EDITORIAL SUMMARY
[18F]6-Fluoro-L-DOPA ([18F]FDOPA), is used for diagnostic PET imaging. Using this protocol, radiofluorination of the electron-rich catechol ring can be achieved in the presence of the amino acid group by Cu-mediated fluorination of a pinacol boronate precursor.
[18F]6-Fluoro-L-DOPA ([18F]FDOPA) is a diagnostic radiopharmaceutical for positron emission tomography (PET) imaging that is used to image Parkinson’s disease, brain tumors, and focal hyperinsulinism of infancy. Despite these important applications, [18F]FDOPA PET remains underutilized because of synthetic challenges associated with accessing the radiotracer for clinical use, stemming from the need to radiofluorinate a highly electron rich catechol ring in the presence of an amino acid. To address this longstanding challenge in the PET radiochemistry community, we have developed a one-pot, two-step synthesis of high molar activity [18F]FDOPA by Cu-mediated fluorination of a pinacol boronate (BPin) precursor. The method is fully automated, has been validated to work well at two separate sites (an academic facility with a cyclotron on site and an industry lab purchasing [18F]fluoride from an outside vendor), and provides [18F]FDOPA in reasonable radiochemical yield (2.44 ± 0.70 GBq, 66 ± 19 mCi, 5 ± 1%), excellent radiochemical purity (>98%) and high molar activity (76 ± 30 TBq/mmol, 2050 ± 804 Ci/mmol), n=26. Herein we report a detailed protocol for the synthesis of [18F]FDOPA which has been successfully implemented at two sites and validated for production of the radiotracer for human use.
INTRODUCTION
Positron emission tomography (PET) imaging is a noninvasive nuclear medicine imaging technique that uses bioactive molecules labeled with positron-emitting radionuclides (radiopharmaceuticals) to quantify physiological processes.1 One such diagnostic radiopharmaceutical is [18F]6-fluoro-L-DOPA ([18F]FDOPA)2,3 and, since the first use of [18F]FDOPA PET for imaging the human brain in the early 1980s,4 it has been used for imaging of large amino acid transport and dopaminergic neurons. Reflecting this, [18F]FDOPA PET finds application in Parkinson’s disease,5 neuro-oncology,6,7 and focal hyperinsulinism of infancy.8
Despite the utility of [18F]FDOPA PET in these important applications in diagnostic nuclear medicine, the radiopharmaceutical remains underutilized because of challenges associated with synthesizing the radiotracer for clinical use.3 These issues stem primarily from difficulties in radiofluorinating a highly electron rich aromatic ring, and the need to protect (and deprotect) both catechol and amino acid functionalities. Historically [18F]6-fluoro-L-DOPA was synthesized via electrophilic aromatic substitution (SEAr) using electrophilic fluorinating agents (e.g. [18F]F2 or [18F]acetyl hypofluorite) and an appropriate precursor such as a stannane (Figure 1a).9,10 New variants of such methods continue to be reported,11 but such SEAr reactions are challenging because of the need for specialized equipment, modest site- and chemoselectivities, and low molar activity products.3
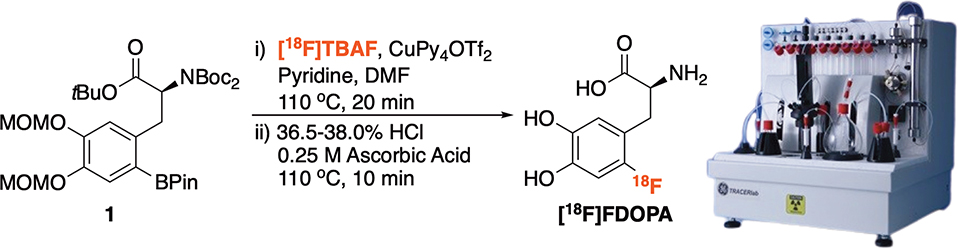
Figure 1 |.
Radiosyntheses of [18F]FDOPA and motivation for this work (adapted from Ref. 43 with permission from The Royal Society of Chemistry).
In contrast to electrophilic reagents like [18F]F2, [18F]fluoride is readily available in multi-Curie amounts and high molar activity from small medical cyclotrons and is used daily in radiochemistry production facilities all over the world. As such, significant research has been aimed at developing a synthesis of [18F]FDOPA using nucleophilic [18F]fluoride. However, the incompatibility of 18F- and the electron rich catechol ring has complicated efforts to develop an operationally simple synthesis of [18F]FDOPA from nucleophilic [18F]fluoride to date. The typical approach involves a multistep synthesis involving initial nucleophilic radiofluorination of a benzaldehyde precursor with an appropriate leaving group.3 Depending on the choice of precursor, the synthesis then either involves coupling of the amino acid side chain12,13,14,15 or oxidation of the 18F-labelled aldehyde intermediate (usually with mCPBA) and hydrolysis of the resulting ester.16,17,18 In either case, final deprotection with concentrated HI or HBr generates [18F]FDOPA (Figure 1b). This strategy has been used to synthesize [18F]FDOPA in good yields and molar activity, and commercial cassette solutions are available.14,19,20 However, operational complexity, stemming from the need to conduct multiple steps after labelling with 18F and use of corrosive acids for deprotection, has limited such methods to certain synthesis modules or manual radiochemistry set-ups. As such, there remains a need for a simple one-pot, two-step (fluorination + deprotection) synthesis of [18F]FDOPA using nucleophilic [18F]fluoride that is high yielding, uses milder reagents, and can be easily automated using standard radiochemistry synthesis modules.
Copper-mediated radiofluorination technique overview
The last few years have seen renewed interest in developing new fluorine-18 radiochemistry (for recent reviews, see: 21,22,23,24), and novel methods enabling radiofluorination of hypervalent iodine reagents,25,26,27,28 organoborons,29,30 stannanes,31 Pd-32 and Ni-complexes,33 and phenols34 with nucleophilic [18F]fluoride have enabled facile radiolabelling of electron rich arenes. Of these new approaches, Cu-mediated fluorination has emerged as a powerful labeling technique that has been widely adopted by the PET radiochemistry community. Originally introduced by Sanford in 2013 for fluorination of iodonium salts and organoborons,35,36 the first report by Sanford and Scott adapting the method for radiolabeling demonstrated the Cu-mediated 18F-fluorination of (mesityl)(aryl)iodonium salts.25 Subsequently, a method for the Cu-mediated 18F-fluorination of pinacol boronate (BPin) esters was reported by Gouverneur,29 while Sanford and Scott have also disclosed 18F-fluorination of organoborons,30 organostannanes31 and aromatic C-H bonds.37 These methods enable direct introduction of nucleophilic [18F]fluoride into electron rich arenes and are ideally suited for the synthesis of [18F]FDOPA. A number of these approaches have been used to synthesize [18F]FDOPA in preliminary proof-of-concept studies (Figure 1c),25,31,33,38,39,40,41,42 and we recently developed a method that is compliant with current Good Manufacturing Practice (cGMP) and the subject of this Protocol (Figure 1d).43 This method has been validated for production of human doses and can be used as a starting point for creating regulatory filings (e.g. a Chemistry, Manufacturing and Controls (CMC) section for an Investigational New Drug (IND) application or a New Drug Application (NDA)). A comparison of these new methods for preparing [18F]FDOPA with the historical approaches is provided in Figure 1, along with a summary of the advantages and limitations of each strategy.
Protocol Outline
This protocol describes a one-pot, two-step synthesis of [18F]FDOPA from a BPin precursor using a General Electric (GE) TRACERLab FXFN synthesis module (Figure 2). The method is also compatible with other synthesis modules and/or manual synthesis setups. High molar activity [18F]fluoride is prepared on a cyclotron (Step 9) and used to prepare [18F]FDOPA via the Cu-mediated radiofluorination of commercially available BPin precursor (1) (Steps 10 – 15). Following radiofluorination, the MOM and Boc protecting groups are removed under mild conditions (HCl / ascorbic acid) (Step 16). Ascorbic acid is necessary to protect against [18F]FDOPA degradation during deprotection. We hypothesize that this decomposition is likely due to side reactions promoted by Cu(II) salts,44,45 and the addition of ascorbic acid minimizes any degradation by reducing Cu(II) to Cu(I). Purification and reformulation of the final product is then accomplished using hydrophilic interaction liquid chromatography (HILIC). [18F]FDOPA is purified by semi-preparative HPLC using a Phenomenex Luna NH2 5 μ column; the HPLC fraction corresponding to the product is collected and reformulated into sterile saline using a HILIC Strata NH2 solid-phase extraction (SPE) cartridge (Steps 16 – 22). The final drug product is dispensed into a septum-sealed, sterile, pyrogen-free glass vial through a 0.22 μm sterile filter (Millex GV) to afford sterile formulated doses of [18F]FDOPA (Step 23) that are submitted for quality control testing (Steps 24 – 36). The method is fully automated and process verification batches confirmed that doses of [18F]FDOPA made using this method met or exceeded our established quality control criteria (Table 1), and validated the production method for preparation of the radiotracer for human use.
