Abstract
The treatment of chronic, non-cancer musculoskeletal pain has become a topic growing interest as it is believed to be one of the reasons for the current opioid epidemic. The medicinal use of cannabis has a long history as a number of active compounds in cannabis have been shown to interact with the body’s endocannabinoid system to reduce pain. This position paper provides a history on the evolution of cannabis, the science behind its therapeutic effects, and review of the evidence and current guideline recommendations on its use as a treatment for patients with chronic, non-cancer musculoskeletal pain. Results from systematic reviews have demonstrated a statistically significant reduction in chronic pain conditions with cannabinoids, compared with placebo, although the effects might be considered small and did not reach the minimally important difference. More adverse events were reported in the cannabinoid group than in the placebo group with longer than 2 weeks of treatment. There is a lack of evidence on dependence. With changes to policies, patients’ perception has changed to be more positive toward the use of medical cannabis. Current recommendations from North America, Latin America, Europe, Australia and Iran support the use of medical cannabis for chronic, non-cancer pain. Based on the current evidence, it is our position that cannabinoids may be considered as an adjunctive therapy after recommended first- and second-line therapies have failed to provide sufficient efficacy or tolerability. Patients should consider the balance between the desirable and undesirable effects of taking cannabis for chronic pain, and comprehensively consider their own values and preferences, as well as cost-effectiveness factors, based on the information provided by their physician.
Keywords: cannabis, musculoskeletal pain, orthopaedic patients, position statement
The impact of musculoskeletal pain
Musculoskeletal pain is a clear driver of the opioid epidemic, as acute and chronic musculoskeletal pain continue to be among the leading reasons for patients to seek opioid prescriptions from providers.1 While the initiation of this class of analgesics may often be considered when treating severe injuries or intractable pain, its addictive potential and narrow toxic range make it a high risk first-line medication. The opioid epidemic continues to dominate headlines as opioid-related hospitalizations and emergency department visits in Canada have ballooned by over 50% during the last decade, with the majority occurring over the last 3 years.2 Even more staggering is the 500% increase in opioid-related deaths across North America over the last year, with over 50,000 reported fatalities; more than a third of which are related to prescription medications.2,3
Canadian and American guidelines have been putting forth for responsible prescribing of opioids, and discourage their use in the treatment of chronic, non-cancer pain (CNCP), which is defined as persistent or recurrent pain lasting beyond 3 months or having an established diagnosis of a chronic condition that is not associated with cancer.4–7 However, opioids remain the default choice for the majority of orthopaedic providers across North America, with deeply ingrained practice patterns leading to their routine prescription following an orthopaedic injury, surgery, or worsening degenerative bone and joint diseases.
The purpose of this position was to provide an overview of the history of medical cannabis, how cannabis functions based on laboratory findings, and the current evidence and healthcare guidelines on its applications for patients with CNCP. We intended to provide knowledge of medical cannabis and facilitate patient decision-making, in this evidence-based position paper.
The evolution of cannabis use
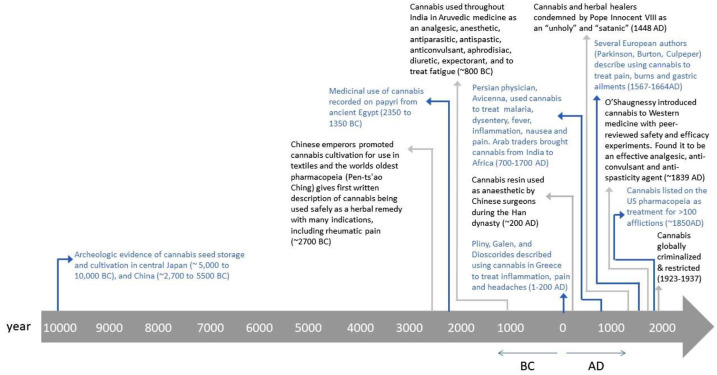
In parallel with the rising opioid crisis, there has been a societal shift in attitudes surrounding another of the world’s most commonly used recreational drugs. With an estimated 200 million current or past users worldwide, cannabis usage has spread to every continent, despite being illegal in almost all countries. Much of the controversy surrounding cannabis has focused on its associated euphoric ‘high’; however, the plant has been integrated throughout civilizations over thousands of years, primarily for non-euphorigenic purposes. Ancient texts describing cannabis use in traditional Chinese medicine date back as early as 2700 BC, and archeologic findings from East Asia indicate that cannabis may have been used by humans up to 10,000 years ago (Figure 1).8 Over centuries, cannabis and its derivatives have been utilized as textile bases, food sources, analgesics, anti-inflammatories, antiemetics, and mood enhancers. By the 1800s, cannabis was a widely grown agricultural crop, mainly to produce sails and ropes using hemp plants that had little to no psychotropic potential. However, in many areas of Asia and the Middle East, marijuana was commonly infused into food and liquids, or smoked as hashish for both medicinal and recreational purposes.
