Abstract
Background:
Levothyroxine is a commonly prescribed medication. Some data suggest that levothyroxine may be initiated for mild degrees of hypothyroidism and used without considering age-specific reference ranges or individual patient factors when prescribing.
Methods:
The electronic medical record of a health care system operating in the Washington, DC and Maryland area was interrogated to determine the number of patients who were being prescribed levothyroxine during the time period 2008–2016, the number of prescriptions supplied to these individuals, an associated diagnosis of hypothyroidism, and whether the prescriptions were new or existing prescriptions. Information was also extracted about the age of patients receiving prescriptions and the thyroid stimulating hormone level documented prior to levothyroxine initiation.
Results:
Although the number of levothyroxine prescriptions provided annually increased over this time period, when corrected for the number of patients in the database, the percentage of patients receiving levothyroxine prescriptions showed a slight downward trend. Levothyroxine was both most frequently prescribed and frequently initiated in those of ages 50–59 years and 60–69 years. The doses of levothyroxine most commonly prescribed were 50 µg and 100 µg and the pattern of levothyroxine doses being used was unaffected by whether a diagnosis of hypothyroidism was documented or not. Levothyroxine prescription initiation was associated with mean thyroid stimulating hormone values that were modestly elevated and in the range of 7.5–13.8 mIU/L.
Conclusion:
This analysis showed that although the percentage of patients being prescribed levothyroxine is stable or slightly declining, with most decrement in those without a diagnosis of hypothyroidism, there is nevertheless continued initiation of levothyroxine in those with mild degrees of thyroid stimulating hormone elevation, and in those of older age, raising concerns about both unnecessary treatment and iatrogenic thyrotoxicosis. Such data suggest the need for great consideration of both the degree of thyroid stimulating hormone elevation and the patient context when considering whether treatment of an elevated thyroid stimulating hormone value, versus ongoing monitoring, is indicated.
Keywords: age, hypothyroidism, levothyroxine, prescribing, TSH
Introduction
Hypothyroidism affects approximately 5% of the United States population, and synthetic thyroxine in the form of levothyroxine (LT4) is the most prescribed therapy for this common condition.1,2 Autoimmune hypothyroidism is generally progressive, with a decrement in endogenous thyroid function occurring over time, such that overt hypothyroidism would occur if the thyroid hormone deficiency is untreated.3 However, at the current time hypothyroidism may initially be diagnosed when it is mild or subclinical, at a time that the serum thyroid stimulating hormone (TSH) is minimally elevated below 10 mIU/L and the serum thyroid hormone levels are normal. Symptoms of hypothyroidism are not always specific for this condition, and each of the symptoms generally associated with hypothyroidism may also have non-thyroid causes.4 As attribution of the cause of symptoms is not a simple matter,5,6 many physicians may err on the side of treating mild TSH elevations with the expectation that symptoms may improve. Thus, treatment with LT4 may be initiated for borderline TSH elevations.7 The authors also hypothesize that LT4 may even be prescribed for unconfirmed serum TSH elevations, or even without TSH elevation, based on the symptoms that the patient is experiencing.
LT4 is being prescribed with increasing frequency, both in the United States8,9 and in other countries.10,11 Several studies have shown that the TSH threshold at which LT4 therapy is initiated has fallen over time, such that an increasing number of cases of subclinical hypothyroidism are being treated and the mean or median TSH value at which therapy is initiated has decreased over time.7,12–14 This trend for increasing treatment and decreasing thresholds has been documented both in the United States9,14 and in Europe.7,12,13 Moreover, a recent study showed that if LT4 therapy was discontinued in patients undergoing treatment, while 39% of patients did in fact become hypothyroid as manifest by an elevated TSH, 61% maintained a normal TSH, perhaps suggesting that their treatment was unnecessary.15 However, the follow-up period in this study was relatively brief at 6–8 weeks, thus hypothyroidism being manifest later during follow-up cannot be excluded.15 Although national databases show a trend for an increasing number of LT4 prescriptions being provided over time,16,17 part of this apparent increase may be accounted for by a reduction in the length of prescriptions by prescribers,10 such that more prescriptions are being generated for the same number of patients each year.
