Abstract
Particularly interesting cysteine histidine-rich (PINCH) protein functions as a shuttling protein in Schwann cells after peripheral nerve damage, during repair and remodeling, and in maintaining neuronal polarity. However, the presence of PINCH in the human CNS during disease has not been addressed. Because HIV-associated damage to cells of the CNS involves dysregulation of neuronal signaling and white matter damage, we hypothesized that PINCH may play a role in neuropathological processes during the course of HIV infection. To determine the expression of PINCH in the CNS, brain, and cerebrospinal fluid (CSF) obtained at autopsy from HIV patients with no CNS alterations, HIV encephalitic (HIVE) patients, and HIV-negative individuals with no CNS alterations were examined for PINCH immunoreactivity. Our results show that PINCH is expressed robustly in the brains and CSF of HIV patients, but is nearly undetectable in HIV-negative individuals. However, HIVE patients’ CSF contained significantly less PINCH than HIV patients with no CNS alterations. PINCH immunolabeling was significantly more intense in the white matter than in the grey matter and was associated exclusively with neuronal cell bodies or processes, or with the extracellular matrix. Given the recently discovered importance of PINCH in maintaining neuronal fitness, our observations that PINCH is robustly expressed in the CNS of HIV patients suggests an important role for PINCH in HIV-associated neurodegenerative processes. Understanding mechanisms by which PINCH functions during HIV-associated CNS alterations will provide new insight into potential treatments to limit neurological alterations in HIV.
Keywords: HIV encephalitis, CNS, white matter, demyelination, neurodegeneration
A significant proportion of central nervous system (CNS) diseases such as Alzheimer’s disease, HIV, amyotrophic lateral sclerosis, and multiple sclerosis involve neuronal dysfunction. We believe that a newly discovered protein called PINCH (particularly interesting new cysteine-histidine-rich protein; Rearden, 1994; for review see Wu, 2005) may play an important role in the neuropathogenic process.
Recent evidence for PINCH expression in the peripheral nervous system shows increased expression and aberrant patterns in postinjury Schwann cells and dorsal root ganglia neurons, suggesting that PINCH plays a role during neuronal damage and myelin loss (Campana et al., 2003). PINCH recruits integrin linked-kinase (ILK) to sites of integrin activation and is required for proper ILK-integrin localization to focal adhesions (Barillari et al., 1999; Li et al., 1999; Zhang et al., 2002). Moreover, PINCH contains both nuclear localization and export sequences, and was identified as an abundant shuttling protein important for integrin and growth factor signaling (Wu, 1999). PINCH also interacts directly with Nck-2, a Src homologue adaptor protein, to regulate several key components of growth factor kinase signaling pathways (Tu et al., 1998).
We used HIV encephalitis (HIVE) as a model to investigate the potential contribution of PINCH in neuronal dysfunction. Unlike other neurological conditions such as Alzheimer’s and Parkinson’s diseases, in HIV neural functioning is compromised by indirect insults that may not include substantial neuronal loss (for review see Ellis et al., 2007). Neuronal dysfunction without neuronal death involves pruning and/or aberrant sprouting of processes, resulting in signaling alterations that disrupt neuronal communication with the extracellular matrix. HIV enters the CNS early after initial infection and likely enters again at various points during the course of disease, especially during viremic episodes. Combination antiretroviral therapy (cART) has been successful at treating systemic HIV infection, and, although HIV is now viewed as a chronic manageable disease, the neurological manifestations have changed dramatically to include not only cases of severe dementia but, more commonly, a range of milder impairments in the absence of HIVE or AIDS (Tozzi et al., 2004).
With the emerging importance of integrin signaling in synaptogenesis, activation of glial cells, and blood–brain barrier stabilization in the CNS (for review see Banks et al., 2006; see also Milner and Campbell, 2002), we hypothesized that PINCH plays an important role in neuronal functioning during HIV infection of the brain. In support of our hypothesis, a recent report by Guo et al. (2007) describes a key role for the PINCH/ILK complex in neuronal polarity and related signaling pathways. Therefore, we analyzed the distribution of PINCH in the adult CNS and found abundant PINCH expression in the neurons and extracellular matrix of HIV patients’ brains. Interestingly, PINCH patterns of distribution were distinctly different between HIVE patients and HIV patients with no CNS alterations. Moreover, levels of PINCH were significantly increased in the cerebrospinal fluid (CSF) of both groups of HIV patients. Interestingly, however, levels of PINCH in the CSF of HIVE patients were significantly lower than those in HIV patients without CNS alterations. Our results strongly suggest PINCH’s involvement in the signaling processes that occur during HIV infection of the brain. In this context, HIV proteins such as gp120 are reported to contribute directly to dysfunction of CNS cells through mechanisms involving integrin signaling (Shrikant et al., 1996). Variations in CSF levels of PINCH in HIV patients during disease progression may indicate CNS alterations, pointing to PINCH as a potential biomarker for early neuronal signaling changes in the CNS.
MATERIALS AND METHODS
Tissue and Fluid Acquisition
Tissue from a total of 66 patients from the groups described below was selected for this study (Table I). The UCSD Human Subjects Protection Program and Temple University Office for Human Subjects Protections approved all studies conducted. Post-mortem frontal cortex tissue and CSF samples were obtained from 20 age-matched normal controls with no CNS alterations (mean age 43 years), from 22 seropositive HIV patients without evidence of CNS involvement (mean age 47 years), and from 24 seropositive HIV patients with HIVE (mean age 38 years; HIV Neurobehavioral Research Center, California NeuroAIDS Tissue Network, UCSD). All cases had detailed clinical histories and neuropathological post-mortem gross and histological examination. Exclusion criteria for cases included a post-mortem time >24 hr or a history of non-HIV-related neurological disorders, such as CNS infections (other than JC virus), neurosyphillis, schizophrenia, head trauma, etc. Among the 24 HIVE cases, 19 had HIVE only and 5 were diagnosed with both HIVE and progressive multifocal leukoencephalopathy (PML).
TABLE I.
Summarized Patient Characteristics*
| n = (brain, CSF) | Neuropathology | Age (mean ± SEM) | % Male | |
|---|---|---|---|---|
| Control | 10, 10 | No CNS Alts | 43 ± 2 | 40 |
| HIV no Alts | 10, 12 | No CNS Alts | 47 ± 2 | 70 |
| HIVE | 14, 10 | HIVE, PML | 38 ± 2 | 80 |
No CNS alts, no central nervous system alterations reported; HIV, human immunodeficiency virus; PML, progressive multifocal leukoencephalopathy; HIVE, HIV encephalitis.