Figure 1.
Timeline of cannabis use throughout human civilization.
Medicinal applications of cannabis remained largely unknown to the West until the mid-1800s, when William O’Shaughnessy, an Irish physician working in Calcutta, India, first reported a series of basic animal experiments and human cases on various Indian Ayruvedic medicinal claims.8–10 Based on his studies on cannabis extracts and tinctures, O’Shaughnessy recommended its use for spasticity, pain and epilepsy-related convulsions.8–10 Following this, the availability of cannabis extracts in over-the-counter medications, as well as its general use, proliferated rapidly throughout North America and Europe and, by 1850, cannabis was listed in the United States (US) Pharmacopeia as a treatment option for approximately 100 symptoms. On the other hand, many in the West were concerned about users’ report of cannabis-induced hallucinations and an overall dulling of senses.10 This led to its moral and political opposition in the early 20th century.8
By 1906, the Pure Food and Drug Act required any cannabis-containing product to clearly identify its components and, by 1912, a certification was required for the importing of any cannabis-containing product.10 Both in the US and globally, cannabis has remained under the most restricted schedule category of controlled substances, with the 1970 Drug Abuse Prevention and Control Act and 1971 World Health Organization (WHO) treaty defining it as having a high potential for abuse with no currently accepted medicinal use and a lack of safety data to permit its use under medical supervision.11,12
Despite this, various countries have challenged this designation and have permitted limited medical and recreational use of cannabis based on a growing body of scientific evidence and sociopolitical shift in attitudes.13 The 17 October 2018 marked the day that Canada became the second country to fully legalize the use of cannabis (Uruguay was the first in 2013).14 Since then, several other countries have enacted similar legislation or are considering doing so in the near future, including South Africa, New Zealand, and the United Kingdom. In addition, the WHO now recommends reclassifying cannabis under international treaties to permit its medical use.12,14 Currently, in the US, recreational and medical use of cannabis is legal in 10 states, with an additional 23 states providing legal medical access for a limited list of conditions.8,13
Surrounding this shift in cannabis legislation, more emphasis has been put on generating new scientific evidence on the efficacy and safety of cannabinoids for an array of medical indications, including its use as an opiate alternative.
Basic science of cannabis
The cannabis plant’s flowers and leaves have been found to contain about 500 distinct compounds and 144 different cannabinoids, of which delta-9-tetrahydrocannabinol (∆9-THC, THC) and cannabidiol (CBD) are the most common (Box 1).15–18 The relative proportions and effective concentrations of these and other possibly active cannabinoids varies, and are not standardized outside of plants legally cultivated by licensed producers in North America.19,20
Box 1.
Key terms.
|
• ECS – endocannabinoid system, a complex network of receptors and transmitters that has been implicated in a number of physiological functions, both in the central and peripheral nervous systems as well as peripheral organs. • Endocannabinoid – naturally occurring ECS receptor agonists, produced by humans. AEA (N-arachidonoyl-ethanolamide, anandamide) and 2-AG (2-arachidonyl glycerol) are the best studied endocannabinoids, and are derivatives of arachidonic acid. • Cannabinoid – chemical compound that acts on the endocannabinoid system (ECS). The cannabis plant produces active and inactive cannabinoids, and many active cannabinoids can mimic the actions of the bodies own ECS receptor agonist, but these may also be synthetically derived. May be further differentiated as phytocannabinoid (from the cannabis plant) and synthetic cannabinoid (developed in laboratories to mimic naturally produced endo- or phyto- cannabinoids). • THC – ∆9-tetrahydrocannabinol; a cannabinoid used for medicinal purposes and non-medicinally for its intoxicating effects • CBD – cannabidiol, a cannabinoid with contrasting mechanisms of action and therapeutic indications to THC. Not intoxicating at typical doses |
Among the chemical constituents of cannabis, THC is the most considerably studied and responsible for the majority of the physical and psychotropic effects.21 The average amount of THC is typically 10–12.5% (range 1–30%) in cannabis plants found on markets in Canada. CBD, the second most prevalent cannabinoid in most strains of cannabis (with typical concentrations of < 1%), has effects counter to those of THC, with less psychoactive potential and more calming and anti-inflammatory effects.22 The interaction between THC and CBD is complex, and there may be benefits to using them in combination as CBD may temper the undesired psychotropic side-effects associated with THC.22,23 Other cannabinoids are present in the plant with lesser amounts (0.5%) and pre-clinical research suggest that they may have their own independent effects. In vitro and in vivo mouse studies have indicated that cannabinol (CBN) may been associated with prolonged sleep,24 and that non-psychoactive cannabigerol (CBG) and tetrahydrocannabinolic acid (THCA) may protect against neurodegeneration.25,26 THCA is a THC precursor that have many of the same molecular targets as THC that play a role in anti-inflammation, immunomodulation, neuroprotection and antineoplastic actions, and is not associated with potentially undesired psychoactive effects.26 The remainder of the plant consists of non-cannabinoid compounds, such as terpenes and flavonoids, that have little, if any, psychotropic properties, but may have additional therapeutic actions (e.g. anti-oxidant, anti-anxiety, anti-inflammatory, anti-bacterial, anti-neoplastic).16,21 Laboratory studies have indicated that the interaction between the various cannabinoids and non-cannabinoid compounds from the plant may result in increased effectiveness while decreasing negative side-effects when administered together, indicating that trying to isolate individual compounds from cannabis may not be the best approach.23,27–30 Furthermore, in living plants, the phytocannabinoids exist as both inactive monocarboxylic acids (e.g. THCA) and active decarboxylated forms (e.g. THC), with biologic activation occurring when heated to a temperature above 120°C.31–33 This leads to a transformation into a number of active compounds, many of which contribute further to the varied physiological effects as they interact with the body’s endocannabinoid system (ECS).
