Dear Editor,
LIF, a multi-functional cytokine, is frequently overexpressed in many human cancers, including breast, colorectal, and pancreatic cancers (Liu et al., 2013; Li et al., 2014; Yu et al., 2014; Pascual-Garcia et al., 2019; Shi et al., 2019; Wang et al., 2019). LIF overexpression is frequently associated with poor prognosis in human cancers (Liu et al., 2013; Li et al., 2014; Yu et al., 2014). LIF functions through binding to LIF receptor complex composed of LIF receptor (LIF-R) and glycoprotein gp130 (Taga and Kishimoto, 1997; Heinrich et al., 2003; Watanabe et al., 2006). LIF overexpression induces activation of several oncogenic signaling pathways in a cell/tissue type-specific manner, including STAT3, PI3K/AKT, and mTOR, which in turn promotes proliferation, metastasis, and therapeutic resistance of cancer cells (Liu et al., 2013; Li et al., 2014; Yu et al., 2014; Shi et al., 2019). Recent studies have suggested that LIF is a potential important target for cancer therapy, especially for cancers with LIF overexpression. LIF neutralization antibodies (LIF neu Abs) have been reported to block LIF signaling and largely abolish the promoting effect of LIF on cancer progression (Li et al., 2014; Yue et al., 2016; Shi et al., 2019).
Here, we reported a group of small-molecule compounds, including EC330, EC357, and EC363, as potential LIF inhibitors. EC330, EC357, and EC363 were designed based on the structure–activity relationship (SAR) studies on human breast cancer MCF7 cells with LIF overexpression (Nair et al., 2018). An initial screening was performed using a library of steroidal molecules. The SAR established through this screening includes following criteria: (i) the steroidal skeleton of the molecule is an antiprogestin, (ii) the molecule has the specific 17α-difluoro acetylenic moiety, and (iii) nonpolar substituents at position 11 are preferred over polar substituents. EC330, EC357, and EC363 have all these features. The structures of these compounds are shown in Figure 1A. EC364, which belongs to the same class of lipophilic steroidal molecules, does not have the specific 17α-difluoro acetylenic moiety and is expected to have no inhibitory activity towards LIF.
Figure 1.
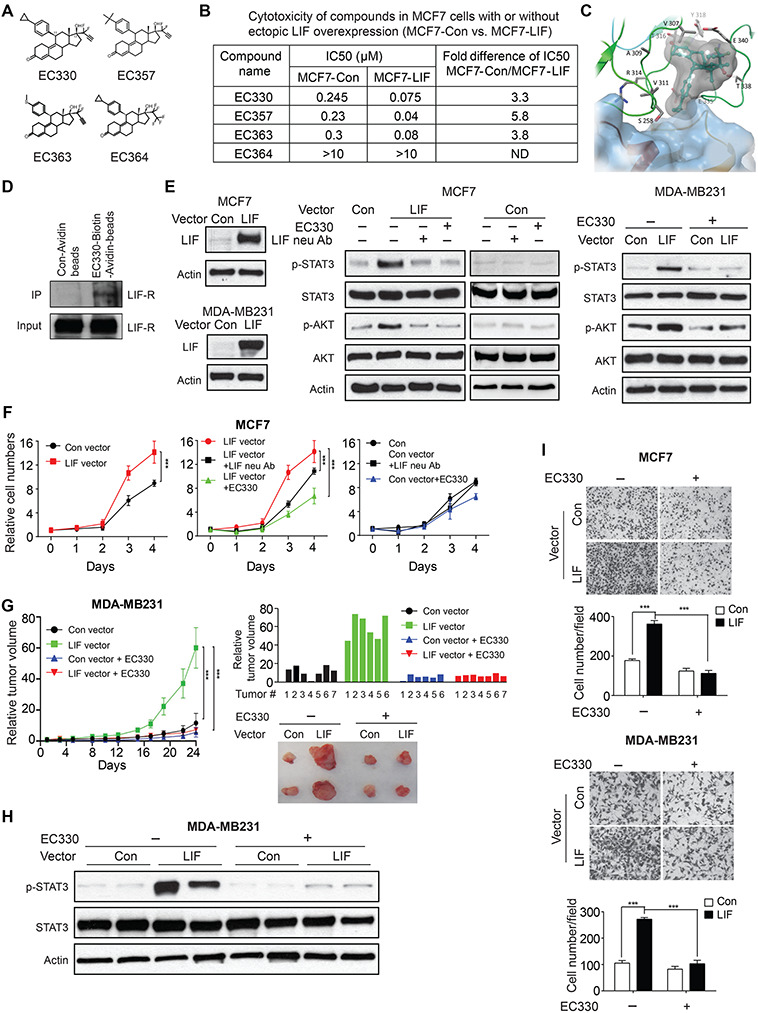