Figure 2 |.
Radiosyntheses of [18F]FDOPA and the TRACERLab automated synthesis module (synthesis module schematics (Supplementary Figures 1 and 2) and timelists (Supplementary Method 1) are provided in the Supplementary Information).
Table 1|.
[18F]FDOPA Process Verification Batch QC Data using the One-pot Methoda
| QC Test | Specifications | Result (n = 3)b |
|---|---|---|
| Radioactivity Conc. | ≥0.37 Gbq/batch | 3.85 ± 0.59 GBq |
| F-DOPA Conc. | ≤5μg/mL | 0.69 ± 0.47 μg/mL |
| H-DOPA | ≤50μg/mL | Not detected |
| HO-DOPA | ≤50μg/mL | Not detected |
| Molar activity | ≥ 18.5 TBq/mmol | 141 ± 77 TBq/mmol |
| Radiochemical Purity | >90% | 99.7 ± 0.3% |
| Radiochemical Purity 2 h post-EOS | >90% | 99.1 ± 1.2% |
| Radiochemical Purity 4 h post-EOS | >90% | 96.5 ± 1.0% |
| Radiochemical Identity | RRTc = 0.9–1.1 | 1.02 ± 0.002 |
| Enantiomeric Purity | ≥ 95% L-FDOPA | >99% |
| Visual Inspection | Clear, colorless, no ppt | Pass |
| pH | 4.5–7.5 | 5.5 ± 0 |
| Radionuclidic Identity | T1/2 = 105–115 min | 112 ± 2 min |
| Residual TBA+ | ≤260 μg/mL by Dragendorff reagent | < 260 μg/mL |
| Residual DMF | ≤880 ppm | 106 ± 56 ppm |
| Residual MeCN | ≤410 ppm | 179 ± 78 ppm |
| Residual Cu | ≤34 ppm | 0.11 ± 0.02 ppm |
| Filter membrane integrity | ≥50 psi | 56 ± 1 psi |
| Bacterial endotoxins | ≤ 2.00 EUd/mL | <2.00 EUd/mL |
| Sterility | No microbial growth | Pass |
Some data in this Table is reproduced from Ref. 43 with permission from The Royal Society of Chemistry.
Grouped data are reported as mean ± standard deviation
Relative retention time (RRT) = [HPLC retention time of [18F]FDOPA / HPLC retention time of FDOPA reference standard]
EU = endotoxin units.
Protocol variations
Radiofluorination can be accomplished using Cu(Py)4(OTf)2 (Step 13A) or Cu(OTf)2 (Step 13B). To simplify automation, we prefer using the less hygroscopic Cu(Py)4(OTf)2. Two variations have also been utilized for removal of the MOM and Boc groups from protected [18F]FDOPA that perform similarly (see Anticpated Results) and can be selected based upon available equipment and site radiochemistry preferences. The first is a standard one-pot synthesis that uses a standard TRACERLab FXFN synthesis module set-up (see Supplementary Figure 1 for layout and Supplementary Method 1 for timelist); the deprotection cocktail (HCl / ascorbic acid) is added to the crude reaction mixture following fluorination (Step 16A). Following deprotection, the entire reaction mixture is then injected onto the column for purification of [18F]FDOPA by semi-preparative HPLC. The second variation is a pseudo-one-pot synthesis involving pre-purification of the protected-[18F]FDOPA intermediate prior to deprotection (Step 16B). Briefly, the radiofluorination reaction mixture is diluted in an aqueous solution containing ascorbic acid and/or ethylenediaminetetraacetic acid (EDTA) to reduce and coordinate copper, respectively, then passed through a reversed phase C18 plus or Oasis® Hydrophilic-Lipophilic-Balanced (HLB) SPE cartridge to trap the lipophilic protected [18F]FDOPA intermediate. Protected [18F]FDOPA is then eluted from the SPE cartridge with ethanol back into the reactor, resulting in a partially purified solution of protected [18F]FDOPA. Deprotection then proceeds as for Step 16A. This pre-purification requires a modified TRACERlab FXFN synthesis module (see Supplementary Figure 2 for configuration and Supplementary Method 1 for timelist), and has two benefits: 1) it removes most of the copper, potentially reducing the extent of oxidative [18F]FDOPA degradation during deprotection, and 2) it removes N-N-dimethylformamide (DMF), which simplifies semi-preparative HPLC. The net result is a more consistent radiochemical yield (RCY) run-to-run. It is also possible to synthesize [18F]FDOPA using a manual procedure (Box 1). This method uses lower amounts of starting [18F]fluoride than automated clinical-scale production and can be used in facilities without access to automated synthesis modules. It is also appropriate when only a small amount of [18F]FDOPA is required for a chemistry or animal imaging experiment. Lastly, Cu-mediated radiofluorination is showcased for the synthesis of [18F]FDOPA in this Protocol. However, the method is readily adaptable to the synthesis of other PET radiotracers after appropriate development of radiolabelling and deprotection conditions.
Box 1 | Manual Synthesis of [18F]FDOPA using low levels of [18F]fluoride ● TIMING Approximately 2 h.
This procedure can be used in facilities without access to automated synthesis modules or when a small amount of [18F]FDOPA is required for a chemistry or animal imaging experiment.
Procedure
Slowly transfer a solution of [18F]fluoride (e.g. ~3.7 GBq, 100 mCi) in [18O]H2O through a QMA cartridge, trapping the [18F]fluoride.
Elute [18F]fluoride from the QMA cartridge into a synthesis module reactor or manual radiochemistry setup with 0.5 mL Eluent Solution (see Reagent Setup) and azeotropically dry it. Resolubilize in DMF (4 mL) – depending on time and elution efficiency, strength will be ~10 – 20 mCi / mL.
Add 0.4 mL of the [18F]fluoride stock solution to a glass vial containing BPin precursor 1 (4 μmol), Cu(OTf)2 (20 μmol) and pyridine (500 μmol) in DMF (1 mL). Heat at 110 °C for 20 min.
Cool the reaction to 50 °C and dilute it with 0.10M ascorbic acid/0.01M EDTA solution (see Reagent Setup). Stir for 30 s, and then load the reaction mixture onto an HLB cartridge, trapping protected [18F]FDOPA on the cartridge. Elute protected [18F]FDOPA from the HLB cartridge back into a clean vial using ethanol (2 mL).
Add Alternative Deprotecting solution (see Reagent Setup) and heat at 110 °C for 20 min.
Allow mixture to cool to 50 °C before purifying and reformulating [18F]FDOPA according to Steps 18 – 23of the main Protocol.
Complete QC testing as required for chemistry or animal studies according to the methods described in Steps 24 – 36 of the main Protocol.
Typical radiochemical yields of [18F]FDOPA are 35 – 55% over 2 steps using this manual method.
MATERIALS
BIOLOGICAL MATERIALS
Pharmaceutical microorganism BioBall(s)® (Biomérieux p/n 56022 (aspergillus niger), 56024 (bacillus subtillis), 56026 (candida albicans), 56029 (clostridium sporogenes), 56040 (pseudomonas aeruginosa) and 56045 (staphylococcus aureus).
! CAUTION BioBall(s) are not hazardous but this product is classified as an infectious substance as defined. If accidental contact with materials occurs laboratory staff must follow the local first aid procedures that are normally applied following exposure to microorganisms.
REAGENTS
[18O]H2O – ABX or Rotem
18F- from 18O(p,n)18F nuclear reaction using a cyclotron
! CAUTION Fluorine-18 represents a radiological hazard and must only be used with institutional, state and/or federal authorization, and according to ALARA (as low as reasonably achievable) principles. Properly shield yourself against radiation by performing all reactions involving radioactivity in a lead-shielded fume hood or hot-cell, and follow appropriate institutional, state and/or federal radiation safety guidelines. When handling radioactive material, ensure that all appropriate personal protective equipment is worn, and that shielding, dosimeters and survey meters are always used.
Tetrabutylammonium trifluoromethanesulfonate (TBAOTf) – Aldrich 99.8%+ part number (p/n): 86888
Cesium carbonate(Cs2CO3) – Aldrich 99% p/n: 441902
! CAUTION May cause eye and skin irritation. May cause respiratory and digestive tract irritation.
Sodium bicarbonate (NaHCO3) – Fisher p/n: S6297
! CAUTION Causes eye and skin irritation. May cause respiratory tract irritation.