The ECS is implicated in inflammation, bone development, and pain. Also, the ECS is involved in other physical functions or systems including memory, learning, reproduction, appetite, psychiatric symptoms, psychomotor behaviour, digestion, sleep and wake cycles, the regulation of emotion, and synaptic plasticity.34 This has led to concerns regarding the non-specific effects of cannabis, including undesired side-effects. The ECS is comprised of two main receptors, cannabinoid receptor type-1 (CB1) and type-2 (CB2), as well as endogenous ligands that bind and activate these receptors [N-arachidonoylethanolamide (AEA) and 2-arachidonyl glycerol (2-AG)]. Although found throughout the body, including the brain, endothelium, gastrointestinal lining, lungs, bone and muscle, there is considerable variation in receptor expression. CB1 receptors are highly localized to the central and peripheral nervous systems, whereas CB2 receptor expression is greatest in the immune tissues and can also be found in bone and muscle.33–35 In addition, a number of cannabinoids and other constituent compounds are believed to bind to target receptors outside of the ECS, such as the serotonin 1A receptor (or 5-HT1A receptor),36 voltage gated sodium channels37 and G protein-coupled receptor 55 (or GPR55),38 furthering the complexity of determining the impact of cannabis use.
Can cannabinoids treat chronic musculoskeletal pain?
A recent British Medical Journal (BMJ) evidence review indicated, with moderate certainty, that nabiximols had a higher odds of providing a 30% reduction in pain scores compared with placebo for patients with chronic pain [odds ratio 1.46, 95% confidence intervals (CI) 1.16–1.84; reduction in pain scores was statistically significant, but clinically non-significant], but was no more effective than placebo in treating symptoms associated with multiple sclerosis.39,40 The authors of the review also concluded that cannabinoids may provide a 50% reduction in seizure frequency and alleviate symptoms of chemotherapy-associated nausea and vomiting; however, there was only low certainty in the results for these indications.39 The inclusion of low-quality trials, heterogeneous studies, and failure to perform a comprehensive assessment of methodological quality may result in misleading findings from meta-analyses.40–46 Given this, although there are clinical trials that have investigated other indications for the therapeutic use of cannabinoids, including post-traumatic stress disorder, anxiety, sleep and schizophrenia, among others, there is currently insufficient evidence to estimate efficacy in these areas.39,44,47,48 Among the indications examined, the treatment of pain is the most pertinent for orthopaedic patients, who suffer from a combination of acute pain associated with musculoskeletal injuries and the perioperative period, as well as chronic pain associated with degenerative conditions such as arthritis or long-standing back pain. This is reflected in the fact that, under previous Canadian medical cannabis access programs, 65% of Canadians authorized to possess medicinal cannabis claimed to need it for severe arthritis. Similarly, in one US pain clinic, up to 80% of cannabis users report myofascial pain as their primary diagnosis.16,49 Especially amid the opioid epidemic, the use of cannabinoids for specific analgesic indications may represent the most promise for their clinical integration; however, a thorough look at the safety and efficacy of the evidence surrounding these indications is required.