EC330 inhibits the LIF/LIF-R signaling and blocks the promoting effects of LIF on growth and migration of cancer cells. (A) Chemical structure of compounds EC330, EC357, EC363, and EC364. (B) Cytotoxicity of EC330, EC357, EC363, and EC364 in MCF7 cells with or without ectopic LIF overexpression. Cells were treated with compounds for 24 h. (C) EC330 binds to hLIF-R in the presence of LIF (blue surface) as predicted by molecular modeling studies. (D) EC330 interacts with LIF-R. Cellular lysates of MCF7-LIF cells were incubated with Avitin-biotin-EC-330 or Control-Avidin beads. The ability of Avitin-biotin-EC330 to bind to LIFR was analyzed by Avidin beads pull-down followed with western blot assays. (E) Ectopic LIF expression increased the levels of p-STAT3Y705 and p-AKTS473 in MCF7 and MDA-MB231 cells, which was abolished by treatment of LIF neu Abs (200 ng/ml in MCF7 cells) or EC330 (1 μM) for 2 h. (F) Ectopic LIF expression in MCF7 cells promoted the proliferation of cells (left panel), which was largely abolished by LIF neu Abs (200 ng/ml) and EC330 (5 nM) (middle and right panels). (G) EC330 (1 mg/kg, i.p., 5 times/week for 24 days) preferentially inhibited the growth of xenograft tumors formed by MDA-MB231 cells with ectopic LIF expression (n ≥ 6/group). Left panel: growth curves of xenograft tumors. Right upper panel: relative tumor volume for all xenograft tumors of each group at the end of the experiment. Right lower panels: representative images of xenograft tumors. (H) The levels of p-STAT3 and total STAT3 in MDA-MB231 xenograft tumors. (I) EC330 preferentially inhibits the migration of cancer cells with LIF overexpression. MCF7 and MDA-MB231 cells with or without ectopic LIF expression were treated with EC330 (5 nM for MCF7 and 15 nM for MDA-MB231 cells). The migration abilities of cells were determined by trans-well assays. Data are presented as mean ± SD. ***P < 0.001.
Our results showed that EC330, EC357, and EC363, but not EC364, displayed cytotoxicity in MCF7 cells. IC50 values of EC330, EC357, and EC363 are at the range of 0.2–0.3 μM when cells were treated with these compounds for 24 h (Figure 1B). Interestingly, all three compounds exhibited a much stronger cytotoxicity in MCF7 cells with ectopic LIF overexpression (MCF7-LIF); their IC50 values were 3–5-fold lower in MCF7-LIF cells than in control MCF7 cells transfected with empty vectors (MCF7-Con) (Figure 1B). In contrast, EC364 did not show obvious cytotoxicity in both MCF7-Con and MCF7-LIF cells at concentrations up to 10 μM (Figure 1B). In another pair of human breast cancer cell lines, MDA-MB231 with or without ectopic LIF expression (MDA-MB231-LIF and MDA-MB231-Con), the IC50 of EC330 in MDA-MB231-LIF cells was ~2-fold lower than that in MDA-MB231-Con cells (Supplementary Figure S1). These results indicate that EC330 preferentially targets cancer cells with LIF overexpression.
