oncol. 2016;27(suppl 5):v103-v110. doi: 10.1093/annonc/mdw327 [DOI] [PubMed] [Google Scholar]
- 34.American Society of Clinical Oncology Hereditary breast and ovarian cancer. Accessed September 30, 2019. https://www.cancer.net/cancer-types/hereditary-breast-and-ovarian-cancer
- 35.Marino MA, Gucalp A, Leithner D, et al. Mammographic screening in male patients at high risk for breast cancer: is it worth it? Breast Cancer Res Treat. 2019;177(3):705-711. doi: 10.1007/s10549-019-05338-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao Y, Goldberg JE, Young TK, Babb JS, Moy L, Heller SL. Breast cancer screening in high-risk men: a 12-year longitudinal observational study of male breast imaging utilization and outcomes. Radiology. 2019;293(2):282-291. doi: 10.1148/radiol.2019190971 [DOI] [PubMed] [Google Scholar]
- 37.Woods RW, Salkowski LR, Elezaby M, Burnside ES, Strigel RM, Fowler AM. Image-based screening for men at high risk for breast cancer: benefits and drawbacks. Clin Imaging. 2020;60(1):84-89. doi: 10.1016/j.clinimag.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page EC, Bancroft EK, Brook MN, et al. ; IMPACT Study Collaborators . Interim results from the IMPACT study: evidence for prostate-specific antigen screening in BRCA2 mutation carriers. Eur Urol. 2019;76(6):831-842. doi: 10.1016/j.eururo.2019.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palli D, Masala G, Mariani-Costantini R, et al. A gene-environment interaction between occupation and BRCA1/BRCA2 mutations in male breast cancer? Eur J Cancer. 2004;40(16):2474-2479. doi: 10.1016/j.ejca.2004.07.012 [DOI] [PubMed] [Google Scholar]
- 40.Rudolph A, Chang-Claude J, Schmidt MK. Gene-environment interaction and risk of breast cancer. Br J Cancer. 2016;114(2):125-133. doi: 10.1038/bjc.2015.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiechle M, Engel C, Berling A, et al. Effects of lifestyle intervention in BRCA1/2 mutation carriers on nutrition, BMI, and physical fitness (LIBRE study): study protocol for a randomized controlled trial. Trials. 2016;17:368. doi: 10.1186/s13063-016-1504-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonds NI, Ghazarian AA, Pimentel CB, et al. Review of the gene-environment interaction literature in cancer: what do we know? Genet Epidemiol. 2016;40(5):356-365. doi: 10.1002/gepi.21967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans DG, Clancy T, Hill J, Tischkowitz M. Is there really an increased risk of early colorectal cancer in women with BRCA1 pathogenic mutations? Clin Genet. 2016;89(3):399. doi: 10.1111/cge.12687 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Male BRCA1 and BRCA2 PV carriers by study group/country.
eTable 2. BRCA1 and BRCA2 PV spectrum of male carriers included in this study.
eTable 3. Self-reported ethnicity in male BRCA1/2 PV carriers (regardless of cancer diagnosis) within the CIMBA dataset.
eTable 4. Proband/Family member status in breast, prostate and other cancers in male BRCA1/2 PV carriers within the CIMBA dataset.
eTable 5. Family history (FH) of male breast, female breast and prostate cancers in male BRCA1/2 PV carriers (regardless of cancer diagnosis) within the CIMBA dataset.
eTable 6. Cancer diagnosis in male BRCA1/2 PV carriers within the CIMBA dataset and ORs in predicting BRCA2 PV carrier status.
eTable 7. Analysis restricted to the first cancer diagnosis according to cancer site and BRCA1/2 PV in the 1,376 affected male carriers within CIMBA dataset and ORs in predicting BRCA2 PV carrier status.
eTable 8. Cancer sites other than breast and prostate according to BRCA1/2 PV status and mean age at diagnosis.
eTable 9. Analysis restricted to the total cancers reported in the 1,376 affected male BRCA1/2 PV carriers within the CIMBA dataset and ORs in predicting BRCA2 PV carrier status.
eTable 10. Analyses restricted to affected individuals after omitting cancer diagnoses occurring more than 5 years prior to study recruitment.
eFigure 1. Age distribution graphs in the whole series of 6,902 male BRCA1 and BRCA2 PV carriers (A) and in the 1,376 affected male BRCA1 and BRCA2 PV carriers (B). Age is considered at cancer diagnosis for affected individuals and at last follow-up for unaffected individuals.