Although limited clinical evidence supports the use of cannabinoids for chronic, non-cancer related pain, studies continue to emerge rapidly; therefore, we performed a systematic review that included 36 randomized controlled trials (RCTs, 4006 participants) comparing cannabinoids to placebo on patients with CNCP (MEDLINE search strategy in Supplement).50–86 We found that, compared with placebo, cannabinoids showed a statistically significant reduction in pain scores. Within the first 2 weeks of treatment, cannabinoids had a greater reduction in pain, measured on a 0–10 visual analog scale, compared with placebo (–0.54, 95% CI –0.76 to –0.31). This difference remained statistically significant at 2 months (–0.68, 95% CI –0.96 to –0.40) and, though the effect estimate decreased, it still remained significant by 6 months (–0.43, 95% CI –0.75 to –0.10).50 Based on the recommended minimally important difference (MID) of 10 mm on a 100 mm visual analogue scale (VAS) when reporting treatment effects regarding pain, which is equivalent to 1 cm on a 10 cm VAS87; the differences in our findings did not reach the threshold of MID at any duration of treatment. There is little evidence that cannabinoids increase the risk of experiencing serious adverse events, although non-serious adverse events may be common when longer than 2 weeks’ treatment. Serious adverse events were rare and risks were similar across the cannabinoid (74/2176, 3.4%) and placebo groups (53/1640, 3.2%) at the longest follow-up. The most commonly reported adverse events included dizziness, drowsiness, dry mouth, nausea, fatigue, headache and euphoria. We did not find significant differences between subgroups of natural extracts of marijuana (THC, CBD, combination of THC and CBD) and synthetic cannabis (ajulemic acid, nabilone, dronabinol) on pain reduction or adverse events between.50
Although there are concerns regarding potential dependence associated with long-term and high-dose cannabinoids for pain, there is a lack of evidence.88–91 An answer to such a question requires a minimum 6 months follow up from an RCT or large observational study.92
Based on the current available research findings, low-to-moderate-quality evidence shows that cannabinoids are associated with a small, but statistically significant reduction in chronic pain. In addition, there is a need for further research to investigate the optimal route and composition of cannabinoids in the musculoskeletal pain setting, including large, high-quality RCTs to better understand the risks and benefits of cannabinoids in this patient population.
Making sense of current guideline recommendations
The overarching message emerging from both the Canadian and American Medical Associations remains one of extreme caution.93–95 They emphasize that, while there may be a limited role for cannabis in select patients with terminal illness or chronic disease refractory to conventional therapies, there is an overall lack of clinical evidence for most of the purported indications for cannabis. This includes musculoskeletal pain, which has been a driver of prescriptions since 2001, when Health Canada first granted access through the Marijuana Medical Access Regulations (MMAR). Current recommendations from North America, Latin America, Europe, Australia and Iran are consistent in supporting the use of medical cannabis for chronic, non-cancer pain, with comprehensive knowledge sharing and consideration of a patient’s own values and preferences. Physicians are suggested to take a shared decision-making approach to discuss the prescription of cannabinoids with their patients.89–92,96–103
Changes to policies regarding the use of medical cannabis have occurred worldwide. These changes might be related to a transition in the public perception of cannabis and attitudes towards cannabis.104,105 A survey enrolling approximately 1000 participants, among whom two-thirds were diagnosed with chronic pain, in the US found a level of 75% symptom relief with cannabis prescription. In the same study, over one-third of the near 2600 qualitative responses conveyed health benefits of the medical cannabis in treating their condition.106
Conclusion
Cannabinoids are being approved globally for pain management, especially in the last decade. Controversies and uncertainties on the trade-off between the benefits and harms of medical cannabis still remain. Whether or not cannabinoids can be used as an opioid-deterrent requires further investigation. Our current evidence review serves as a position paper to inform orthopaedic surgeons on the appropriate use of medical cannabis for their patients presenting with chronic musculoskeletal pain. Based on the current evidence, cannabinoids may be considered as an adjunctive therapy after recommended first- and second-line therapies have failed to provide sufficient efficacy or tolerability. In general, the evidence on cannabinoids in musculoskeletal patients based on high-quality clinical trials are limited. Prescribers need to supervise all patients taking cannabinoids for any condition.
Supplemental Material
Supplemental material, TAB937968_Supplemental_Material_CLN for Medical cannabis for orthopaedic patients with chronic musculoskeletal pain: does evidence support its use? by Herman Johal, Christopher Vannabouathong, Yaping Chang, Meng Zhu and Mohit Bhandari in Therapeutic Advances in Musculoskeletal Disease
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: A research grant was received from Spectrum Therapeutics, A Canopy Growth Company.
ORCID iDs: Christopher Vannabouathong  https://orcid.org/0000-0002-9694-6364
https://orcid.org/0000-0002-9694-6364
Yaping Chang  https://orcid.org/0000-0002-0549-5087
https://orcid.org/0000-0002-0549-5087
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Herman Johal, Department of Surgery, McMaster University, 237 Barton Street East, Floor 5N, Hamilton, ON L8L 2X2, Canada.
Christopher Vannabouathong, OrthoEvidence Inc., Burlington, ON, Canada.
Yaping Chang, OrthoEvidence Inc., 3228 South Service Road, Suite 206, Burlington, ON L7N 3J6, Canada.
Meng Zhu, OrthoEvidence Inc., Burlington, ON, Canada.
Mohit Bhandari, Department of Surgery, McMaster University, Hamilton, ON, Canada; OrthoEvidence Inc., Burlington, ON, Canada.