Neuropathological Examination of Brain Tissue
Brains were removed at autopsy, and, after gross examination, representative tissue sections from the frontal cortex were immersion fixed in 4% formalin and embedded in paraffin. Paraffin blocks were serially sectioned at 4 μm thickness, placed on electromagnetically charged slides, and stained with hematoxylin and eosin and Luxol fast blue for routine histopathological analysis. The diagnosis of HIVE was based on criteria established by the American Academy of Neurology and the HIV Neurobehavioral Research Center group, as previously described (Langford et al., 2004; Del Valle and Pina-Oviedo, 2006; Cherner et al., 2007). Briefly, diagnoses included areas of myelin pallor, perivascular cuffs of mononuclear inflammatory cells, multinucleated giant cells, microglial nodules, reactive astrogliosis, and detection of virus either by quantitative RT-PCR or by immunohistochemical detection of the HIV capsid protein, p24. Quantitative RT-PCR was conducted to determine copies of HIV RNA/mg tissue, and anti-HIV p24 antibody was used to detect virus in brain tissue sections, as previously described (Langford et al., 2002).
Immunohistochemical Analyses of PINCH Expression
Immunohistochemistry was performed using the avidin-biotin-peroxidase methodology according to the manufacturer’s instructions, with modifications (Vector Laboratories, Burlingame, CA). Our modified protocol included deparaffination in xylene, rehydration through alcohol up to water, nonenzymatic antigen retrieval in citrate buffer pH, 6.0 for 1 hr at 95–97°C, and quenching of endogenous peroxidase with 10% H2O2 in methanol for 20 min. After thoroughly rinsing with PBS, sections were blocked with normal goat serum for 1 hr at room temperature. Sections were incubated overnight in a humidified chamber with a rabbit polyclonal antibody against human PINCH (6 mg/ml) that was generated by using a full-length 6-histidine recombinant PINCH protein as the immunogen, as previously described (Wang-Rodriguez et al., 2002). Tissue sections were then incubated in biotinylated goat anti-rabbit secondary antibody (1:200; Vector Laboratories) followed by avidin-biotin D-horseradish peroxidase (HRP; ABC Elite; Vector Laboratories) and developed with diaminobenzidine (DAB; 0.2 mg/ml) in 50 mM Tris, pH 7.4. For antibody specificity controls, additional sections of HIVE, HIV with no CNS alterations, and normal brain were treated and incubated as described previously; however, the primary antibody was omitted. Any positive signal would be interpreted as nonspecific binding, rendering the data invalid.
Double-Labeling Immunofluorescence and Deconvolution
For double-immunofluorescence labeling, the first part of our protocol is similar to the methodology described above, with modifications. After overnight incubation with the rabbit polyclonal anti-PINCH primary antibody, sections were rinsed with PBS and incubated with a fluorescein isothiocyanate (FITC)-conjugated secondary antibody for 2 hr at room temperature in the dark. After washing thoroughly with PBS, the sections were reblocked with normal horse serum and incubated overnight at room temperature with mouse monoclonal primary antibodies for specific cellular markers. These antibodies included an antiglial fibrillary acidic protein (GFAP; clone 6F2; 1:100 dilution; Dako, Carpinteria, CA) for labeling of astrocytes, a pan-neuronal cocktail for phosphorylated neurofilaments (clone SMI-311; 1:2,000 dilution; Sternberger Monoclonals, Berkeley, CA) for labeling of neurons, and myelin basic protein (MBP; clone SMI-99; 1:1,000 dilution; Sternberger Monoclonals, Baltimore, MD) for labeling of myelin. Sections were then incubated with a second rhodamine-tagged secondary antibody for 2 hr at room temperature in the dark. Finally, sections were coverslipped with an aqueous-based mounting media containing DAPI for nuclear labeling (Vectashield; Vector Laboratories) and visualized with a Nikon ultraviolet inverted microscope and processed with deconvolution software (Slidebook 4.0; Intelligent Imaging, Denver, CO).
Western Analyses of PINCH
As described previously (Langford et al., 2004), 0.1 g frozen tissues from frontal cortex was homogenized on ice in HEPES buffer (1 mM HEPES, 5 mM benzamidine, 2 mM 2-mercaptoethanol, 3 mM EDTA, 0.5 mM magnesium sulfate, 0.05% sodium azide, 1 mM sodium orthovanadate, and 0.01 mg/ml leupeptin). Protein concentrations were determined by the bicinchoninic acid assay (BCA Protein Assay Kit; Pierce, Rockford, IL) following the manufacturer’s protocol, and 25 μg of protein was loaded per well. For CSF, constant volumes of 3 μl CSF were loaded per well. Proteins were separated by electrophoresis for 1 hr at 200 V on 4–12% Bis-Tris NuPage Gels (Invitrogen, Carlsbad, CA). Each membrane was immunolabeled with a monoclonal antibody against human PINCH (1:1,000; BD Biosciences, San Jose, CA), followed by horseradish peroxidase (HRP)-tagged secondary antibodies (1:5,000; American Qualex, San Clemente, CA). Polyclonal antigrowth factor receptor-bound protein (Grb2) antibody (1:1,000; Cell Signaling Technologies, Danvers, MA) was used as a loading control. Enhanced chemiluminescence was detected with the Western Lightning Chemiluminescence Reagent Plus Kit (PerkinElmer Life Sciences, Boston, MA) and recorded using the Bio-Rad VersaDoc Imaging System model 3000 (Bio-Rad, Hercules, CA).
Statistical Analysis
Western Blots were analyzed with Quantity One analysis software (Bio-Rad). Where appropriate, data were analyzed by using Fischer’s PLSD or one-way ANOVA with post hoc Dunnett’s or Tukey-Kramer tests in GraphPad Prism (GraphPad Software, San Diego, CA). All results are expressed as mean ± SEM (n = 3).
RESULTS
PINCH Is Expressed Robustly in the Brains of HIV Patients
Given the importance of PINCH in the peripheral nervous system during nerve injury and its involvement in Schwann cell signaling (Campana et al., 2003), we hypothesized that PINCH may be involved in neuronal dysfunctions common in HIV-associated CNS alterations such as HIVE, PML, and HIV-associated leukoencephalopathy (HAL). Immunohistochemical analyses demonstrated that PINCH expression was undetectable in normal brain from HIV-negative samples (Fig. 1A,B). Interestingly, HIV-positive patients without reported brain pathology showed a moderate and patchy labeling pattern of PINCH in both white and grey matter (Fig. 1C,D). On the other end of the spectrum, expression of PINCH was robust and widespread in brains from patients with HIVE, in both the white and the grey matter (Fig. 1E,F). Figure 1 illustrates the changing patterns of PINCH expression in these different conditions. Samples of HIVE tissue where the PINCH antibody was omitted rule out nonspecific binding (Fig. 1G,H). Western analyses of brain homogenate from HIV-negative controls, HIV cases with no CNS alterations, and HIVE cases confirmed increased levels of PINCH protein in HIV infection of the CNS (Fig. 2). PINCH levels in the brains of HIV patients with no CNS alterations and HIVE were significantly greater than levels in control (P < 0.05, P < 0.01, respectively; Fig. 2). Additionally, PINCH levels detected in the brains of HIV patients without CNS alterations were lower than those in HIVE patients, but this did not reach statistical significance. Clearly, PINCH protein expression is up-regulated in the brains of HIV patients, and its distribution patterns change with progression to HIVE.