Since LIF functions through binding to its specific receptor LIF-R to recruit gp130 to form a high-affinity receptor complex (Chollangi et al., 2012; Yue et al., 2015), we investigated whether EC330 interferes with the LIF/LIF-R interaction by molecular docking studies. All five potential binding sites (numbered as site 1–site 5) on human LIF-R (hLIF-R) identified through Sitemap (Supplementary Figure S2A) were used. The standard precision (SP) docking studies suggested that the binding of EC330 at site 3 is more energetically favorable compared with other sites. One of the best docked poses obtained from the SP docking (docking score −2.56 kcal/mol) was then utilized for the induced fit docking (IFD) studies to investigate the ligand-induced conformational changes of the receptor. Multiple poses generated from IFD studies suggested that the ligand-induced conformational changes occur to loops close to the LIF binding region. In all of the generated poses, the ligand made large steric clashes with LIF. The top scoring pose (MM-GBSA ~−76 kcal/mol) was selected for further analysis. EC330 is sandwiched between two loops at the N-terminus of the D4 domain with its difluro-acetylenic group facing to the bulk solvent (Figure 1C). The keto group of EC330 is engaged in two hydrogen bonds with the side chain of T308 and the backbone of T316, respectively. Moreover, several van der Waals contacts with the surrounding residues were found to stabilize the ligand binding. The stability of the ligand at the binding site was confirmed by a 25-ns molecular dynamics (MD) simulation (Supplementary Figure S2B). To confirm that EC330 interacts with LIF-R, we performed in vitro pull-down assays by incubating total cellular lysate of MCF7-LIF cells with Avidin-biotin-EC330 or Control-Avidin beads. The ability of EC330 to interact with LIF-R was determined by using Avidin pull-down assays followed with western blot assays. Results elucidated that EC330 interacts with LIF-R (Figure 1D). These results suggest that EC330 binding is energetically favorable at the LIF/LIF-R binding interface and EC330 could prevent the binding of LIF to LIF-R.
LIF activates STAT3 and PI3K/AKT pathways (Graf et al., 2011; Yue et al., 2015). LIF increases phosphorylation of STAT3 at Tyr705 (p-STAT3Y705) and AKT at Ser473 (p-AKTS473), leading to activation of STAT3 and AKT, respectively (Li et al., 2014; Yue et al., 2016; Ali et al., 2017; Bressy et al., 2018; Ke et al., 2018). As shown in Figure 1E, ectopic LIF expression increased the levels of p-STAT3Y705 and p-AKTS473 without affecting the total STAT3 and AKT levels in MCF7 cells, which was blocked by LIF neu Abs. Notably, EC330 treatment largely blocked the activation of STAT3 and AKT by LIF, but did not affect the total STAT3 and AKT levels (Figure 1E). Similarly, ectopic LIF expression in MDA-MB231 cells activated STAT3 and AKT, which was largely abolished by EC330 (Figure 1E). Consistently, treating MCF7 and MDA-MB231 cells with recombinant human LIF protein activated STAT3 and AKT, which was largely abolished by EC330 (Supplementary Figure S3A). Previously, we found that LIF activated the mTOR signaling in breast cancer cells (Li et al., 2014). Indeed, in both MCF7 and MDA-MB231 cells, treatment with recombinant human LIF increased the phosphorylation levels of p70 S6K at Thr389 (p-p70-S6KT389). Notably, the increase of p-p70-S6KT389 by LIF in both cell lines was largely abolished by EC330 (Supplementary Figure S3B). LIF belongs to the IL6 superfamily (Heinrich et al., 2003). Both LIF and IL6 activate STAT3 (Heinrich et al., 2003). Interestingly, EC330 did not affect STAT3 activation by IL6 (Supplementary Figure S3C). These results indicate that EC330 inhibits the activation of STAT3, PI3K/AKT, and mTOR signaling pathways by LIF.
LIF promotes cancer cell proliferation (Li et al., 2014). Here, we examined the effect of EC330 on proliferation of MCF7 and MDA-MB231 cells with or without ectopic LIF expression. MCF7 and MDA-MB231 cells with ectopic LIF expression showed significantly accelerated proliferation compared with their isogenic control cells (Figure 1F; Supplementary Figure S4). Furthermore, LIF neu Ab displayed a much stronger inhibitory effect on proliferation of MCF7-LIF cells than that of MCF7-Con cells, validating that LIF promotes proliferation of MCF7-LIF cells and can be blocked by LIF neu Ab (Figure 1F). Treating these two pairs of isogenic cell lines with EC330 greatly inhibited proliferation of cells with LIF overexpression and exhibited a much weaker inhibitory effect on control cells (Figure 1F; Supplementary Figure S4). These results demonstrate that EC330 preferentially inhibits the proliferation of cancer cells with LIF overexpression.