References
- 1. Sabatino MJ, Kunkel ST, Ramkumar DB, et al. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am 2018; 100: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O’Connor S, Grywacheski V, Louie K. Opioid-related harms in Canada. Ottawa: Canadian Institute for Health Information, 2018. [DOI] [PubMed] [Google Scholar]
- 3. Katz J. Drug deaths in America are rising faster than ever. The New York Times, 5 June 2017. [Google Scholar]
- 4. The Canadian Orthopaedic Association. COA position statement: opioids and orthopaedic surgical practice, https://coa-aco.org/wp-content/uploads/2017/01/COA-Position-Statement-Opioids-and-Orthopaedic-Surgical-Practice-2018-June-ENG.pdf (2018, accessed 21 January 2020).
- 5. Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017; 189: E659–E666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris BJ, Mir HR. The opioid epidemic: impact on orthopaedic surgery. J Am Acad Orthop Surg 2015; 23: 267–271. [DOI] [PubMed] [Google Scholar]
- 7. Turk DC, Okifuji A. Pain terms and taxonomies. In Loeser D, Butler SH, Chapman JJ, Turk DC. (eds) Bonica’s Management of Pain. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2001, pp.18–25. [Google Scholar]
- 8. Hand A, Blake A, Kerrigan P, et al. History of medical cannabis. J Pain Manage 2016; 9: 387–394. [Google Scholar]
- 9. O’Shaughnessy W. On the preparations of the Indian Hemp, or Gunjah: cannabis indica their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Prov Med J Retrosp Med Sci 1843; 5: 363. [PMC free article] [PubMed] [Google Scholar]
- 10. Pisanti S, Bifulco M. Medical cannabis: a plurimillennial history of an evergreen. J Cell Physiol 2019; 234: 8342–8351. [DOI] [PubMed] [Google Scholar]
- 11. United States Congress. Comprehensive drug abuse prevention and control act of 1970. Washington, DC: United States Congress, 1970. [Google Scholar]
- 12. Mayor S. WHO proposes rescheduling cannabis to allow medical applications. BMJ 2019; 364: l574. [DOI] [PubMed] [Google Scholar]
- 13. Bridgeman MB, Abazia DT. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T 2017; 42: 180–188. [PMC free article] [PubMed] [Google Scholar]
- 14. National Post Wire Services. Nations going to pot: where cannabis is legal around the world, and where it isn’t. Financial Post, 13 May 2019. [Google Scholar]
- 15. Balducci C, Nervegna G, Cecinato A. Evaluation of principal cannabinoids in airborne particulates. Anal Chim Acta 2009; 641: 89–94. [DOI] [PubMed] [Google Scholar]
- 16. Health Canada. Information for health care professionals: cannabis (marihuana, marijuana) and the cannabinoids: dried or fresh plant and oil for administration by ingestion or other means psychoactive agent, https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-medication/cannabis/information-medical-practitioners/information-health-care-professionals-cannabis-cannabinoids-eng.pdf (2018, accessed 21 January 2020).
- 17. Yamaori S, Kushihara M, Yamamoto I, et al. Characterization of major phytocannabinoids, cannabidiol and cannabinol, as isoform-selective and potent inhibitors of human CYP1 enzymes. Biochem Pharmacol 2010; 79: 1691–1698. [DOI] [PubMed] [Google Scholar]
- 18. Zhu HJ, Wang JS, Markowitz JS, et al. Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J Pharmacol Exp Ther 2006; 317: 850–857. [DOI] [PubMed] [Google Scholar]
- 19. Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in cannabis (cannabaceae). Am J Bot 2004; 91: 966–975. [DOI] [PubMed] [Google Scholar]
- 20. Mehmedic Z, Chandra S, Slade D, et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci 2010; 55: 1209–1217. [DOI] [PubMed] [Google Scholar]
- 21. Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry 2001; 178: 101–106. [DOI] [PubMed] [Google Scholar]
- 22. Hudson R, Puvanenthirarajah N. Cannabis for pain management: pariah or panacea? Univ West Ont Med J 2018; 87: 58–61. [Google Scholar]
- 23. McPartland JM, Russo EB. Cannabis and cannabis extracts: greater than the sum of their parts? Journal of Cannabis Therapeutics 2001; 1: 103–132. [Google Scholar]
- 24. Yoshida H, Usami N, Ohishi Y, et al. Synthesis and pharmacological effects in mice of halogenated cannabinol derivatives. Chem Pharm Bull (Tokyo) 1995; 43: 335–337. [DOI] [PubMed] [Google Scholar]
- 25. Gugliandolo A, Pollastro F, Grassi G, et al. In vitro model of neuroinflammation: efficacy of cannabigerol, a non-psychoactive cannabinoid. Int J Mol Sci 2018; 19: 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moreno-Sanz G. Can you pass the acid test? Critical review and novel therapeutic perspectives of Δ9-tetrahydrocannabinolic acid A. Cannabis Cannabinoid Res 2016; 1: 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ben-Shabat S, Fride E, Sheskin T, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol 1998; 353: 23–31. [DOI] [PubMed] [Google Scholar]
- 28. McPartland JM, Russo EB. Non-phytocannabinoid constituents of cannabis and herbal synergy. In: Pertwee R. (ed.) Handbook of Cannabis. Oxford: Oxford University Press, 2014, pp.280–295. [Google Scholar]