Potassium acetate (KOAc) – Sigma Aldrich ACS >99%, p/:n 236497
Acetic acid (AcOH) – Fisher, p/n:A35–500, or Sigma Aldrich PharmaGrade, p/n: ARK2183
! CAUTION Acetic acid causes severe eye and skin burns as well as severe digestive and respiratory tract burns. It may be harmful if absorbed through the skin. It has a flammable liquid and vapor, while glacial acetic acid solidifies below 62°F (17°C). AcOH is corrosive to metal.
Acetonitrile (MeCN), HPLC grade – fisher p/n: A998–4, or Sigma Aldrich HPLC grade, p/n: 34851
! CAUTION MeCN is a flammable liquid and vapor. It Causes eye irritation, and may be harmful if swallowed, inhaled, or absorbed through the skin. It may also cause skin and respiratory tract irritation, as well as liver and kidney damage. MeCN is metabolized to cyanide in the body, which may cause headaches, dizziness, weakness, unconsciousness, convulsions, coma and possible death.
L-Ascorbic acid – Aldrich p/n: 255564
Ethylenediamine tetraacetic acid tetrasodium salt dehydrate, Sigma Aldrich p/n: 03695
36.5 – 38.0% Hydrochloric acid – Aldrich p/n: h1758
! CAUTION Hydrochloric acid causes eye and skin burns, digestive and respiratory tract burns and may be fatal if inhaled or swallowed. Repeated or prolonged exposure may cause erosion of exposed teeth. Corrosive to metal.
Tetrakispyridine copper(II) trifluoromethanesulfonate (Cu(py)4(OTf)2) – Aldrich 95% p/n: 734527
Copper(II) trifluoromethanesulfonate (Cu(OTf)2) – Aldrich 95% p/n: 283673
▲ CRITICAL Cu(OTf)2 is hygroscopic but can be handled in air for quick transfers.
N,N-Dimethylformamide (DMF), 99.8% extra dry – Acros; p/n: 448381000 [Note: DMF29,30,43,46 or N,N-dimethylacetamide (DMA)38,42,52 can be used as the reaction solvent for this chemistry. Both give comparable yields of [18F]FDOPA in our experience and have similar residual solvent limits,47 but with ease of routine synthesis in mind we selected DMF for this protocol because it is available in convenient 25 mL single use ampules].
! CAUTION DMF is a flammable liquid and vapor. DMF may cause harm to the unborn child, and is also harmful if absorbed through skin or inhaled. It is a lachrymator (substance which increases the flow of tears) and also causes eye and skin irritation. DMF may cause respiratory tract irritation, liver and kidney damage, and/or central nervous system effects.
FDOPA BPin precursor (1) – ABX p/n: 1312.0020. This can also be synthesised as described in Box 2.
Pyridine, anhydrous – Aldrich, 99.8%, p/n: 270970
Box 2 | Preparation of BPin Precursor (1) ● TIMING Approximately 45 h.
Procedure (Full experimental details including detailed descriptions of synthesis methods, purification strategies and characterization data (NMR and mass spectra) are provided in Supplementary Method 2)

Procedure
Treat L-DOPA (10.0 g, 50.7mmol) with perchloric acid (6.54 mL, 76.0 mmol) in tert-butyl acetate (120 mL) overnight to generate (S)-tert-butyl 2-amino-3-(3,4-dihydroxyphenyl)propanoate (16.4 g); use this product in Step 2 without further purification.
Dissolve (S)-tert-butyl 2-amino-3-(3,4-dihydroxyphenyl)propanoate (16.4 g) in ethanol (250 mL) and add Boc2O (11.1 g, 50.7 mmol). After 30 min at room temperature (rt), purify the resulting catechol (2) by flash chromatography on silica (heptane/EtOAc, 0–70%). The expected yield over two steps is 5.46 g (30%).
Dissolve Catechol 2 (5.44 g, 14.0 mmol) in dichloromethane (DCM) (70 mL) and add N,N-diisopropylethylamine (DIPEA) (4.9 mL, 28 mmol). Cool the mixture to 0 oC and add bromomethyl methyl ether (MOMBr) (2.3 mL, 28.0 mmol). Stir the reaction at rt for 45 min and then add more DIPEA (4.9 mL, 28.0 mmol) and MOMBr (2.3 mL, 28.0 mmol). After a total reaction time of 4 h, purify the product by flash chromatography on silica (heptane/EtOAc, 0–30%) to yield 4.95 g of bis-MOM protected derivative 3 (80% yield).
Dissolve the Bis-MOM protected derivative 3 (5.04 g, 11.4 mmol) in DCM (67 mL) and cool to 0 oC. Add Phenyliodine bis(trifluoroacetate) (PIFA) (5.89 g,13.7 mmol) and iodine (2.9 g, 11.4 mmol), and then allow the reaction to warm up to rt and stir for 1.5 h. Purify by flash chromatography on silica (heptane/EtOAc, 0–30%); the expected yield of 4, is 6.11 g (94%).
Dissolve Iodide 4 (4.97 g, 8.76 mmol), bis(pinacolato)diboron ((BPin)2) (5.56 g, 21.9 mmol), Pd(dppf)Cl2 (4.81 g, 6.57 mmol) and potassium acetate (KOAc) (2.58 g, 26.3 mmol) under Ar in DMF. Stir the mixture for 2.5 h at 80 oC then purify by flash chromatography on silica (heptane/EtOAc, 0–22%) to get 2.78 g of pinacol ester 5 (50% yield).
Dissolve Pinacol ester 5 (2.78 g, 4.90 mmol), Boc2O (5.35 g, 24.5 mmol) and 4-dimethylaminopyridine (DMAP) (300 mg, 2.5 mmol) in MeCN, and stir the mixture at rt overnight. Purification by semi-preparative HPLC (HPLC: Agilent Infinity II 1290 preparative LCMS; Column: Waters XSelect CSH C18, 30 × 150 mm, 5μ; Isocratic mobile phase: H2O:MeCN (95:5); flow rate: 50 mL/min) provided 1.17 g of BPin precursor 1 (33% yield).
■ PAUSE POINT BPin precursor 1 can be stored for >1 year at −20 oC
Results (BPin precursor 1)
1H NMR (600 MHz, DMSO-d6) δ = 7.30 (s, 1H), 6.73 (s, 1H), 5.16–5.12 (m, 3H), 5.07 (d, J = 6.7 Hz, 1H), 5.03 (dd, J = 11.5, 3.9 Hz, 1H), 3.80 (dd, J = 13.2, 3.9 Hz, 1H), 3.37 (s, 6H), 2.90 (dd, J = 13.3, 11.5 Hz, 1H), 1.44 (s, 9H), 1.30–1.26 (m, 30H).
ESI-MS: m/z = 690 [(M+Na+)].
Notes
BPin precursor 1 is also commercially available from ABX Advanced Biochemicals (Part No. 1312.0020).
! CAUTION Pyridine is a flammable liquid and vapor associated with a strong stench. Pyridine causes severe eye and skin irritation with possible burns, and respiratory tract irritation. It may be harmful if swallowed, inhaled, or absorbed through the skin, and may also cause central nervous system depression.
Ethanol, 200 proof, dehydrated – Akorn dehydrated ethanol for injection, p/n: 11635 or Seton Dehydrated Alchohol Injection, USP NDC: 13925–177-10.
Absolute Ethanol, USP –Sigma Aldrich HPLC p/n: 45928–4L
! CAUTION Ethanol is a flammable liquid and vapor that causes severe eye and respiratory tract irritation as well as moderate skin irritation. This substance has caused adverse reproductive and fetal effects in humans. Ethanol may also cause central nervous system depression, liver, kidney and heart damage.