We further compared the effect of EC330 on the growth of subcutaneous xenograft tumors formed by MDA-MB231 cells with or without ectopic LIF expression in BALB/c nude mice. Consistent with our previous reports (Li et al., 2014; Yu et al., 2014), LIF overexpression significantly promoted the growth of xenograft tumors (Figure 1G). To test the effect of EC330 on the growth of these xenograft tumors, when tumor volumes reached ~30 mm3, mice were treated with EC330 (1 mg/kg body weight) or PBS via intraperitoneal (i.p.) injection 5 times/week for 24 days (n ≥ 6/group). EC330 treatment did not clearly affect the body weight of mice, but significantly inhibited the growth of xenograft tumors. Notably, EC330 significantly inhibited the growth of xenograft tumors with LIF overexpression but exhibited a much weaker inhibitory effect on the growth of tumors without LIF overexpression (Figure 1G). The activation of STAT3 signaling by LIF was confirmed in the xenograft tumors with LIF overexpression; the levels of p-STAT3Y705 were much higher in MDA-MB231-LIF tumors than that in MDA-MB231-Con tumors (Figure 1H). Notably, EC330 largely blocked STAT3 activation by LIF in MDA-MB231-LIF xenograft tumors (Figure 1H). Taken together, these results indicate that EC330 inhibits LIF function in promoting tumor growth in vivo.
LIF promotes migration and metastasis of cancer cells (Li et al., 2014; Yue et al., 2016). Here, the effect of EC330 on migration ability was compared between above-mentioned cancer cells with or without ectopic LIF expression by trans-well migration assays. Consistent with previous reports (Li et al., 2014; Yue et al., 2016), ectopic LIF expression promoted migration of MCF7 and MDA-MB231 cells (Figure 1I). Notably, EC330 largely abolished the promoting effect of LIF on migration of these cells; EC330 treatment significantly inhibited migration of MCF7 and MDA-MB231 cells with LIF overexpression but had a much weaker effect on their control cells (Figure 1I). These results demonstrate that EC330 inhibits LIF function in promoting migration of cancer cells.
Together, these data showed that EC330 efficiently inhibited LIF function in promoting proliferation and migration of cancer cells and tumor growth. Results from molecular docking studies indicated that the molecular target of EC330 is LIF-R (Figure 1C; Supplementary Figure S2). The interaction of EC330 with LIF-R was confirmed (Figure 1D). Notably, EC330 inhibited the proliferation of cancer cells with ectopic LIF overexpression to a similar extent as LIF neu Ab did (Figure 1F). LIF neu Abs can block LIF function (Figure 1E and F) and is a promising approach to target LIF in cancer cells (Pascual-Garcia et al., 2019; Shi et al., 2019; Wang et al., 2019). Both monoclonal antibodies and small-molecule agents have their unique properties. For example, monoclonal antibodies are generally intravenously administered, whereas some small-molecule agents can be orally administered (Imai and Takaoka, 2006). Monoclonal antibodies are generally more specific than small-molecule inhibitors (Imai and Takaoka, 2006). Monoclonal antibodies can also activate immune effector cells to eliminate tumor cells (Imai and Takaoka, 2006). On the other hand, small-molecule agents usually have a better ability to penetrate into tissues and tumors (Imai and Takaoka, 2006). Combination of distinct classes of inhibitors for the same target may not only increase their efficacy, but also assist in overcoming resistance to one class of inhibitors.
In summary, given that LIF is frequently overexpressed in many cancers and its overexpression promotes cancer progression, LIF is an attractive target for cancer therapy. EC330 displays an inhibitory activity towards the LIF/LIF-R signaling and blocks the promoting effects of LIF on tumor development and progression, which can be potentially developed for targeted therapy of cancers with LIF overexpression.