- 29. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol 2011; 163: 1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Russo EB, McPartland JM. Cannabis is more than simply Δ9-tetrahydrocannabinol. Psychopharmacology (Berl) 2003; 165: 431–432. [DOI] [PubMed] [Google Scholar]
- 31. Dussy FE, Hamberg C, Luginbühl M, et al. Isolation of Δ9-THCA-A from hemp and analytical aspects concerning the determination of Δ9-THC in cannabis products. Forensic Sci Int 2005; 149: 3–10. [DOI] [PubMed] [Google Scholar]
- 32. Guy GW, Whittle BA, Robson PJ. The medicinal uses of cannabis and cannabinoids. Le Pharmacien Hospitalier 2008; 43: 56–57. [Google Scholar]
- 33. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers 2007; 4: 1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zou S, Kumar U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci 2018; 19: 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol 2008; 20(Suppl. 1): 10–14. [DOI] [PubMed] [Google Scholar]
- 36. De Gregorio D, McLaughlin RJ, Posa L, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2019; 160: 136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Watkins AR. Cannabinoid interactions with ion channels and receptors. Channels (Austin) 2019; 13: 162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Henstridge CM. Off-target cannabinoid effects mediated by GPR55. Pharmacology 2012; 89: 179–187. [DOI] [PubMed] [Google Scholar]
- 39. Freeman TP, Hindocha C, Green SF, et al. Medicinal use of cannabis based products and cannabinoids. BMJ 2019; 365: I1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stockings E, Campbell G, Hall WD, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain 2018; 159: 1932–1954. [DOI] [PubMed] [Google Scholar]
- 41. Lim K, See YM, Lee J. A systematic review of the effectiveness of medical cannabis for psychiatric, movement and neurodegenerative disorders. Clin Psychopharmacol Neurosci 2017; 15: 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maharajan MK, Yong YJ, Yip HY, et al. Medical cannabis for chronic pain: can it make a difference in pain management? J Anesth 2019; 34: 95–103. [DOI] [PubMed] [Google Scholar]
- 43. Pratt M, Stevens A, Thuku M, et al. Benefits and harms of medical cannabis: a scoping review of systematic reviews. Syst Rev 2019; 8: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015; 313: 2456–2473. [DOI] [PubMed] [Google Scholar]
- 45. Dijkman BG, Abouali JAK, Kooistra BW, et al. Twenty years of meta-analyses in orthopaedic surgery: has quality kept up with quantity? J Bone Joint Surg Am 2010; 92: 48–57. [DOI] [PubMed] [Google Scholar]
- 46. Glasziou PP, Sanders SL. Investigating causes of heterogeneity in systematic reviews. Stat Med 2002; 21: 1503–1511. [DOI] [PubMed] [Google Scholar]
- 47. European Monitoring Centre for Drugs and Drug Addiction. Medical use of cannabis and cannabinoids: questions and answers for policymaking. Lisbon: European Monitoring Centre for Drugs and Drug Addiction, 2018. [Google Scholar]
- 48. National Academies of Sciences Engineering and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, et al. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academies Press, 2017. [PubMed] [Google Scholar]
- 49. Aggarwal SK, Carter GT, Sullivan MD, et al. Characteristics of patients with chronic pain accessing treatment with medical cannabis in Washington State. J Opioid Manag 2009; 5: 257–286. [DOI] [PubMed] [Google Scholar]
- 50. Johal H, Devji T, Chang Y, et al. Cannabinoids in chronic non-cancer pain: a systematic review and meta-analysis. Clin Med Insights Arthritis Musculoskelet Disord 2020; 13: 1179544120906461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 2007; 68: 515–521. [DOI] [PubMed] [Google Scholar]
- 52. Ball S, Vickery J, Hobart J, et al. The cannabinoid use in progressive inflammatory brain disease (CUPID) trial: a randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Health Technol Assess 2015; 19: vii–viii, xxv–xxxi, 1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain 2004; 112: 299–306. [DOI] [PubMed] [Google Scholar]
- 54. Blake DR, Robson P, Ho M, et al. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006; 45: 50–52. [DOI] [PubMed] [Google Scholar]
- 55. Carroll CB, Bain PG, Teare L, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology 2004; 63: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 56. Collin C, Ehler E, Waberzinek G, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res 2010; 32: 451–459. [DOI] [PubMed] [Google Scholar]
- 57. Corey-Bloom J, Wolfson T, Gamst A, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ 2012; 184: 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Vries M, van Rijckevorsel DCM, Vissers KCP, et al. Tetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled study. Clin Gastroenterol Hepatol 2017; 15: 1079–1086.e4. [DOI] [PubMed] [Google Scholar]