Fig. 1.
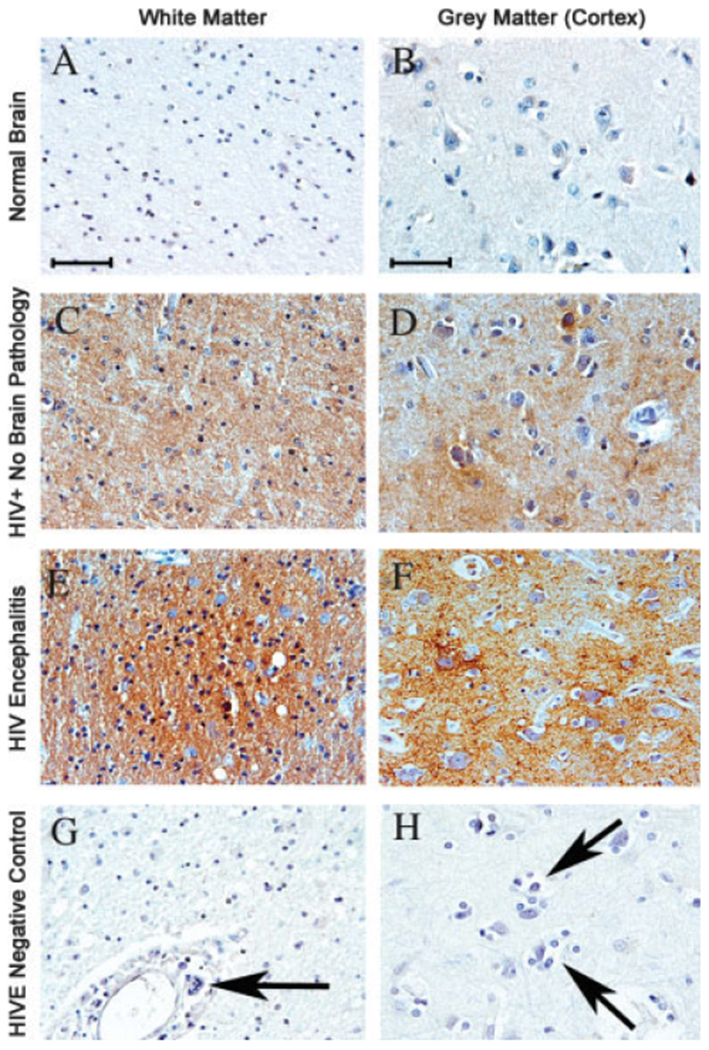
Immunohistochemical analyses of PINCH expression in HIV patients. All images are from frontal lobe and immunolabeled with anti-PINCH antibody (brown) and counterstained with hematoxylin. A,B: Expression of PINCH in the white and grey matter, respectively, of normal HIV-seronegative human brain samples is undetectable. C,D: In HIV-positive patients with no neurological complications, PINCH expression is increased with a patchy appearance in both the cortex and the subcotical white matter, respectively. E,F: In cases of HIVE, PINCH immunoreactivity is robust and widespread. G,H: In an HIVE case showing characteristic perivascular cuffing by mononuclear cells and a multinucleated giant cell (G, arrow) and cortical microglial nodule (H, arrows), primary anti-PINCH antibody was omitted to demonstrate antibody specificity. Scale bars = 10 μm.
Fig. 2.
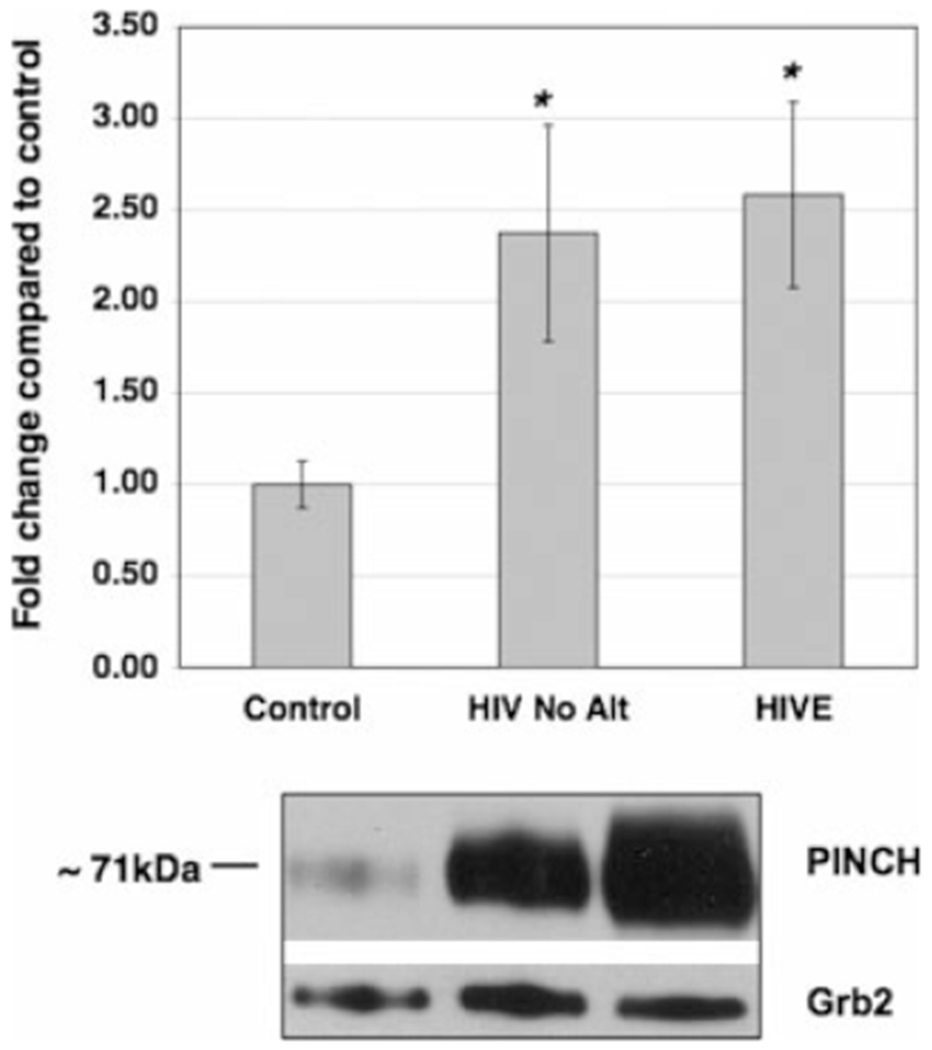
Western analyses of PINCH levels in brain tissue from HIVE patients, HIV patients with no CNS alterations, and non-HIV controls. PINCH protein levels in the brains of HIV patients with no CNS alterations (n = 10; HIV No Alt) and HIVE (n = 14) were significantly greater than levels in non-HIV patients (n = 10; Control; *P < 0.05 and 0.01, respectively) by one-way ANOVA with Dunnett’s post hoc test compared with untreated control. The lower panel is a representative Western blot of brain homogenate reacted with anti-PINCH antibody detecting a band at 71 kDa that corresponds to PINCH dimer. Grb2 was used as a loading control.