The current analysis examined the prescription of LT4 using the electronic medical record (EMR) of a large health care system (MedStar) operating in the Washington, DC and Maryland area. The MedStar Health System is a non-profit healthcare organization founded in 1998. It operates several physician practice groups and also 10 hospitals in the Baltimore–Washington metropolitan area. Approximately 5000 physicians provide medical care within this system. The MedStar Health System also operates the MedStar Health Research Institute, which employs scientists and investigators engaged in translational and health sciences research. The patient population is likely representative of the general population in terms of age, sex, and socioeconomic status, based on datasets such as the United States Census.18 The EMR, which is the system in use by all MedStar physicians, was interrogated to determine whether the number of prescriptions being provided for LT4 was changing over time, what the associated diagnosis was, and also to examine prescriptions according to patient age and sex.
Methods
The study was approved by the joint Georgetown University–MedStar Institutional Review Board (study number 2017-0335). Waiver of the need to obtain informed consent from participants was granted. Data extraction was performed by the Biostatistics and Biomedical Informatics component of the Clinical and Translation Science Award program at Georgetown University using Medstar Health Research Institute Databases, including Centricity and Explorys as appropriate. Centricity is an ambulatory care electronic medical record system which can be used for clinical research, including performing retrospective cohort studies. Explorys is a system that interacts with electronic medical records systems and allows for secure storage and analysis of large patient data sets in a manner compliant with ethical regulations. The databases were searched for the years 2008–2016, a period during which significant changes to the database were not occurring. Adult outpatients 18 years and older were included in the search. All LT4 products were searched for, including the following: Levothyroxine, Synthroid, Unithroid, Levoxyl, and Levothroid. The doses of LT4 products that were included were 25, 50, 75, 88, 100, 112, 125, 137, 150, 175, 200, 300 µg. Data regarding other thyroid hormone preparations such as armour thyroid, desiccated thyroid extract, liothyronine (Cytomel) were not collected as these were a small proportion of thyroid hormone prescriptions.
Diagnoses of hypothyroidism, and all diagnoses potentially associated with hypothyroidism, were documented using International Classification of Diseases (ICD) codes. Both ICD -9 and ICD-10 codes were noted. These included, for example, diagnoses of thyroid cancer, Hashimoto’s thyroiditis, and thyroidectomy. The design of the EMR encourages physicians to be as complete and comprehensive as possible in assigning all relevant diagnoses. Such diagnoses were noted if they were present at any time and linked to any visits in the patient’s electronic chart. Patients with any of the multiple diagnostic codes for hyperthyroidism were excluded from the analysis, as were hospitalized patients, pediatric patients, and pregnant patients. Prescriptions were classified as pre-existing if any dose of LT4 had been prescribed before, even if there had been a hiatus or a change in dose. Prescriptions were classified as new if no dose of LT4 had been prescribed before. All LT4 prescriptions were normalized to a 90-day period.
The following information was extracted from the electronic medical record:
The number of patients in the database annually;
The number of patients being prescribed levothyroxine in the database annually;
The average age and age distribution of the patients in the database annually;
The average age and age distribution of the patients in the database being prescribed levothyroxine annually;
The number of levothyroxine prescriptions in the database annually;
The number of levothyroxine prescriptions per patient annually;
The duration of each levothyroxine prescription in number of days on an annual basis;
The sex distribution of patients being prescribed levothyroxine annually;
The presence or absence of a diagnosis of hypothyroidism in the patients being prescribed levothyroxine annually;
The average levothyroxine dose and distribution of levothyroxine doses annually;
The average TSH value and TSH distribution associated with the first levothyroxine prescription in each patient annually.
Statistical analysis
This was a study to determine whether LT4 prescribing was increasing within this population in the same way that has been described nationally, and to determine which factors seemed to be associated with any LT4 prescribing trends observed in this geographic area of the United States. All data extracted from the EMR was summarized using descriptive statistics (mean, standard deviation, median, range for continuous variables, and frequencies and percentages for categorical variables). Factors examined included patient age and sex, and TSH values. Prescriptions were divided according to whether they were written for a diagnosis of hypothyroidism, or written for patients without a diagnosis of hypothyroidism. LT4 prescribing across the age spectrum was examined. Statistical analyses were conducted using the statistical expertise of the Department of Biostatistics and Bioinformatics at MedStar Health Research Institute.