0.9% sodium chloride solution, USP – Hospira, p/n: 55277
Sterile water for injection, USP, Hospira, cat# 4887–50
Water, ACS Reagent for ultratrace, p/n: 14211–1L-F
Water, for HPLC, Sigma Aldrich, p/n: 34877
Water, Milli-Q – obtained in house from a Milli-Q Ultrapure Water system
Drierite® - Acros, pn: 350010020
EQUIPMENT
Synthesis and Purification of [18F]6-Fluoro-L-DOPA
TRACERLab FXFN radiochemistry synthesis module (or equivalent automated synthesis module that can be configured for Cu-mediated radiofluorination chemistry)
10cc sterile multi-dose vial: Hollister-Stier p/n: 7515ZA
2cc sterile dose vial:
0.2 μm millex GV filter: Millipore p/n: SLGV013SL
0.2 μm millex FG filter: Millipore p/n: SLFG025LS
Phenomenex Strata® NH2 200mg SPE cartridge: Phenomenex p/n: 8B-5009-FBJ
Waters Light QMA SPE cartridge: Waters p/n: WAT023525
Waters HLB Short Plus cartridge: Waters p/n: WAT186000132
Luna NH2 5μ 10×250mm semi-preparative HPLC column: Phenomenex p/n: 00g-4378-n0
Luna NH2 guard cartridge discs: Phenomenex p/n: 00G-4454-N0PRP-214513
Syringes: 1, 3, 5 and 10 mL
Sterile Needles: 21–23 gauge
Venting Needle Filtered: IMI p/n: 31–20
Aspirating Needle, 19 gauge, 3” cannula: IMI p/n: 32–21
20 mL scintillation vials with caps
4 mL scintillation vials with caps
Small stirrer bar
Heating mantle
200 mL bottles with caps
1 L bottles with caps
Sonicator
Electronic pH sensor
Jars
1–200 μL pipetter with disposable tips
Quality Control of 6-[18F]Fluoro-L-DOPA
Shimadzu HPLC (or equivalent analytical HPLC system)
Shimadzu GC (or equivalent analytical GC system)
Luna NH2 5μ 4.6×150 mm analytical HPLC column: Phenomenex p/n: 00f-4378-e0
Charles River Endosafe (or equivalent device for measuring bacterial endotoxin levels)
Capintec dose calibrator (or equivalent dose calibrator compatible with fluorine-18)
pH paper
Dragendorff stain48
20 mL culture tubes of fluid thioglycolate media (FTM): BD p/n 220889
15 mL culture tubes of tryptic soy broth (TSB): BD p/n/ 221823
REAGENT SETUP
TBAOTf/Cs2CO3 eluent solution (Step 11): Weigh out 150 mg TBAOTf and 2 mg Cs2CO3 in a 20 mL scintillation vial. Add 10 mL water and a stirrer bar and cover (but do not seal) the scintillation vial. Heat while stirring until solution is near-boiling and all solids have dissolved. Remove stirrer bar, cap vial and allow to cool to room temperature (18 – 24 oC). A small amount of crystalline material may form on cooling around lip of container, but this will re-dissolve in the solution over time. Store this eluent at room temperature for up to 3 months.
FDOPA BPin precursor (1) stock solution (4 μmol/200 μL): Using a syringe, transfer 1.5 mL of anhydrous DMF into the sealed vial containing 20 mg dry BPin precursor (1). Vortex for at least 1 min to dissolve all precursor. Precursor stock solution can be stored for up to 3 months in the −20 °C freezer, vial placed in a sealed jar containing Drierite®.
FDOPA reactant solution (Step 13A): Weigh out 13.5 ± 0.5 mg of Cu(py)4(OTf)2 into a 4 mL glass vial. Add 0.8 mL of anhydrous DMF then cap vial and fully dissolve the solid by vortexing for 30 sec. Add 33.2 μL anhydrous pyridine followed by 0.2 mL of precursor stock solution. Agitate briefly, and do not heat this solution at any stage of preparation. Prepare freshly for each synthesis and use immediately after preparation.
Alternative FDOPA reactant solution (Step 13B): Weigh out 7.5 ± 0.5 mg of Cu(OTf)2 into a 4 mL glass vial. Add 1.0 mL of anhydrous DMF then cap vial and fully dissolve the solid by vortexing for 30 sec. Add 80 μL anhydrous pyridine followed by 0.2 mL of precursor stock solution. Agitate briefly, and do not heat this solution at any stage of preparation. Prepare freshly for each synthesis and use immediately after preparation.
0.25M ascorbic acid solution: Weigh out 440 mg ascorbic acid in a 20 mL scintillation vial and dissolve in 10 mL water. Cap vial and store in refrigerator for up to 1 month.
0.10M ascorbic acid solution: Weigh out 176 mg ascorbic acid in a 20 mL scintillation vial and dissolve in 10 mL water. Cap vial and store in refrigerator for up to 1 month.
0.10M ascorbic acid/0.01M EDTA solution (Step 16B, i): Weigh out 176 mg ascorbic acid and 42 mg EDTA in a 20 mL scintillation vial and dissolve in 10 mL HPLC grade water. Cap vial and store in refrigerator for up to 1 month.
Deprotecting solution (Step 16A): Mix 0.2 mL 0.25M ascorbic acid solution with 0.6 mL 36.5 – 38.0% HCl. Use immediately after preparation.
Alternative Deprotecting solution (Step 16B, iii): Mix 0.25 mL of 0.10M ascorbic acid solution with 0.25 mL of 36.5 – 38.0% HCl. Use immediately after preparation.
Semi-preparative HPLC eluent #1 (90% MeCN) (Steps 3, 4 and 18): Dissolve approximately 1 g KOAc in 100 mL water, add 1 mL AcOH followed by 900 mL MeCN. Once made up, ensure the pH of the solution lies between 7.0 and 8.0 using a pH sensor. Adjust accordingly with KOAc/AcOH if outside of range. Sonicate before using. Store for up to 1 week at room temperature.
Semi-preparative HPLC eluent #2 (75% MeCN) (Steps 3 and 18): Dissolve approximately 1 g KOAc in 250 mL water, add 10 mL AcOH followed by 750 mL MeCN. Once made up, ensure the pH of this solution lies between 5.0 and 5.5 using a pH sensor. Adjust accordingly with KOH/AcOH if outside of range. Sonicate before using. Store for up to 1 week at room temperature.
Analytical HPLC eluent (70% MeCN) (Step 27): Dissolve approximately 1 g KOAc in 300 mL water, add 1 mL AcOH followed by 700 mL MeCN. Once made up, ensure the pH of this solution lies between 5.0 and 5.5 using a pH sensor. Adjust accordingly with KOAc/AcOH if outside of range. Sonicate before using. Store for up to 1 week at room temperature.
Chiral Analytical HPLC eluent (30% EtOH) (Step 29): Dissolve approximately 1 g KOAc in 700 mL water, add 1 mL AcOH followed by 300 mL EtOH. Once made up, ensure the pH of this solution lies between 5.0 and 5.5 using a pH sensor. Adjust accordingly with KOAc/AcOH if outside of range. Sonicate before using. Store for up to 1 week at room temperature.
0.5 M NaHCO3 solution: Dissolve 8.4 g NaHCO3 in 200 mL water. Can be stored sealed at room temperature for up to 1 month.
EQUIPMENT SETUP
Waters Light QMA cartridge (Step 6): Flush sequentially with 10 mL absolute ethanol, 10 mL 0.5M sodium bicarbonate solution, and 10 mL water (Milli-Q or ACS Reagent for ultratrace). Attach it to the synthesis module.
Waters HLB Short Plus cartridge (for alternate method with HLB purification between fluorination and deprotection, Step 6): Flush sequentially with 10 mL absolute ethanol, 10 mL HPLC grade water. Attach to the synthesis module in the “intermediate cartridge” position.
Strata® NH2 200mg SPE cartridge (Step 6): Flush sequentially with 10 mL absolute ethanol, 10 mL water, and 10 mL acetonitrile. Attach it to the reformulation module of the synthesis module.
Preparation of semi-preparative (Luna NH2 5μ 10 × 250 mm) HPLC column: Assemble guard column as per manufacturer instructions and attach to semi-preparative HPLC column. Flush column for 15 – 30 min at 5 mL/min with 90% MeCN (Semi-preparative HPLC eluent #1) prior to installation in the synthesis module. Replace guard column cartridges as needed (every 1–3 months depending on frequency of use). We also recommend flushing the column with water containing 0.1% trifluoroacetic acid for 15 min at 5mL/min once every 5 runs.
Cleaning of TRACERLab FXFN synthesis module: Follow manufacturer instructions/facility standard operating procedures in cleaning, disinfecting, and drying the synthesis module. We recommend using 70% ethanol as disinfectant. Both the glass and glassy carbon reactors can be used in the synthesis of [18F]FDOPA.
If using a TRACERLab FXFN synthesis module, the vials should be filled as follows (otherwise this section can be used as a reference): Vial 1: Eluent Solution (0.5 mL, Step 11); Vial 2: Acetonitrile (1 mL, Step 12); Vial 3: FDOPA Reactant Solution (0.5 mL, Step 13A) or Alternative FDOPA Reactant Solution (1.25 mL, Step 13B); Vial 4: Deprotecting Solution (0.8 mL, Step 16A) or Alternative Deprotecting Solution (0.5 mL, Step 16Biii); Vial 5: Ascorbic acid/EDTA solution (10 mL, Step 16Bi, only if using the alternate method with HLB purification between fluorination and deprotection); Vial 6: acetonitrile (3 mL for one-pot procedure, 2 mL for alternate method with HLB purification); Vial 7: 10 mL of USP saline; Vial 8: 3 mL of dehydrated ethanol; Intermediate Vial: absolute ethanol (2 mL, Step 16Bii, only if using the alternate method with HLB purification); Dilution flask: acetonitrile (100 mL).