PINCH Is Expressed Robustly in Neuronal Processes and the Extracellular Matrix of White Matter in HIVE Patients
Localization of PINCH immunoreactivity in HIV revealed robust and widely distributed immunolabeling of the white matter in frontal cortex, with less intense reactivity in the grey matter, of HIVE patients (Fig. 3B), whereas PINCH was undetectable in the control sections (normal brain; Fig. 3A). In the white matter, PINCH expression was particularly robust in areas of inflammation, as demonstrated in Figure 3C. For example, an area illustrating typical perivascular cuffing of mononuclear cells and a multinucleated giant cell (Fig. 3C, arrow) observed in HIVE is surrounded by robust immunolabeling of PINCH in the extracellular matrix (Fig. 3D, arrowheads) and most likely associated with neuronal processes, as demonstrated in the next set of experiments. Finally, PINCH is expressed in neurons that exhibit morphological signs of damage, such as pycnotic nuclei (Fig. 3E, arrow). These changes seem to be specific, in that, in numerous tissue sections, abnormal neurons (Fig. 3E, arrow) lay adjacent to normal neurons in which expression of PINCH is undetectable (Fig. 3E, arrowhead). Western analyses of brain homogenate enriched for white vs. grey matter confirmed significantly increased PINCH levels in white matter (P < 0.001; Fig. 4).
Fig. 3.
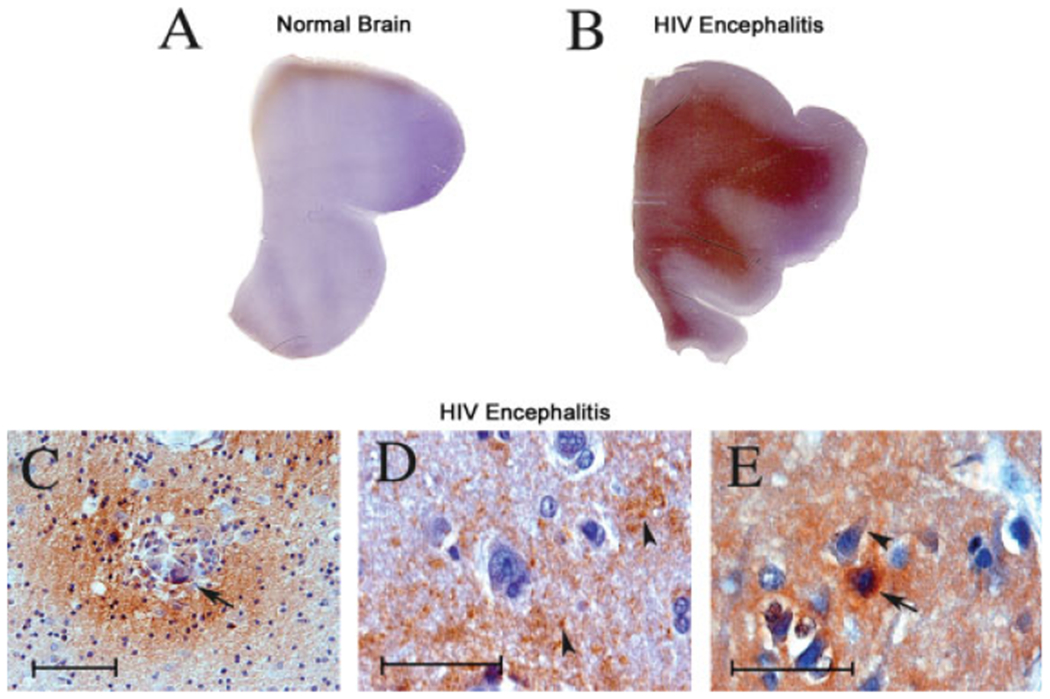
Patterns of PINCH expression in white vs. grey matter in HIVE. Full montages of the lobe cortex are immunolabeled with anti-PINCH antibody and counterstained with hematoxylin from a normal brain (A) and an HIVE case (B) demonstrating high levels of PINCH in HIVE compared with the normal brain in which PINCH is absent. Higher magnification images demonstrate the pattern and localization of PINCH expression, which is robust in close proximity to perivascular cuffs of inflammatory cells and decreases gradually to areas with less inflammation (C; arrowhead indicates a multinucleated giant cell). D: Extracellular PINCH is present in HIVE brains. E: PINCH is also robustly expressed in neurons showing morphological alterations such as picnotic nuclei (arrowhead) but less intense in morphologically normal adjacent neurons (arrow). Scale bars = 10 μm.
Fig. 4.