Results
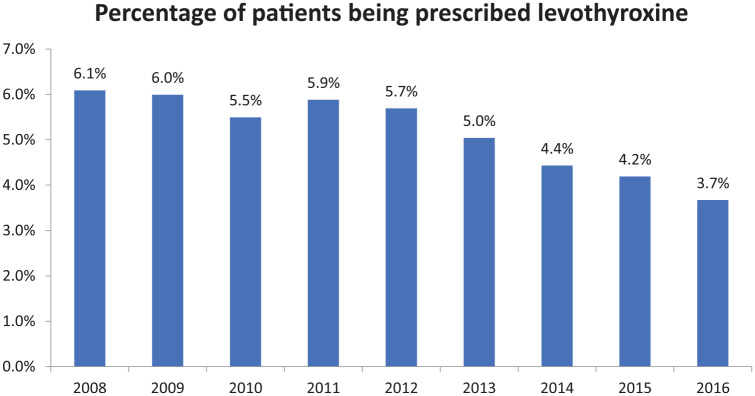
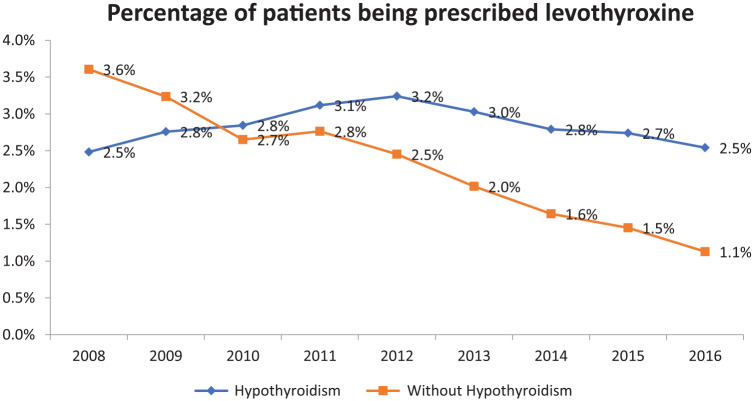
The number of outpatients within the MedStar system increased from 135,150 in 2008 to 547,433 patients in 2016. The number of outpatients being prescribed LT4 increased from 8229 in 2008 to 20,089 in 2016. Using the outpatient population as a denominator, the percent of patients being prescribed LT4 was 6.1% in 2008 and decreased to 3.7% in 2016 (see Figure 1). Outpatients were also divided according to whether or not they had a diagnosis of hypothyroidism documented in their EMR. The percentage of patients being prescribed LT4 with a documented diagnosis of hypothyroidism increased from 2.5% in 2008 to 3.2% in 2012 and then decreased to 2.5% in 2016. The percentage of patients being prescribed LT4 without a diagnosis of hypothyroidism decreased from 3.6% in 2008 to 1.1% in 2016 (see Figure 2).
Figure 1.
Percentage of patients being prescribed levothyroxine between 2008 and 2016.
Figure 2.
Percentage of patients being prescribed levothyroxine between 2008 and 2016, divided according to whether they carried a diagnosis of hypothyroidism or not.
LT4 doses being prescribed
When the LT4 prescriptions were categorized according to the dose being prescribed, the most commonly prescribed doses were 50, 75, 100, and 125 µg. This pattern was seen regardless of whether or not a diagnosis of hypothyroidism was documented in the patient’s chart (see Tables 1 and 2). The 50 µg dose was prescribed for the highest percentage of patients, the percentage being 14.6–16.8% in all patients, 15.1–16.7% in those patients without documentation of hypothyroidism, and 14.1–17.1% in patients with documentation of hypothyroidism.
Table 1.
Levothyroxine prescriptions divided by the percentage of each dose that was prescribed on an annual basis.