PROCEDURE
Synthesis and Purification of [18F]FDOPA ● TIMING ~ 3 hours
! CAUTION All hazardous laboratory chemicals should be used by trained personnel under the supervision of Environmental Health and Safety. Radioactivity should be used only with institutional, state and/or federal authorization and according to ALARA (as low as reasonably achievable) principles. Properly shield yourself against radiation by performing all reactions involving radioactivity in a lead-shielded fume hood or hot-cell, and follow appropriate institutional, state and/or federal radiation safety guidelines. When handling radioactive material, ensure that all appropriate personal protective equipment is worn, and that shielding, dosimeters and survey meters are always used.
▲ CRITICAL Check all equipment is in working order, has been appropriately cleaned, disinfected and dried, and that all necessary system suitability checks have been conducted according to local standard operating procedures prior to synthesizing [18F]FDOPA.
Hot Cell and TRACERLab FXFN Preparation
▲ CRITICAL Preparation for the synthesis of [18F]FDOPA should begin at least 15–30 min before start of cyclotron production of [18F]fluoride.
-
1
Turn on the TRACERLab FXFN module and computer. Start the Tracerlab FXFN software program and fill the module’s dewar with liquid nitrogen. Verify the machine has been cleaned, disinfected and dried using approved methods (clean if necessary) and that all components are in working order.
-
2
Remove and clean the synthesis module reactor with 1) 0.5M NaHCO3 solution, 2) water, 3) ethanol and 4) acetone, then rinse with acetone and thoroughly dry. After drying, equip the reactor with a stirrer bar and reinstall on the TRACERLab.
-
3
Install HPLC eluents on the TRACERLab system (Reservoir 1: 90% MeCN; Reservoir 2: 75% MeCN).
-
4
Attach a guard column to the semi-preparative HPLC column and install on the TRACERLab module. Flush the column with 90% acetonitrile (Reservoir 1) for 15–30 min at 5 mL/min.
-
5
Start the FDOPA production method in the synthesis module software (see Supplementary Figures 1 and 2 for synthesis module schematics, and Supplementary Method 1 for synthesis module timelists).
-
6
Attach the QMA, optional HLB, and Strata® NH2 cartridges to the synthesis module. See Equipment Setup for preconditioning protocols.
-
7
Fill vials according to instructions in Equipment Setup. Fill dilution flask with 100 mL acetonitrile. If using the alternate procedure involving purification with an HLB cartridge between fluorination and deprotection, also add 2 mL of absolute ethanol to the intermediate vial.
-
8
Aseptically assemble the final dose vial by inserting an inlet needle and 13 mm Millex GV filter and a vent needle with FG filter according to local procedures. Attach the TRACERLab product delivery line to the final dose vial via the 13 mm GV filter.
▲ CRITICAL STEP Final dose vials should be assembled using aseptic technique in a Class 5 laminar airflow hood (or equivalent such as a certified clean room) and in compliance with local drug manufacturing and/or pharmacy regulations.
Preparation of [18F]fluoride
! CAUTION Properly shield yourself against radiation by performing all reactions involving radioactivity in a lead-shielded fume hood or a hot cell.
-
9
Produce fluorine-18 in the cyclotron via the 18O(p,n)18F nuclear reaction. Load 2.5 mL of 18OH2 into the fluorine target, and irradiate with a 55 μA proton beam for 30 min (or as needed). Upon completion of irradiation, transfer the solution of [18F]fluoride in 18OH2 to the target vial of the TRACERLab FXFN synthesis module [Note: Steps 1–8 and 9 can occur simultaneously].
! CAUTION Cyclotrons should only be operated by appropriately trained and qualified personnel. At our facility, the production radiochemist(s) operate the cyclotron, but certain jurisdictions require a dedicated cyclotron operator. Local laws and radiation safety practices should be followed at all times.
Synthesis of [18F]FDOPA
! CAUTION Properly shield yourself against radiation by performing all reactions involving radioactivity in a lead-shielded fume hood or a hot cell.
-
10
Slowly transfer the solution of [18F]fluoride in [18O]H2O through the QMA cartridge, trapping the [18F]fluoride and recovering the [18O]H2O for proper disposal or recycling.
-
11
Elute [18F]fluoride from the QMA cartridge into the TRACERLab reactor with 0.5 mL Eluent Solution (see Reagent Setup).
? TROUBLESHOOTING
-
12
Add 1 mL of MeCN to the reactor and then heat to 100 °C while applying vacuum and/or argon flow to azeotropically dry the [18F]fluoride.
-
13
After drying is complete, cool the reactor to 50 °C and add either Cu(Py)4(OTf)2 or Cu(OTf)2.
| Reagent Setup Name | Volume (mL) | |
| Cu(Py)4(OTf)2 | FDOPA reactant solution | 0.5 |
| Cu(OTf)2 | Alternative FDOPA Reactant Solution | 1.25 |
<CRITICAL STEP> Radiofluorination can performed using either Cu(Py)4(OTf)2 or Cu(OTf)2. The readily available and inexpensive combination of Cu(OTf)2 and pyridine promotes this reaction as effectively as the more costly Cu(OTf)2(py)4, but the latter is easier to handle day-to-day given the hygoscopic nature of Cu(OTf)2.
-
14
Stir at 50 °C for 5 min.
-
15
Increase the reactor temperature to 110 °C and continue heating for 20 min (Note: shorter reaction times can be employed with only a modest detriment to radiochemical yield, but 20 mins is the optimal reaction time for this chemistry46).
? TROUBLESHOOTING
-
16
Do the deprotection reaction either without pre-purification (one-pot method, option A) or with pre-purification of the protected-[18F]FDOPA intermediate prior to deprotection (alternate one-pot method with HLB purification, option B).
(A) One-pot Method
Cool the reactor to 50 °C, add the Deprotecting Solution to the reactor (see Reagent Preparation) and heat at 100 °C for 10 min.
(B) Alternate One-pot Method with HLB purification between Fluorination and Deprotection
Cool the reactor to 50 °C and add ascorbic acid/EDTA solution (see Reagent Preparation) into the reactor. Stir for 30 s, and then load the reaction mixture onto the HLB cartridge, trapping protected [18F]FDOPA on the cartridge.
Elute protected [18F]FDOPA from the HLB cartridge back into the reactor using ethanol (2 mL) from the intermediate vial.
Add the Alternative Deprotecting Solution to the reactor (see Reagent preparation), and heat at 100 °C for 10 min.
? TROUBLESHOOTING
-
17
Cool reactor to 50 °C and add 2 mL acetonitrile.
Purification and Reformulation of [18F]FDOPA
-
18
Load reactor contents onto a 5 mL HPLC loop, then inject onto the HPLC column. Purify by semi-preparative HPLC. Flow rate should be set to 5 mL/min, reservoir 1 (90% MeCN). Elute column for 10 minutes at 5 mL/min. Switch eluent to reservoir 2 (75% acetonitrile) and continue to elute column at 5 mL/min.
? TROUBLESHOOTING
-
19
Collect the HPLC product fraction corresponding to [18F]FDOPA into a dilution flask containing MeCN (100 mL).
▲ CRITICAL STEP Collect the peak corresponding to [18F]FDOPA at the appropriate retention time (~22 – 23 min, see Figure 3). Start collecting when the radioactivity peak exceeds ~500 – 1000 counts and collect for approximately 2 min from the time liquid begins dripping into collection vial (fraction should elute in ≤ 1.5 min).
Figure 3 |.
Semi-preparative HPLC traces for [18F]FDOPA prepared using A: the one-pot method (Reproduced from Ref. 43 with permission from The Royal Society of Chemistry), and B: the alternate one-pot method with HLB purification between fluorination and deprotection
? TROUBLESHOOTING
-
20
Briefly stir the dilution flask contents, then transfer the solution through the Strata® NH2 SPE cartridge, trapping [18F]FDOPA. Dry with argon flow for at least 2 min.
▲ CRITICAL STEP Drying step is important to remove residual MeCN.
? TROUBLESHOOTING
-
21
Wash the Strata® NH2 cartridge with 3 mL absolute ethanol, then dry with argon flow for at least 3 min.
▲ CRITICAL STEP Washing and drying steps are important to ensure there is no residual MeCN in final dose.
? TROUBLESHOOTING
-
22
Elute Strata® NH2 SPE cartridge with 10 mL 0.9% saline into a product collection vial.