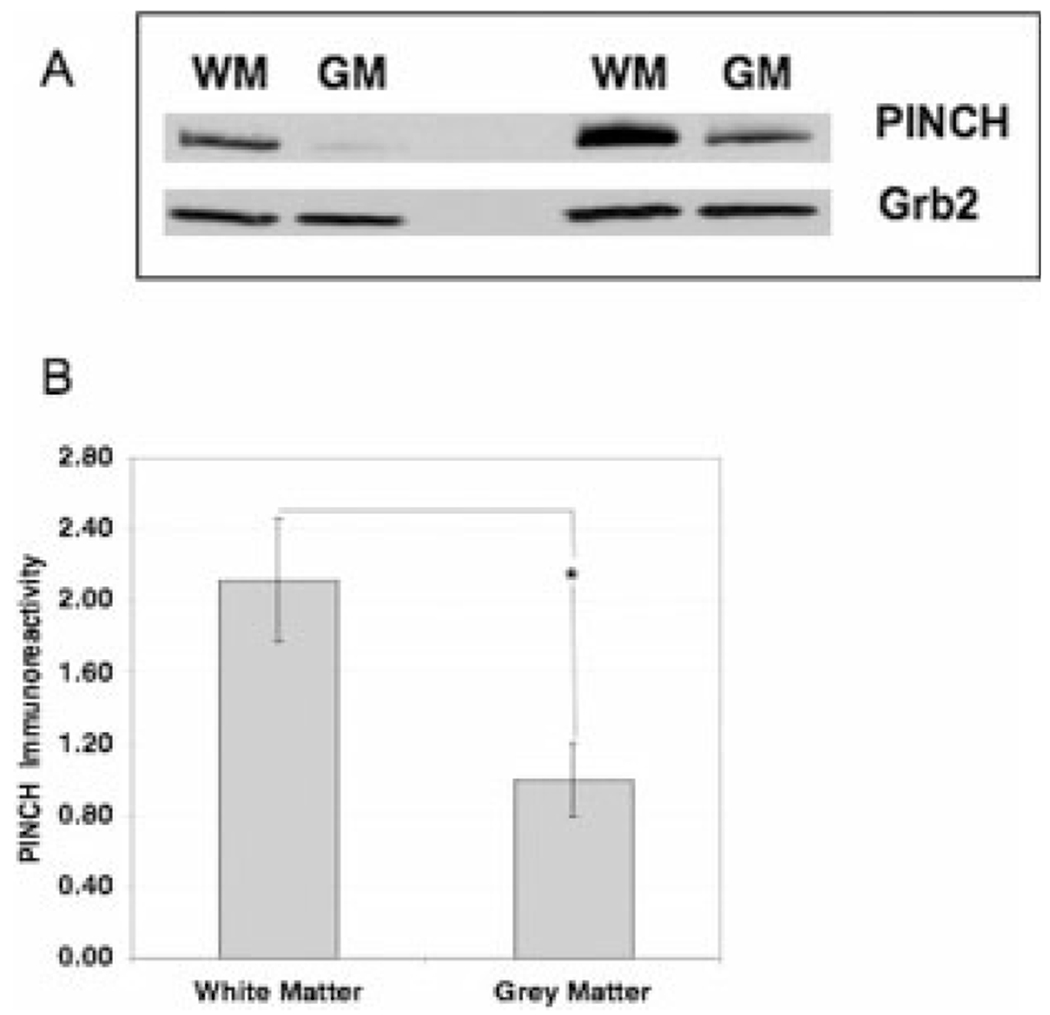
Western analyses of PINCH protein expression in white matter vs. grey matter. A: Western blot of frontal cortex homogenate from two representative HIVE cases enriched for either white matter (WM) or grey matter (GM) showing an immunoreactive band corresponding to the PINCH dimer at approximately 71 kDa. Grb2 was used as a loading control. B: Analyses of PINCH immunoreactivity in the white matter is significantly greater (*P < 0.001) than in the grey matter in HIVE cases (n = 14).
To confirm the cell type in which PINCH was expressed in brains of HIV patients, double-immunohistochemical analyses were conducted. Immunolabeling of the frontal cortex of HIVE brains revealed PINCH expression in neuronal cells, particularly concentrated in proximity to the cellular membrane (Fig. 5A, arrow) and out into the axon (Fig. 5A, arrowhead). Double-immunofluorescence labeling in a serial section confirmed expression of PINCH in neuronal bodies and into the axon (Fig. 5B, arrow, arrowhead, respectively). Given the intense immunoreactivity observed in the white matter (Figs. 3, 4), double immunolabeling for PINCH and myelin basic protein was conducted. As illustrated in Figure 5A, PINCH immunoreactivity was observed both in the neuronal cell bodies (Fig. 5C, arrow) and in the axonal processes (Fig. 5C, arrowheads). Double immunolabeling of a serial section confirmed that PINCH localized in a punctate beadlike pattern along the processes (Fig. 5D, arrows) but did not colocalize with myelin surrounding the processes (Fig. 5D, arrowheads). Likewise, PINCH was expressed robustly by neuronal cells, as illustrated by a PINCH-positive neuron (Fig. 5E, arrow) surrounded by PINCH-negative astrocytes (Fig. 5E, arrowhead). Astrocytes labeled with anti-GFAP showed no expression of PINCH (Fig. 5F, arrow). Instead, PINCH localized around the astrocytes in neuronal processes (Fig. 5F, arrowheads). Our results show that PINCH is robustly expressed in the brains of individuals infected with HIV and that expression is limited to neuronal cell bodies and processes and extracellular matrix. Moreover, PINCH is not expressed by astrocytes and does not colocalize with myelin.
Fig. 5.
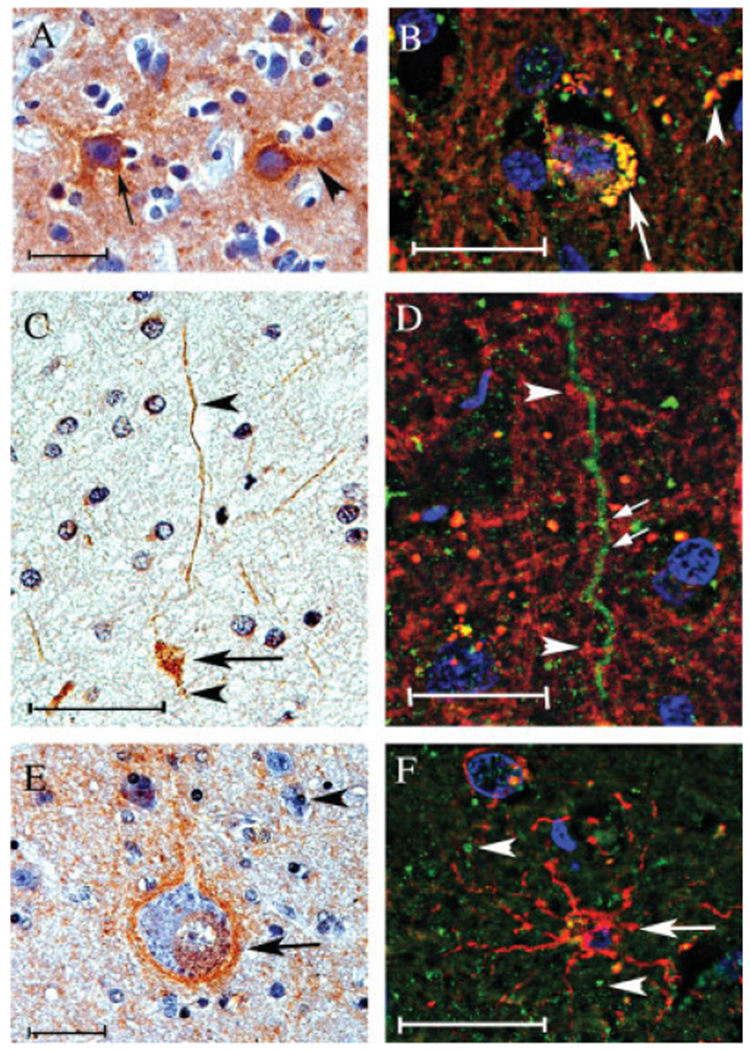
Double-immunofluorescence analyses of PINCH cellular localization in the frontal cortex of HIV patients. A, C, and E are reacted with anti-PINCH antidody and counterstained hematoxylin. B, D, and F are doubly labeled with anti-PINCH antibody (green) and antineurofilament, antimyelin basic protein, or anti-GFAP, (red, respectively). A: PINCH immunoreactivity (brown) is observed in neuronal cell bodies (arrow) and in neuronal processes (arrowhead) in an HIVE brain. B: Neuronal cells displayed both PINCH (green) and neurofilament (red) immunoreactivity, with colocalization (yellow) observed in neuronal cell bodies (arrow) and in neuronal processes (arrowhead). C: PINCH (brown)-immunoreactive neuronal cell bodies (arrow) and processes (arrowhead). D: Neuronal cell processes displayed PINCH immunoreactivity with a punctate, beadlike pattern (arrows, green) but did not colocalize with the surrounding myelin basic protein (red). E: Neuronal cell body and process displaying PINCH immunoreactivity (arrow, brown) surrounded by astroglial cell nuclei (arrowhead). F: Astroglial cell labeled with GFAP (red) showing no colocalization with PINCH (green). Scale bars = 10 μm.