| Percentage of each dose prescribed per year | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| LT4 Dose (µg) | Year |
||||||||
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | |
| 25 | 6.35 | 6.61 | 6.70 | 7.78 | 8.10 | 8.64 | 8.48 | 8.52 | 8.35 |
| 50 | 15.00 | 14.60 | 14.91 | 15.56 | 16.51 | 16.82 | 16.40 | 16.63 | 16.51 |
| 75 | 14.70 | 13.74 | 14.15 | 14.34 | 14.60 | 14.78 | 15.15 | 15.19 | 15.49 |
| 88 | 7.09 | 7.29 | 7.63 | 7.96 | 8.52 | 8.59 | 8.73 | 8.74 | 9.23 |
| 100 | 15.96 | 15.67 | 14.63 | 14.76 | 14.23 | 14.10 | 14.37 | 14.30 | 14.27 |
| 112 | 8.12 | 8.53 | 8.60 | 8.64 | 7.99 | 8.15 | 8.16 | 8.08 | 8.48 |
| 125 | 10.57 | 10.87 | 10.68 | 9.77 | 9.93 | 9.45 | 9.41 | 9.23 | 9.02 |
| 137 | 4.20 | 4.86 | 4.93 | 4.76 | 4.72 | 4.39 | 4.54 | 4.80 | 4.62 |
| 150 | 8.79 | 8.53 | 8.23 | 7.74 | 7.31 | 6.96 | 6.81 | 6.77 | 6.60 |
| 175 | 4.70 | 4.61 | 4.99 | 4.61 | 4.35 | 4.53 | 4.45 | 4.31 | 4.17 |
| 200 | 4.16 | 4.23 | 4.11 | 3.67 | 3.44 | 3.34 | 3.29 | 3.25 | 3.00 |
| 300 | 0.37 | 0.47 | 0.44 | 0.40 | 0.30 | 0.24 | 0.22 | 0.19 | 0.26 |
Gray shading indicates the most frequently prescribed doses. LT4, levothyroxine.
Table 2.
Levothyroxine prescriptions divided by the percentage of each dose that was prescribed on an annual basis, and subdivided by whether a diagnosis of hypothyroidism was documented or not.
| LT4 dose (µg) | Percentage of each LT4 dose prescribed per year |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2008 |
2009 |
2010 |
2011 |
2012 |
2013 |
2014 |
2015 |
2016 |
||||||||||
| No hypo | Hypo | No hypo | Hypo | No hypo | Hypo | No hypo | Hypo | No hypo | Hypo | No hypo | Hypo | No hypo | Hypo | No hypo | Hypo | No hypo | Hypo | |
| 25 | 6.8 | 5.7 | 7.3 | 5.8 | 7.3 | 6.2 | 8.1 | 7.5 | 8.1 | 8.1 | 9.0 | 8.4 | 8.5 | 8.5 | 8.5 | 8.5 | 8.2 | 8.4 |
| 50 | 15.6 | 14.2 | 15.1 | 14.1 | 15.0 | 14.9 | 15.4 | 15.7 | 16.0 | 16.8 | 16.7 | 16.9 | 15.7 | 16.8 | 15.6 | 17.1 | 15.5 | 16.9 |
| 75 | 14.8 | 14.5 | 14.0 | 13.4 | 14.6 | 13.8 | 15.0 | 13.8 | 14.7 | 14.5 | 15.2 | 14.5 | 15.9 | 14.8 | 15.4 | 15.1 | 15.7 | 15.4 |
| 88 | 7.7 | 6.3 | 7.2 | 7.3 | 8.2 | 7.2 | 8.2 | 7.8 | 9.2 | 8.0 | 9.1 | 8.3 | 9.1 | 8.5 | 9.4 | 8.4 | 9.7 | 9.0 |
| 100 | 16.2 | 15.6 | 15.8 | 15.5 | 15.3 | 14.1 | 16.2 | 13.6 | 14.9 | 13.7 | 14.7 | 13.8 | 14.9 | 14.1 | 14.7 | 14.1 | 15.1 | 14.0 |
| 112 | 8.3 | 7.8 | 8.7 | 8.3 | 8.8 | 8.4 | 9.0 | 8.3 | 8.1 | 7.9 | 8.2 | 8.1 | 8.7 | 7.9 | 8.8 | 7.7 | 9.0 | 8.3 |
| 125 | 10.1 | 11.2 | 11.0 | 10.8 | 11.1 | 10.3 | 9.1 | 10.3 | 10.0 | 9.9 | 9.3 | 9.6 | 9.4 | 9.4 | 9.1 | 9.3 | 9.0 | 9.0 |
| 137 | 3.6 | 5.0 | 4.3 | 5.5 | 4.4 | 5.4 | 4.4 | 5.1 | 4.7 | 4.7 | 4.2 | 4.5 | 4.2 | 4.7 | 4.8 | 4.8 | 4.7 | 4.6 |
| 150 | 8.7 | 9.0 | 8.1 | 8.9 | 7.2 | 9.1 | 7.3 | 8.1 | 7.0 | 7.5 | 6.7 | 7.1 | 6.8 | 6.8 | 6.4 | 7.0 | 6.4 | 6.7 |
| 175 | 4.1 | 5.5 | 4.1 | 5.1 | 4.6 | 5.3 | 4.1 | 5.0 | 4.1 | 4.5 | 4.2 | 4.7 | 4.0 | 4.7 | 4.4 | 4.3 | 4.2 | 4.2 |
| 200 | 3.7 | 4.8 | 3.9 | 4.6 | 3.2 | 4.9 | 2.9 | 4.3 | 2.8 | 3.9 | 2.6 | 3.8 | 2.6 | 3.6 | 2.7 | 3.5 | 2.5 | 3.2 |
| 300 | 0.3 | 0.4 | 0.3 | 0.6 | 0.3 | 0.5 | 0.3 | 0.4 | 0.3 | 0.3 | 0.2 | 0.3 | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 |
Gray shading indicates the most frequently prescribed doses.