- 59. Ellis RJ, Toperoff W, Vaida F, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009; 34: 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hagenbach U, Luz S, Ghafoor N, et al. The treatment of spasticity with Δ9-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord 2007; 45: 551–562. [DOI] [PubMed] [Google Scholar]
- 61. Karst M, Salim K, Burstein S, et al. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. JAMA 2003; 290: 1757–1762. [DOI] [PubMed] [Google Scholar]
- 62. Killestein J, Hoogervorst ELJ, Reif M, et al. Safety, tolerability, and efficacy of orally administered cannabinoids in MS. Neurology 2002; 58: 1404–1407. [DOI] [PubMed] [Google Scholar]
- 63. Langford RM, Mares J, Novotna A, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol 2013; 260: 984–997. [DOI] [PubMed] [Google Scholar]
- 64. Leocani L, Nuara A, Houdayer E, et al. Sativex® and clinical–neurophysiological measures of spasticity in progressive multiple sclerosis. J Neurol 2015; 262: 2520–2527. [DOI] [PubMed] [Google Scholar]
- 65. Malik Z, Bayman L, Valestin J, et al. Dronabinol increases pain threshold in patients with functional chest pain: a pilot double-blind placebo-controlled trial: dronabinol increases pain threshold in patients. Dis Esophagus 2017; 30: 1–8. [DOI] [PubMed] [Google Scholar]
- 66. Markovà J, Essner U, Akmaz B, et al. Sativex® as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind, placebo-controlled randomised clinical trial. Int J Neurosci 2019; 129: 119–128. [DOI] [PubMed] [Google Scholar]
- 67. Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 ‘N of 1’ studies. Anaesthesia 2004; 59: 440–452. [DOI] [PubMed] [Google Scholar]
- 68. Novotna A, Mares J, Ratcliffe S, et al. ; Sativex Spasticity Study Group. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol 2011; 18: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 69. Nurmikko TJ, Serpell MG, Hoggart B, et al. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain 2007; 133: 210–220. [DOI] [PubMed] [Google Scholar]
- 70. Rog DJ, Nurmikko TJ, Friede T, et al. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005; 65: 812–819. [DOI] [PubMed] [Google Scholar]
- 71. Schimrigk S, Marziniak M, Neubauer C, et al. Dronabinol is a safe long-term treatment option for neuropathic pain patients. Eur Neurol 2017; 78: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Selvarajah D, Gandhi R, Emery CJ, et al. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes Care 2010; 33: 128–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014; 18: 999–1012. [DOI] [PubMed] [Google Scholar]
- 74. Skrabek RQ, Galimova L, Ethans K, et al. Nabilone for the treatment of pain in fibromyalgia. J Pain 2008; 9: 164–173. [DOI] [PubMed] [Google Scholar]
- 75. Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004; 329: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Toth C, Mawani S, Brady S, et al. An enriched-enrolment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assignment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain 2012; 153: 2073–2082. [DOI] [PubMed] [Google Scholar]
- 77. Turcotte D, Doupe M, Torabi M, et al. Nabilone as an adjunctive to gabapentin for multiple sclerosis-induced neuropathic pain: a randomized controlled trial. Pain Med 2015; 16: 149–159. [DOI] [PubMed] [Google Scholar]
- 78. van Amerongen G, Kanhai K, Baakman AC, et al. Effects on spasticity and neuropathic pain of an oral formulation of Δ9-tetrahydrocannabinol in patients with progressive multiple sclerosis. Clin Ther 2018; 40: 1467–1482. [DOI] [PubMed] [Google Scholar]
- 79. Wade DT, Makela P, Robson P, et al. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler 2004; 10: 434–441. [DOI] [PubMed] [Google Scholar]
- 80. Wade DT, Robson P, House H, et al. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil 2003; 17: 21–29. [DOI] [PubMed] [Google Scholar]
- 81. Ware MA, Wang T, Shapiro S, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010; 182: E694–E701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weber M, Goldman B, Truniger S. Tetrahydrocannabinol (THC) for cramps in amyotrophic lateral sclerosis: a randomised, double-blind crossover trial. J Neurol Neurosurg Psychiatry 2010; 81: 1135–1140. [DOI] [PubMed] [Google Scholar]
- 83. Wissel J, Haydn T, Müller J, et al. Low dose treatment with the synthetic cannabinoid nabilone significantly reduces spasticity-related pain: a double-blind placebo-controlled cross-over trial. J Neurol 2006; 253: 1337–1341. [DOI] [PubMed] [Google Scholar]
- 84. Zadikoff C, Wadia PM, Miyasaki J, et al. Cannabinoid, CB1 agonists in cervical dystonia: failure in a phase IIa randomized controlled trial. Basal Ganglia 2011; 1: 91–95. [Google Scholar]