Our results showing that brain levels of PINCH protein are increased in HIV and that patterns of distribution vary depending on the presence or absence of HIVE point to its potentially important role during the neuropathogenic process. However, examining patterns of PINCH in the brain in vivo is highly impractical. Numerous studies have shown that the CSF content of proteins such as erythropoietin, neurofilament, enolase, and tau, to name a few, provides valuable information regarding the status of CNS cellular functioning and neuropathological changes (Selakovic et al., 2005; Brettschneider et al., 2006). Together with these data, the presence of significant PINCH in the extracellular matrix led us to test whether PINCH was detectable in the CSF from HIV patients.
Increased Levels of PINCH Are Detected in CSF From Patients With HIV
To determine first whether PINCH could be detected in CSF, and second whether levels varied among the control group, HIV with no CNS alterations, and HIVE, Western analyses for PINCH were conducted on CSF from these three groups. PINCH levels in CSF from both HIV groups were significantly increased compared with those from the HIV-negative control group (P < 0.05; Fig. 6). A representative Western blot of PINCH in CSF from each group clearly shows increased levels of PINCH in HIV and HIVE compared with control (Fig. 6). Interestingly, levels of PINCH in the CSF of HIVE patients were significantly lower than in HIV patients with no CNS alterations (P < 0.01; Fig. 6). Taken together, these results show for the first time that PINCH is detectable in human CSF and that increased CSF PINCH levels are observed in patients with HIV. Further analyses are currently underway to determine the significance of increased expression levels and altered distribution patterns of PINCH in HIV patients.
Fig. 6.
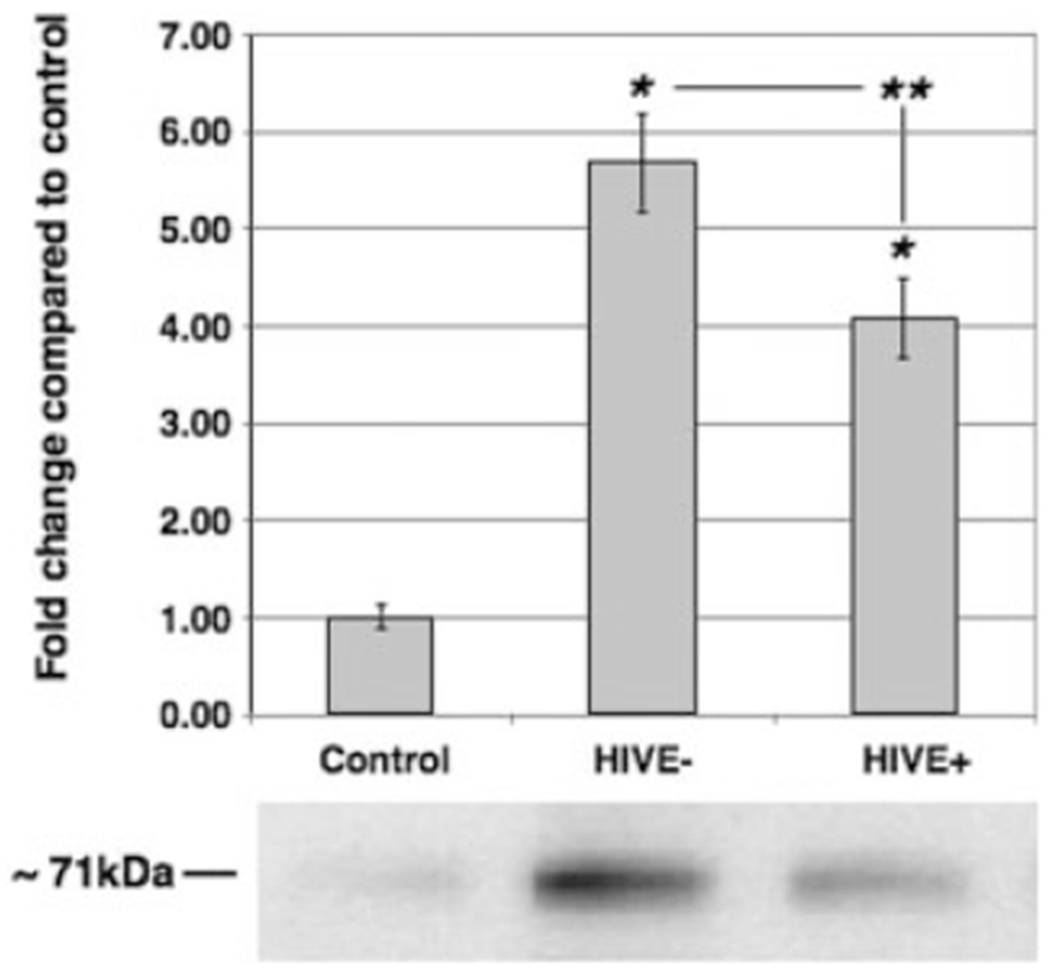
Western analyses of PINCH protein levels in CSF. Western analyses of PINCH levels in CSF from non-HIV controls (n = 10), HIV No Alts (HIVE−; n = 12), and HIVE (n = 10). *P < 0.05 by one-way ANOVA, **P < 0.01 with Tukey-Kramer post hoc test for HIV compared with HIVE. The lower panel is a representative Western blot of CSF reacted with anti-PINCH antibody detecting a band at 71 kDa that corresponds to the PINCH dimer.