-
23
Transfer the formulated [18F]FDOPA through the 13 mm GV sterile filter into the sterile product vial. Aseptically remove 0.5 mL of the batch and place it in a 2cc sterile dose vial for quality control (QC) testing (see Table 1 for results of QC testing).
Quality Control of [18F]FDOPA
! CAUTION Properly shield yourself against radiation by performing all QC testing procedures involving radioactivity in a lead cave, shielded fume hood or hot cell.
Pre-release QC Testing ● TIMING 0.5 hours
-
24
Conduct a visual inspection of the QC sample to ensure the dose is clear, colorless and free of particulate matter.
-
25
Analyze the pH of the [18F]FDOPA dose by applying a small amount of the dose to a pH-indicator strip and compare it to the scale provided. Dose pH is required to be between 4.5 and 7.5.
-
26
Determine residual TBA+ levels in [18F]FDOPA doses using Dragendorff stain and confirm that they are less than the European Pharmacopeia (EP) requirement of <0.26 mg/mL TBA+ (no USP limits currently exist for TBA+).
-
27
Analyze [18F]FDOPA by analytical HPLC (Figure 4 and Supplementary Figure 3) to determine identity, radiochemical and chemical purity (column: Luna NH2 5μ 4.6 × 150 mm column; mobile phase: 70% MeCN 10 mM KOAc, pH 5.2; flow rate: 1.5 mL/min). Radiochemical purity should be >90% and there should be <50 μg/mL of OH-DOPA and H-DOPA by-products which result from competing hydroxy- and proto-deborylation, respectively (see ref 43 for more details). Identity is confirmed by comparing the retention time of the radiolabelled product with that of the corresponding unlabelled FDOPA reference standard.
Figure 4|.
Analytical HPLC trace of [18F]FDOPA using a Luna NH2 analytical column (RAD top, 282nm UV bottom). Reproduced from Ref. 43 with permission from The Royal Society of Chemistry.
? TROUBLESHOOTING
-
28
Use analytical HPLC data to calculate molar activity. Molar activity (Am) is calculated using the equation Am = A/n, Where A is the activity of [18F]FDOPA (expressed in Bq or Ci) and n is the molar amount of F-DOPA. Am needs to be ≥ 18.5 TBq/mmol (>500 Ci/mmol).
-
29
Analyze [18F]FDOPA by chiral HPLC to determine enantiomeric purity detector (column: Astec Chirobiotic T 5 micron 250 × 4.6 mm analytical column; mobile phase: 30% ethanol 10mM KOAc pH 5.13, flow rate: 1.5mL/min). Enantiomeric purity is determined by comparison to 6F-D,L-DOPA and/or 6F-L-DOPA reference standards (Figure 5). Enantiomeric purity of [18F]FDOPA needs to be >95% of the L enantiomer.
-
30
Analyze levels of residual solvents in [18F]FDOPA doses using a Shimadzu GC-2010 with an AOC-20 autoinjector, split/splitless inlet, a flame ionization detector (or equivalent), and a Restek column (Stabilwax 30 m 0.25 mm, 0.25 m G16 stationary phase). Limits of residual solvents are based upon the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines (MeCN: ≤410 ppm; DMF: ≤880 ppm).47
Figure 5|.
Chiral HPLC trace of production [18F]FDOPA (RAD, bottom, black) 6F-D,L-DOPA reference standard (282nm, teal, middle) and 6F-L-DOPA reference standard (282nm, pink, top) using a Chirobiotic T analytical column. Reproduced from Ref. 43 with permission from The Royal Society of Chemistry.
? TROUBLESHOOTING
-
31
Confirm radionuclidic identity by determining the half-life of the [18F]FDOPA dose and compare it to the known half-life of fluorine-18 (109.77 min). Measure radioactivity at 2 time points using a Capintec dose calibrator (or equivalent) and determine half-life (T1/2 = -ln2(Time Difference / (ln(ending activity/starting activity)))). Calculated half-life must be 105–115 min.
-
32
Determine integrity of the 13 mm GV sterile filter using the bubble point test. The filter from the dose (with needle still attached) is connected to a nitrogen supply via a regulator. The needle is then submerged in water and the nitrogen pressure is gradually increased. If the pressure can be raised above the filter acceptance pressure (50 psi for 0.9% saline) without seeing a stream of bubbles, the filter is considered intact.
-
33
Determine endotoxin content in [18F]FDOPA doses according to the US Pharmacopeia using a Charles River Laboratories EndoSafe® Portable Testing System (or equivalent). Doses must contain ≤175 Endotoxin Units (EU), or ≤17.5 EU/mL.
Post-release QC Testing ● TIMING 14 days
Stability testing
-
34
If stability testing of [18F]FDOPA is necessary (e.g. to establish expiration for an Investigational New Drug (IND) filing), repeat Step 27 at desired times after end-of-synthesis (EOS). In this work we analyzed doses of [18F]FDOPA at 2 and 4 h post-EOS and radiochemical purity was found to be 99.1 ± 1.2% and 96.5 ± 1.0%, respectively (see Table 2 and 4 h HPLC trace in Supplementary Figure 4). In this initial work, we set expiration at 4 h post-EOS, but this could be extended with additional HPLC analysis if required.
Table 2|.
Troubleshooting tablea
| Step | Problem | Possible Reason | Solution |
|---|---|---|---|
| 11 | [18F]Fluoride is retained on QMA cartridge | Connections to QMA cartridge are leaking or wrong cartridge used | Check connections, replace as needed |
| 15 | Low incorporation of 18F | Equipment malfunction, decomposition of reactants | Ensure synthesis module is clean and functional, check and replace reactants as needed |
| 15 | BPin precursor 1 ceases to be commercially available | Economic / patent considerations | Prepare Bpin precursor 1 according to the procedure provided in Box 2 |
| 16 | Low purity after deprotection | Ascorbic acid is degrading | If it becomes an issue, check and replace ascorbic acid if needed. |
| 18 | Slow (or no) loading of crude reaction onto HPLC loop | HPLC loop is blocked | Clean HPLC loop |
| 18 | More radioactive impurities in HPLC trace | Not enough acid in Deprotection Solution | Increase the amount of 36.5 – 38.0% HCl in Deprotecting Solution by 0.1 mL increments, or use fresh 36.5 – 38.0% HCl. |
| 19, 27 | Low chemical and/or radiochemical purity | [18F]FDOPA was not adequately purified | Clean or replace guard column and semi-preparative HPLC column |
| 20,21, 30 | Too much MeCN in [18F]FDOPA product | MeCN used during reformulation was not adequately removed | Optimize rinsing and drying in Steps 20 and 21 |
Many of the problems in this table can be be avoided by following cGMP.
Sterility testing
-
35
Sterility testing is a post-release test for short-lived radiopharmaceuticals. Within 24 h of end-of-synthesis, inoculate 20 mL culture tubes of fluid thioglycolate media (FTM) and 15 mL culture tubes of tryptic soy broth (TSB) with 1 mL samples of [18F]FDOPA. After inoculation, the tubes are inverted 2 or 3 times to mix and incubated (along with positive and negative controls) for 14 days (FTM is incubated at 30 – 35oC, TSB tubes are incubated at 22 oC). FTM is used to test for anaerobes, aerobes and microaerophiles while TSB is used to test for non-fastidious and fastidious microorganisms.
| Tube | Media | Innoculate | Required result to be indictive of sterility |
| Dose 1 | FTM | [18F]FDOPA | No growth |
| Negative Control 1 | FTM | Sterile water for injection | No growth |
| Positive Control 1a | FTM | BioBall® microorganism sample | Growth |
| Dose 2 | TSB | [18F]FDOPA | No growth |
| Negative Control 2 | TSB | Sterile water for injection | No growth |
| Positive Control 2 a | TSB | BioBall® microorganism sample | Growth |
positive controls can be run during sterility testing or in a separate growth promotion experiment. Follow local standard operating procedures and regulatory requirements.
-
36
Visually inspect the culture tubes on the 3rd, 7th and 14th days of the test period and compare these to the positive and negative standards. Positive standards need to show growth (turbidity) in the tubes, and [18F]FDOPA doses/negative controls need to show no culture growth after 14 days to be indicative of sterility.
TROUBLESHOOTING
Troubleshooting advice can be found in Table 2.
● TIMING
Reagent and Equipment set-up: 180 min
Steps 1 – 8, hot-cell and TRACERLab FXFN Preparation: 30 min.
Step 9, cyclotron production of [18F]fluoride: approximately 30 min.
Steps 10 – 23, synthesis, purification and reformulation of [18F]FDOPA: 110 min.
Steps 24 – 33, pre-release QC testing of [18F]FDOPA: 30 min.