[Supplementary material is available at Journal of Molecular Cell Biology online. K.V.D. thanks Japan Society for the Promotion of Science for a postdoctoral fellowship. This study was supported in part by National Institutes of Health (NIH) Grants 1R01CA160558 (to W.H.) and 1R01CA227912 (to Z.F. and W.H.). B.S., G.A., and K.N. are employees of Evestra, Inc. All other authors declare no competing interests. X.Y. performed experiments, analyzed data, and wrote the manuscript; F.W., J.W., R.K.V., and S.V. performed experiments; K.K. analyzed data; B.S. and G.A. performed chemical synthesis; K.V.D. and K.Y.J.Z. performed molecular modeling studies; Z.F. analyzed data and wrote the manuscript; K.N. supervised chemical synthesis; W.H. designed experiments, analyzed data, and wrote the manuscript.]
Supplementary Material
References
- Ali S.A., Kaur G., Kaushik J.K., et al. (2017). Examination of pathways involved in leukemia inhibitory factor (LIF)-induced cell growth arrest using label-free proteomics approach. J. Proteomics 168, 37–52. [DOI] [PubMed] [Google Scholar]
- Bressy C., Lac S., Nigri J., et al. (2018). LIF drives neural remodeling in pancreatic cancer and offers a new candidate biomarker. Cancer Res. 78, 909–921. [DOI] [PubMed] [Google Scholar]
- Chollangi S., Mather T., Rodgers K.K., et al. (2012). A unique loop structure in oncostatin M determines binding affinity toward oncostatin M receptor and leukemia inhibitory factor receptor. J. Biol. Chem. 287, 32848–32859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf U., Casanova E.A., and Cinelli P. (2011). The role of the leukemia inhibitory factor (LIF)-pathway in derivation and maintenance of murine pluripotent stem cells. Gene 2, 280–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich P.C., Behrmann I., Haan S., et al. (2003). Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., and Takaoka A. (2006). Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer 6, 714–727. [DOI] [PubMed] [Google Scholar]
- Ke M., He Q., Hong D., et al. (2018). Leukemia inhibitory factor regulates marmoset induced pluripotent stem cell proliferation via a PI3K/Akt-dependent Tbx3 activation pathway. Int. J. Mol. Med. 42, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yang Q., Yu H., et al. (2014). LIF promotes tumorigenesis and metastasis of breast cancer through the AKT–mTOR pathway. Oncotarget 5, 788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.C., Tsang N.M., Chiang W.C., et al. (2013). Leukemia inhibitory factor promotes nasopharyngeal carcinoma progression and radioresistance. J. Clin. Invest. 123, 5269–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H., Santhamma B., and Nickisch K. (2018). Cytotoxic agents that preferentally target leukemia inhibitory factor (LIF) for the treatment of maligancies and as new contraceptive agents. Evestra, Inc. (assignee). US patent 10,053,485.
- Pascual-Garcia M., Bonfill-Teixidor E., Planas-Rigol E., et al. (2019). LIF regulates CXCL9 in tumor-associated macrophages and prevents CD8+ T cell tumor-infiltration impairing anti-PD1 therapy. Nat. Commun. 10, 2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Gao W., Lytle N.K., et al. (2019). Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 569, 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T., and Kishimoto T. (1997). Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 15, 797–819. [DOI] [PubMed] [Google Scholar]
- Wang M.T., Fer N., Galeas J., et al. (2019). Blockade of leukemia inhibitory factor as a therapeutic approach to KRAS driven pancreatic cancer. Nat. Commun. 10, 3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Umehara H., Murayama K., et al. (2006). Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 25, 2697–2707. [DOI] [PubMed] [Google Scholar]
- Yu H., Yue X., Zhao Y., et al. (2014). LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers. Nat. Commun. 5, 5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X., Wu L., and Hu W. (2015). The regulation of leukemia inhibitory factor. Cancer Cell Microenviron. 2, pii:e877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X., Zhao Y., Zhang C., et al. (2016). Leukemia inhibitory factor promotes EMT through STAT3-dependent miR-21 induction. Oncotarget 7, 3777–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