DISCUSSION
Our data show that, in contrast to normal seronegative controls, PINCH is robustly expressed in the brains and CSF of HIV-infected individuals. Specifically, PINCH was detected in the neuronal nucleus, cytoplasm, and processes and in the extracellular matrix. Interestingly, PINCH distribution patterns differ between HIVE patients and HIV patients with no reported CNS alterations.
These findings raise important questions regarding the role of PINCH in the CNS of patients suffering from HIV, and other neurodegenerative diseases for that matter. What triggers the expression of PINCH in certain neurons, whereas adjacent neurons show no detectable expression of PINCH? What are the neurological consequences of increased PINCH expression in the brain?
Based on the reported functions of PINCH in maintaining cell fitness in other systems, the localization patterns of PINCH observed in the brains of HIV patients may suggest a need for the stabilization of neuronal/extracellular matrix communications and/or neurotrophic factor signaling. Unlike the evenly robust patterns of PINCH expression observed in the white matter of HIVE cases, patterns of PINCH immunoreactivity in the HIV-positive group without CNS alterations showed a more patchy, uneven distribution. Likewise, PINCH did not localize with myelin or cellular components in the white matter, indicating that most PINCH might be extracellular. The disproportionately high levels of extracellular PINCH in HIV brains compared with cellular levels suggest that neurons may be releasing PINCH. Abundant PINCH in the brain extracellular matrix suggests the brain as the source of PINCH in the CSF. However, plasma levels of PINCH in HIV have not been assessed. The broadly distributed and robust immunoreactivity of PINCH in neurons and the extracellular matrix may reflect inability, resulting from various insults during infection, of PINCH to maintain proper subcellular localization. In support of this possibility, a compelling review by Chu et al. (2007) summarizes growing evidence that, in numerous diseases with neurodegenerative components, altered transcription factor trafficking in neurons likely plays an important role in neuronal dysfunction. Although mechanisms controlling PINCH transcription, translation, and trafficking are largely undefined, studies describing the interactions of PINCH with ILK and Nck-2 and its bidirectional nuclear/cytoplasmic shuttling capacity make PINCH an excellent candidate as a key player in neuronal dysfunction. For example, Xu et al. (2005) reported that, through its interaction with ILK, PINCH regulates signal transduction important in cell shape modulation and survival. Moreover, insofar as PINCH expression is required for proper ILK–integrin-mediated cell–extracellular matrix adhesions (for review see Wu, 2005), our findings of increased PINCH expression in neurons in HIV suggest a need for increased cell–extracellular matrix signaling. Our data also expand on findings that PINCH is important in shuttling and signaling in Schwann cells and dorsal root ganglia neurons, axons, and satellite cells after injury (Campana et al., 2003). PINCH links directly to Nck-2, and Nck-2 recognizes numerous growth factor and cell adhesion receptors, such as epidermal growth factor, IRS1, and platelet-derived growth factor receptor β1 to promote proper targeting (Tu et al., 1998). It is in this context that we speculate that the increased expression of PINCH during stressful conditions, such as in HIV infection, whether peripherally or locally, may stimulate neuroprotective signaling requiring neurotrophic factor recruitment and shuttling to sites of damage.
Given the success of cART, and the fact that HIV patients are living longer, changes in the clinical features of HIV-associated neurocognitive disorders are predictable. In fact, successful cART has partially reversed severe HIV dementia in some cases. However, milder forms of neurological impairment are more common in the HIV population, and the mechanisms responsible are poorly understood (for review see Ellis et al., 2007). Upon examination at autopsy, histopathological alterations in individuals who suffered from mild forms of neurological impairments are not easily identifiable. Likewise, in HIV patients with no CNS alterations observed post-mortem, subtle changes and/or disruptions in cellular signaling and cross-talk might have occurred through out disease, albeit at levels undetectable by current methodologies. In this regard, neuronal dysfunction can occur in the absence of neuronal loss. Under these circumstances, reparative measures may be initiated, contributing to the cyclic nature of cognitive impairments observed in some patients (Ellis et al., 2007). Possible functions of PINCH in the brains of HIV patients without reported CNS alterations include 1) indication of the beginning stages neurodegenerative processes and white matter damage, 2) successful shuttling of factors involved with cellular protection or preservation induced by stressful stimuli, 3) early growth factor-associated attempts to repair subtle cellular dysfunction or damage, 4) effects of damage that occurred earlier in life and resolved to some degree, or 5) a combination of these and other mechanisms. For example, the mechanisms through which HIV proteins damage cells of the CNS; the signaling pathways that host cells utilize to protect, repair, and prevent dysfunction; and the reported functional properties of PINCH make a compelling argument that PINCH plays an important role in HIV neuropathogenesis. For example, brain-derived growth factor, insulin-like growth factor, fibroblast growth factors 1 and 2, macrophage inflammatory protein-2, and stromal-derived growth factor are a few of the many host-derived neurotrophic/neuroprotective factors that have been shown to contribute to preservation and maintenance of cellular signaling in the CNS during HIV infection (for brief review see Ellis et al., 2007). In addition, the HIV protein Tat has a remarkable ability to travel between and through cells, and it binds to and signals through the α5β1/αvβ3 integrins (for review see Peruzzi, 2006; see also Barillari et al., 1999). Recent studies have also shown that neuronal protection by FGF1 from the toxic HIV protein gp120 involves AKT/GSK3β signaling (Hashimoto et al., 2002). Importantly, a recent report by Guo et al. (2007) shows that ILK, a key component in PINCH integrin signaling, signals upstream of AKT/GSK3β to maintain neuronal polarity.
Although the role of PINCH’s involvement in HIV in the CNS is not clear at this time, our observations support PINCH’s importance during HIV-associated neuropathogenesis. Importantly, systemic inflammation induced by viremia or viral inflammation in peripheral organs can also affect the brain even in the absence of HIV-driven inflammation in the CNS. The punctate, bead-like PINCH immunolabeling patterns along cellular processes in the white matter and at the grey–white matter junction of HIV patients with no reported neuropathological changes may reflect targeted subcellular localization in response to disruptions in focal adhesion capacity. Moreover, PINCH immunoreactivity in HIVE is present at greater levels in areas of severe inflammation and gradually decreases in areas of low inflammatory response, suggesting that PINCH expression is associated with inflammation and/or the tissue damage associated with the inflammatory response. In this regard, investigations aimed at other disease with CNS inflammatory components are warranted. Studies conducted in a cohort of individuals in vivo over time during the progression of HIV disease until death would provide valuable information onb the patterns of PINCH levels in CSF at various stages of disease. Thus, studies are underway that measure levels of PINCH in the CSF HIV patients undergoing multiple draws throughout the course of HIV progression. Importantly, identifying the factors responsible for the induction, localization, and export of PINCH are crucial to understanding the role of PINCH in HIV and other neurodegenerative CNS diseases.