Steps 34 – 36, post-release QC testing of [18F]FDOPA: Initiate within 24 h of end-of-synthesis, complete within 14 days.
Note: Steps 1–8 and 9 can occur simultaneously.
ANTICIPATED RESULTS
Radiofluorination of organoborons, reported by independently by Gouverneur29 as well as Sanford and Scott,30 has proven one of the most versatile approaches for the late-stage fluorination of bioactive molecules to date. We have further optimized the approach for use with automated radiochemistry synthesis modules,46,49 and additional variants of the original methods have subsequently been reported by Neumaier and colleagues.42,50 The methodology has been rapidly adopted by the PET radiochemistry community, and a number of independent groups have used the technique to synthesize new PET radiopharmaceuticals for pre-clinical and clinical use.51,52,53,54,55,56,57,58,59,60
Given the historical challenges associated with synthesizing [18F]FDOPA from nucleophilic [18F]fluoride, we were motivated to overcome these issues through development of a one-pot, two-step synthesis of high molar activity [18F]FDOPA by Cu-Mediated fluorination of a BPin precursor. We recently reported a method that primarily uses off-the-shelf reagents and a commonly available synthesis module, and validated it for production of [18F]FDOPA for clinical use by preparing process verification batches.43 The one-pot production method provides [18F]FDOPA in reasonable radiochemical yield (3.85 ± 0.59 GBq, 104 ± 16 mCi, 6 ± 1% based upon approximately 66.6 GBq (1800 mCi) of starting [18F]fluoride), excellent radiochemical (>99%, Figure 4) and enantiomeric (>99%, Figure 5) purity, and high molar activity (141 ± 77 TBq/mmol, 3799 ± 2087 Ci/mmol), n = 3. All other quality control testing confirmed that each dose met or exceeded quality control criteria established for human use of PET radiopharmaceuticals (Table 2). The one-pot method with HLB purification between fluorination and deprotection also provides [18F]FDOPA in moderate radiochemical yield (2.26 ± 0.48 GBq, 61 ± 13 mCi, 5 ± 1% based upon 45.6 ± 11.0 GBq (1232 ± 298 mCi) of starting [18F]fluoride), excellent radiochemical purity (>98%, Figure S3 in Supporting Information) and high molar activity (71 ± 17 TBq/mmol, 1909 ± 459 Ci/mmol), n = 23. The method has been validated to work well at two separate sites, an academic facility with a cyclotron on site (University of Michigan) and an industry lab purchasing [18F]fluoride from an outside vendor (AbbVie). We were gratified that the yield of [18F]FDOPA was comparable at each site (UM: 6 ± 1%; AbbVie: 5 ± 1%). Given the operational simplicity of the method, which uses a standard radiochemistry synthesis module, and demonstrated robustness of this protocol, we anticipate the reliability of synthesizing [18F]FDOPA using this method will be high. As such, we expect that this method (or a modification thereof) will be useful to research facilities that own a TRACERLab FXFN or similar system, and who want access to a straightforward procedure for producing [18F]FDOPA.
Supplementary Material
Supplementary Figure 1. Standard TRACERLab FXFN Configuration for One-pot Synthesis of [18F]FDOPA (reproduced with permission of GE Healthcare)
Supplementary Figure 2. Modified TRACERLab FXFN Configuration for alternate synthesis of [18F]FDOPA with HLB purification between fluorination and deprotection (Adapted from Supplementary Figure 1 and reproduced with permission of GE Healthcare)
Supplementary Figure 3. Analytical trace (RAD top, 282 nm UV bottom) of [18F]FDOPA at end-of-synthesis. Column: Luna NH2 5 micron 4.6×150 mm column; mobile phase: 70% MeCN 10 mM KOAc, pH 5.2; flow rate: 1.5 mL/min. [18F]FDOPA prepared using alternate synthesis with HLB purification between fluorination and deprotection.
Supplementary Figure 4. Analytical trace (RAD top, 282 nm UV bottom) of [18F]FDOPA 4 h post-end-of-synthesis. Column: Luna NH2 5 micron 4.6×150 mm column; mobile phase: 70% MeCN 10 mM KOAc, pH 5.2; flow rate: 1.5 mL/min. [18F]FDOPA prepared using alternate synthesis with HLB purification between fluorination and deprotection.
Supplementary Method 1. Synthesis Module Timelists
Supplementary Method 2. Synthesis of BPin Precursor (1)
ACKNOWLEDGMENTS
We acknowledge NIH (R01EB021155 to M.S.S. and P.J.H.S.) and US DOE/NIBIB (DE-SC0012484 to P.J.H.S.) for financial support.
Footnotes
DATA AVAILABILITY STATEMEMNT All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
COMPETING FINANCIAL INTERESTS All authors declare no competing financial interests.
Note: Supplementary information is available in the online version of the paper.
References
- 1.Ametamey SM et al. Molecular Imaging with PET. Chem. Rev 108, 1501–1516 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Taïeb D et al. 18F-DOPA: the versatile radiopharmaceutical. Eur. J. Nucl. Med. Mol. Imaging 43, 1187–1189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pretze M et al. 6-[18F]Fluoro-L-DOPA: a well-established neurotracer with expanding application spectrum and strongly improved radiosyntheses. Biomed. Res. Int. Article 674063 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garnett ES et al. Dopamine visualized in the basal ganglia of living man. Nature 305, 137–138 (1983). [DOI] [PubMed] [Google Scholar]
- 5.Darcourt J et al. 18F-FDOPA PET for the diagnosis of parkinsonian syndromes. Q. J. Nucl. Med. Mol. Imaging 58, 355–365 (2014). [PubMed] [Google Scholar]
- 6.Calabria F & Cascini GL Current status of 18F-DOPA PET imaging in the detection of brain tumor recurrence. Hell. J. Nucl. Med 18, 152–156 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Nandu H Imaging in neuro-oncology. Ther. Adv. Neurol. Dis 11, 1–19 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah P et al. , Persistent hyperinsulinaemic hypoglycaemia in infancy. Semin. Pediatr. Surg 23, 76–82 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Luxen A et al. , Remote, semiautomated production of 6-[18F]fluoro-L-dopa for human studies with PET. Appl. Radiat. Isot 41, 275–281 (1990). [DOI] [PubMed] [Google Scholar]
- 10.Füchtner F et al. Aspects of 6-[18F]fluoro-L-DOPA preparation. Deuterochloroform as a substitute solvent for Freon 11. Nuklearmedizin 47, 62–64 (2008). [PubMed] [Google Scholar]
- 11.Luurtsema G et al. Improved GMP-compliant multi-dose production and quality control of 6-[18F]fluoro-L-DOPA. EJNMMI Radiopharm. Chem 1, article 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemaire C et al. Highly Enantioselective Synthesis of No‐Carrier‐Added 6‐[18F]Fluoro‐L‐dopa by Chiral Phase‐Transfer Alkylation. Eur. J. Org. Chem 2899–2904 (2004). [Google Scholar]
- 13.Shen B et al. , Automated synthesis of n.c.a. [18F]FDOPA via nucleophilic aromatic substitution with [18F]fluoride. Appl. Radiat. Isot 67, 1650–1653 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Lemaire C et al. Automated production at the curie level of no-carrier-added 6-[18F]fluoro-L-dopa and 2-[18F]fluoro-L-tyrosine on a FASTlab synthesizer. J. Labelled Comp. Radiopharm 58, 281–290 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Libert LC et al. Production at the Curie Level of No-Carrier-Added 6-18F-Fluoro-L-Dopa. J. Nucl. Med 54, 1154–1161 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Tierling T et al. A new nucleophilic asymmetric synthesis of 6-[18F]fluoro-DOPA. J. Labelled Comp. Radiopharm 44 (Suppl. 1), S146–S148 (2001). [Google Scholar]
- 17.Wagner FM et al. Three-Step, “One-Pot” Radiosynthesis of 6-Fluoro-3,4-Dihydroxy-L-Phenylalanine by Isotopic Exchange J. Nucl. Med 50, 1724–1729 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Sauvage C et al. Synthesis of 18F-FDOPA via Nucleophilic Pathway on IBA’s Synthera®. J. Labelled Comp. Radiopharm 58 (Suppl. 1), S164 (2015). [Google Scholar]
- 19.http://www.abx.de/Information/Index?viewId=Synthesizer,accessed 30-October-2019.
- 20.http://www.trasis.com/tracers/18ffdopa, accessed 30-October-2019.