- 85. Zajicek JP, Sanders HP, Wright DE, et al. Cannabinoids in multiple sclerosis (CAMS) study: safety and efficacy data for 12 months follow up. J Neurol Neurosurg Psychiatry 2005; 76: 1664–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zajicek JP, Hobart JC, Slade A, et al. ; MUSEC Research Group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J Neurol Neurosurg Psychiatry 2012; 83: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 87. Busse JW, Bartlett SJ, Dougados M, et al. Optimal strategies for reporting pain in clinical trials and systematic reviews: recommendations from an OMERACT 12 workshop. J Rheumatol 2015; 42: 1962–1970. [DOI] [PubMed] [Google Scholar]
- 88. Boehnke KF, Scott JR, Litinas E, et al. Pills to pot: observational analyses of cannabis substitution among medical cannabis users with chronic pain. J Pain 2019; 20: 830–841. [DOI] [PubMed] [Google Scholar]
- 89. Allan GM, Ramji J, Perry D, et al. Simplified guideline for prescribing medical cannabinoids in primary care. Can Fam Physician 2018; 64: 111–120. [PMC free article] [PubMed] [Google Scholar]
- 90. Häuser W, Finn DP, Kalso E, et al. European pain federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. Eur J Pain 2018; 22: 1547–1564. [DOI] [PubMed] [Google Scholar]
- 91. Yadav V, Bever C, Jr, Bowen J, et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American academy of neurology. Neurology 2014; 82: 1083–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. National Institute for Health and Care Excellence. Neuropathic pain: the pharmacological management of neuropathic pain in adults in non-specialist settings [Internet]. London: National Institute for Health and Care Excellence, 2013. [PubMed] [Google Scholar]
- 93. Health Canada. Background Document: public consultation on strengthening Canada’s approach to substance use issues, https://www.canada.ca/content/dam/hc-sc/documents/services/substance-use/canadian-drugs-substances-strategy/strengthening-canada-approach-substance-use-issue/strengthening-canada-approach-substance-use-issue.pdf (2018, accessed 21 January 2020).
- 94. Humphreys K, Saitz R. Should physicians recommend replacing opioids with cannabis? JAMA 2019; 321: 639–640. [DOI] [PubMed] [Google Scholar]
- 95. Owens B. CMA position against separate regulations for medical cannabis draws ire and insults. CMAJ 2018; 190: E574–E575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Acevedo JC, Amaya A, Casasola Ode L, et al. Guidelines for the diagnosis and management of neuropathic pain: consensus of a group of Latin American experts. J Pain Palliat Care Pharmacother 2009; 23: 261–281. [DOI] [PubMed] [Google Scholar]
- 97. Andrews CN, Devlin SM, Le Foll B, et al. Canadian association of gastroenterology position statement: use of cannabis in gastroenterological and hepatic disorders. J Can Assoc Gastroenterol 2019; 2: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Australian Government Department of Health. Therapeutic Goods Administration. Guidance for the use of medicinal cannabis in the treatment of chronic non-cancer pain in Australia. Version 1, https://www.tga.gov.au/sites/default/files/guidance-use-medicinal-cannabis-treatment-chronic-non-cancer-pain-australia.pdf (2017, accessed 21 January 2020).
- 99. Bruce RD, Merlin J, Lum PJ, et al. 2017 HIV medicine association of infectious diseases society of America clinical practice guideline for the management of chronic pain in patients living with human immunodeficiency virus. Clin Infect Dis 2017; 65: 1601–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Canadian Rheumatology Association. Canadian rheumatology association (CRA) position statement on medical cannabis use in rheumatic disease. Mississauga: Canadian Rheumatology Association, 2019. [Google Scholar]
- 101. Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007; 132: 237–251. [DOI] [PubMed] [Google Scholar]
- 102. Moulin D, Boulanger A, Clark AJ, et al. ; Canadian Pain Society. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag 2014; 19: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sahraian MA, Baghbanian SM, Langroodi HG, et al. Primary progressive multiple sclerosis in Iran: a consensus recommendation for diagnosis and management. Mult Scler Relat Disord 2018; 26: 112–120. [DOI] [PubMed] [Google Scholar]
- 104. Marshall B, Bland MK, Hulla R, et al. Considerations in addressing the opioid epidemic and chronic pain within the USA. Pain Manag 2019; 9: 131–138. [DOI] [PubMed] [Google Scholar]
- 105. Banerjee S, McCormack S. Medical cannabis for the treatment of chronic pain: a review of clinical effectiveness and guidelines. (CADTH rapid response report: summary with critical appraisal). Ottawa: Canadian Agency for Drugs and Technologies in Health, https://www.ncbi.nlm.nih.gov/books/NBK546424/ (2019, accessed 8 May 2020). [PubMed] [Google Scholar]
- 106. Piper BJ, Beals ML, Abess AT, et al. Chronic pain patients’ perspectives of medical cannabis. Pain 2017; 158: 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, TAB937968_Supplemental_Material_CLN for Medical cannabis for orthopaedic patients with chronic musculoskeletal pain: does evidence support its use? by Herman Johal, Christopher Vannabouathong, Yaping Chang, Meng Zhu and Mohit Bhandari in Therapeutic Advances in Musculoskeletal Disease