ACKNOWLEDGMENTS
We acknowledge the HIV Neurobehavioral Research Center (HNRC) and the California NeuroAIDS Tissue Network (CNTN) and Amy Paulino for providing tissues and Eliezer Masliah for consultation. We extend a special thanks to Paola Pannizzo for manuscript preparation and technical support. The HNRC is supported by center award MH62512 and the CNTN by award MH059745.
Contract grant sponsor: Sam and Rose Stein Institute on Aging at UCSD; Contract grant sponsor: NIH; Contract grant number: MH071206; Contract grant sponsor: NINDS; Contract grant number: NS055639; Contract grant number: MH62962; Contract grant number: MH59745; Contract grant number: T35HL007491-25; Contract grant number: T35AG026757.
REFERENCES
- Banks WA, Ercal N, Price TO. 2006. The blood-brain barrier in neuro-AIDS. Curr HIV Res 4:259–266. [DOI] [PubMed] [Google Scholar]
- Barillari G, Sgadari C, Fiorelli V, Samaniego F, Colombini S, Manzari V, Modesti A, Nair BC, Cafaro A, Sturzl M, Ensoli B. 1999. The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging the alpha5beta1 and alphav-beta3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood 94:663–672. [PubMed] [Google Scholar]
- Brettschneider J, Widl K, Ehrenreich H, Riepe M, Tumani H. 2006. Erythropoietin in the cerebrospinal fluid in neurodegenerative diseases. Neurosci Lett 404:347–351. [DOI] [PubMed] [Google Scholar]
- Campana WM, Myers RR, Rearden A. 2003. Identification of PINCH in Schwann cells and DRG neurons: shuttling and signaling after nerve injury. Glia 41:213–223. [DOI] [PubMed] [Google Scholar]
- Cherner M, Cysique L, Heaton RK, Marcotte TD, Ellis RJ, Masliah E, Grant I, and the HNRC Group. 2007. Neuropathologic confirmation of definitional criteria for human immunodeficiency virus-associated neurocognitive disorders. J Neurovirol 13:23–28. [DOI] [PubMed] [Google Scholar]
- Chu C, Plowery E, Wang Y, Patel V, Jordan-Scuitto K. 2007. Location, location, location: altered transcription factor trafficking in neurodegeneration. J Neuropathol Exp Neurol 66:873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Valle L, Pina-Oviedo S. 2006. HIV disorders of the brain: pathology and pathogenesis. Front Biosci 11:718–732. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. 2007. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev 8:33–44. [DOI] [PubMed] [Google Scholar]
- Guo W, Jiang H, Gray V, Dedhar S, Rao Y. 2007. Role of the integrin-linked kinase (ILK) in determining neuronal polarity. Dev Biol 306: 457–468. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Sagara Y, Langford D, Everall IP, Mallory M, Everson A, Digicaylioglu M, Masliah E. 2002. Fibroblast growth factor 1 regulates signaling via the glycogen synthase kinase-3beta pathway. Implications for neuroprotection. J Biol Chem 277:32985–32991. [DOI] [PubMed] [Google Scholar]
- Langford D, Grigorian A, Hurford R, Adame A, Ellis RJ, Hansen L, Masliah E. 2004. Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J Neuropathol Exp Neurol 63:1038–1047. [DOI] [PubMed] [Google Scholar]
- Langford TD, Letendre SL, Marcotte TD, Ellis RJ, McCutchan JA, Grant I, Mallory ME, Hansen LA, Archibald S, Jernigan T, Masliah E. 2002. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS 16:1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zhang Y, Wu C. 1999. Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J Cell Sci 112:4589–4599. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. 2002. The integrin family of cell adhesion molecules has multiple functions within the CNS. J Neurosci Res 69:286–291. [DOI] [PubMed] [Google Scholar]
- Peruzzi F 2006. The multiple functions of HIV-1 Tat: proliferation versus apoptosis. Front Biosci 11:708–717. [DOI] [PubMed] [Google Scholar]
- Rearden A 1994. A new LIM protein containing an autoepitope homologous to “senescent cell antigen.” Biochem Biophys Res Commun 201:1124–1131. [DOI] [PubMed] [Google Scholar]
- Selakovic V, Raicevic R, Radenovic L. 2005. The increase of neuron-specific enolase in cerebrospinal fluid and plasma as a marker of neuronal damage in patients with acute brain infarction. J Clin Neurosci 12:542–547. [DOI] [PubMed] [Google Scholar]
- Shrikant P, Benos DJ, Tang LP, Benveniste EN. 1996. HIV glycoprotein 120 enhances intercellular adhesion molecule gene expression in glial cells. Involvement of Janus/kinase signal transducer and protein kinase C signaling pathways. J Immunol 156:1307–1314. [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Murri R, Galgani S, Bellagamba R, Narciso P, Antinori A, Giulianelli M, Tosi G, Fantoni M, Sampaolesi A, Noto P, Ippolito G, Wu AW. 2004. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int J STD AIDS 15:254–259. [DOI] [PubMed] [Google Scholar]
- Tu Y, Li F, Wu C. 1998. Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Mol Biol Cell 9:3367–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Rodriguez J, Dreilinger AD, Alsharabi GM, Rearden A. 2002. The signaling adapter protein PINCH is up-regulated in the stroma of common cancers, notably at invasive edges. Cancer 95:1387–1395. [DOI] [PubMed] [Google Scholar]
- Wu C 1999. Integrin-linked kinase and PINCH: partners in regulation of cell-extracellular matrix interaction and signal transduction. J Cell Sci 112:4485–4489. [DOI] [PubMed] [Google Scholar]
- Wu C 2005. PINCH, N(i)ck and the ILK: network wiring at cell–matrix adhesions. Trends Cell Biol 15:460–466. [DOI] [PubMed] [Google Scholar]
- Xu Z, Fukuda T, Li Y, Zha X, Qin J, Wu C. 2005. Molecular dissection of PINCH-1 reveals a mechanism of coupling and uncoupling of cell shape modulation and survival. J Biol Chem 280:27631–27637. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo L, Chen K, Wu C. 2002. A critical role of the PINCH-integrin-linked kinase interaction in the regulation of cell shape change and migration. J Biol Chem 277:318–326. [DOI] [PubMed] [Google Scholar]