- 21.Brooks AF et al. Late-stage [18F]fluorination: new solutions to old problems. Chem. Sci 5, 4545–4553 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell MG and Ritter T Modern Carbon–Fluorine Bond Forming Reactions for Aryl Fluoride Synthesis. Chem. Rev 115, 612–633 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Preshlock S et al. 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev 116, 719–766 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Deng X et al. Chemistry for Positron Emission Tomography: Recent Advances in 11 C-, 18 F-, 13 N-, and 15 O-Labeling Reactions. Angew. Chem. Int. Ed 58, 2580–2605 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichiishi N et al. , Copper-catalyzed [18F]fluorination of (mesityl)(aryl)iodonium salts. Org. Lett 16, 3224–3227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotstein BH et al. Spirocyclic Hypervalent Iodine(III)-mediated Radiofluorination of Non-activated and Hindered Aromatics. Nature Commun, 5, 4365 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Liang SH et al. Facile 18F labeling of non-activated arenes via a spirocyclic iodonium (III) ylide method and its application in the synthesis of the mGluR5 PET radiopharmaceutical [18F]FPEB. Nat. Prot 14, 1530–1545 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Pike VW Hypervalent Aryliodine Compounds as Precursors for Radiofluorination. J. Label. Compd. Radiopharm 61, 196–227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tredwell M et al. A general copper-mediated nucleophilic 18F-fluorination of arenes. Angew. Chem., Int. Ed 53, 7751–7755 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Mossine AV et al. Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids. Org. Lett 17, 5780–5783 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makaravage KJ et al. , Copper-Mediated Radiofluorination of Arylstannanes with [18F]KF. Org. Lett 18, 5440–5443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee E A fluoride-derived electrophilic late-stage fluorination reagent for PET imaging. Science, 334, 639–642 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee E Nickel-mediated oxidative fluorination for PET with aqueous [18F]fluoride. J. Am. Chem. Soc 134, 17456–17458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann CN et al. Concerted nucleophilic aromatic substitution with 19F− and 18F−. Nature, 534, 369–373 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Ichiishi N et al. , Cu-catalyzed fluorination of diaryliodonium salts with KF. Org. Lett 15, 5134–5137 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye Y et al. , Cu(OTf)2-mediated fluorination of aryltrifluoroborates with potassium fluoride. J. Am. Chem. Soc 135, 16292–16295 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Lee SJ et al. , Copper-Mediated Aminoquinoline-Directed Radiofluorination of Aromatic C-H Bonds with K18F. Angew. Chem. Int. Ed 58, 3119–3122 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preshlock S et al. Enhanced copper-mediated 18F-fluorination of aryl boronic esters provides eight radiotracers for PET applications.Chem. Commun 52, 8361–8364 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Maisonial-Besset A et al. Base/Cryptand/Metal‐Free Automated Nucleophilic Radiofluorination of [18F]FDOPA from Iodonium Salts: Importance of Hydrogen Carbonate Counterion. Eur. J. Org. Chem 7058–7065 (2018). [Google Scholar]
- 40.Kuik W-J. et al. , In Vivo Biodistribution of No-carrier-added 6–18F-Fluoro-3,4-dihydroxy-L-phenylalanine (18F-DOPA), Produced by a New Nucleophilic Substitution Approach, Comaped with Carrier-added 18F-DOPA, Prepared by Conventional Electrophilic Substitution. J Nucl. Med. 56, 106–112 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Zarrad F et al. A practical method for the preparation of 18F-Labeled Aromatic Amino Acids from Nucleophilic [18F]Fluoride and Stannyl Precursors for Electrophilic Radiohalogenation. Molecules, 22, 2231 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zischler J et al. Alcohol-enhanced Cu-mediated Radiofluorination. Chem. Eur. J. 23, 3251–3256 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Mossine AV et al. One-pot Synthesis of High Molar Activity 6-[18F]Fluoro-L-DOPA by Cu-Mediated Fluorination of a BPin Precursor. Org. Biomol. Chem 17, 8701–8705 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCann SD and Stahl SS Copper-Catalyzed Aerobic Oxidations of Organic Molecules: Pathways for Two-Electron Oxidation with a Four-Electron Oxidant and a One-Electron Redox-Active Catalyst Acc. Chem. Res, 48, 1756–1766 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Allen SE et al. Aerobic copper-catalyzed organic reactions. Chem. Rev. 113, 6234–6458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mossine AV et al. Automated synthesis of PET radiotracers by copper-mediated 18F-fluorination of organoborons: Importance of the order of addition and competing protodeborylation. J. Labelled Compd. Radiopharm. 61, 228–236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Connelly J ICH Q3C Impurities: Guideline for Residual Solvents In ICH Quality Guidelines: An Implementation Guide by Teasdale A, Elder D, Nims RW (Eds), John Wiley & Sons, Inc; pp 199–232 (2018). [Google Scholar]
- 48.Bregoff HM et al. Paper chromatography of quaternary ammonium bases and related compounds. J. Biol. Chem, 205, 565–74 (1953). [PubMed] [Google Scholar]
- 49.Mossine AV et al. Development of customized [18F]fluoride elution techniques for the enhancement of copper-mediated late-stage radiofluorination. Sci. Rep 7, article 233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zlatopolskiy BD et al. Copper-mediated aromatic radiofluorination revisited: efficient production of PET tracers on a preparative scale. Chem. Eur. J 21, 5972–5979 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Zhang Z et al. One-step synthesis of 4-[18F]fluorobenzyltriphenylphosphonium cation for imaging with positron emission tomography. J. Labelled Comp. Radiopharm 59, 467–71 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Schäfer D et al. Preparation of No-Carrier-Added 6-[18F]Fluoro-l-tryptophan via Cu-Mediated Radiofluorination. Eur. J. Org. Chem 4621–4628 (2016). [Google Scholar]
- 53.Antunes IF et al. Synthesis and evaluation of the new estrogen receptor b selective radioligand [18F]FHNP: comparison with [18F]FES. J. Nucl. Med 58, 554–549 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Tang T et al. Preparation and evaluation of L- and D-5-[18F]fluorotryptophan as PET imaging probes for indoleamine and tryptophan 2,3-dioxygenases. Nucl. Med. Biol 51, 10–17 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z et al. Design, synthesis and evaluation of 18F-labeled cationic carbonic anhydrase IX inhibitors for PET imaging. J. Enzyme Inhib. Med. Chem 32, 722–730 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaide S et al. Conversion of iodine to fluorine-18 based on iodinated chalcone and evaluation for β-amyloid PET imaging. Bioorg. Med. Chem 26, 3352–3358 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Constantinescu CC et al. Development and In Vivo Preclinical Imaging of Fluorine-18-Labeled Synaptic Vesicle Protein 2A (SV2A) PET Tracers. Mol. Imaging Biol 21, 509–518 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Cole E et al. Radiochemistry challenges and progression for incorporation of 18F into a complex substituted 6-18F-fluoroquinoline BMS-986205 for IDO imaging. J. Nucl. Med 59 (Suppl. 1), 605 (2018). [Google Scholar]
- 59.Bernard-Gauthier V et al. Identification of [18F]TRACK, a Fluorine-18-Labeled Tropomyosin Receptor Kinase (Trk) Inhibitor for PET Imaging. J. Med. Chem 61, 1737–1743 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Elie J et al. Design of selective COX-2 inhibitors in the (aza)indazole series. Chemistry, in vitro studies, radiochemistry and evaluations in rats of a [18F] PET tracer. J. Enzyme Inhib. Med. Chem 34, 1–7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Standard TRACERLab FXFN Configuration for One-pot Synthesis of [18F]FDOPA (reproduced with permission of GE Healthcare)
Supplementary Figure 2. Modified TRACERLab FXFN Configuration for alternate synthesis of [18F]FDOPA with HLB purification between fluorination and deprotection (Adapted from Supplementary Figure 1 and reproduced with permission of GE Healthcare)
Supplementary Figure 3. Analytical trace (RAD top, 282 nm UV bottom) of [18F]FDOPA at end-of-synthesis. Column: Luna NH2 5 micron 4.6×150 mm column; mobile phase: 70% MeCN 10 mM KOAc, pH 5.2; flow rate: 1.5 mL/min. [18F]FDOPA prepared using alternate synthesis with HLB purification between fluorination and deprotection.
Supplementary Figure 4. Analytical trace (RAD top, 282 nm UV bottom) of [18F]FDOPA 4 h post-end-of-synthesis. Column: Luna NH2 5 micron 4.6×150 mm column; mobile phase: 70% MeCN 10 mM KOAc, pH 5.2; flow rate: 1.5 mL/min. [18F]FDOPA prepared using alternate synthesis with HLB purification between fluorination and deprotection.
Supplementary Method 1. Synthesis Module Timelists
Supplementary Method 2. Synthesis of BPin Precursor (1)