LT4, levothyroxine
Patient age at prescription initiation
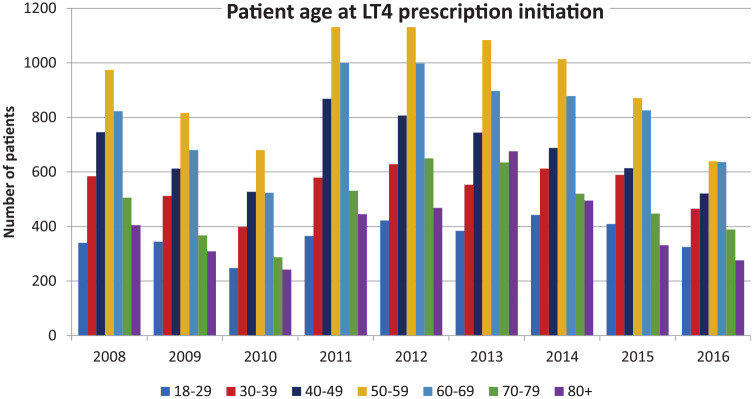
For patients whose LT4 prescription was initiated during the period of observation, the age of the patient at prescription initiation was noted. The mean age at initiation for all patients remained constant over the years 2008–2016 at age 54–57 years [see Table 3(a)]. When patients were divided into age deciles, prescription initiation was observed at all deciles, but with the least number of prescriptions being initiated in the 18–29 and 80 years and older age groups [see Table 4(a) and Figure 3]. When patients were divided according to whether or not a diagnosis of hypothyroidism was documented in their EMR, the trend for age at initiation and age decile at initiation were similar for patients with a hypothyroidism diagnosis and those without [see Tables 3(b) and 4(b) and Tables 3(c) and 4(c) respectively].
Table 3.
Patient age at first initiation of a levothyroxine prescription on an annual basis, with patients divided according to whether they carried a diagnosis of hypothyroidism or not.
| Patient group | Year | Patients n |
Patient age |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean age | SD | Median age | 25th pctl | 75th pctl | Minimum age | Maximum age | |||
| (a) All patients | 2008 | 4376 | 55 | 17 | 55 | 42 | 66 | 18 | 99 |
| 2009 | 3639 | 54 | 17 | 54 | 41 | 65 | 18 | 100 | |
| 2010 | 2905 | 54 | 17 | 54 | 41 | 65 | 18 | 110 | |
| 2011 | 4911 | 55 | 17 | 55 | 43 | 67 | 18 | 101 | |
| 2012 | 5099 | 55 | 17 | 56 | 43 | 68 | 18 | 104 | |
| 2013 | 4967 | 57 | 18 | 57 | 44 | 70 | 18 | 108 | |
| 2014 | 4634 | 55 | 18 | 56 | 41 | 67 | 18 | 114 | |
| 2015 | 4078 | 54 | 17 | 55 | 40 | 66 | 18 | 97 | |
| 2016 | 3237 | 54 | 18 | 55 | 40 | 67 | 18 | 104 | |
| (b) Patients with hypothyroidism | 2008 | 1591 | 52 | 17 | 52 | 39 | 64 | 18 | 99 |
| 2009 | 1645 | 53 | 17 | 53 | 40 | 64 | 18 | 99 | |
| 2010 | 1561 | 53 | 17 | 53 | 40 | 63 | 18 | 109 | |
| 2011 | 2453 | 54 | 17 | 54 | 41 | 65 | 18 | 101 | |
| 2012 | 2924 | 54 | 17 | 55 | 41 | 66 | 18 | 100 | |
| 2013 | 2926 | 55 | 18 | 55 | 42 | 67 | 18 | 108 | |
| 2014 | 2983 | 53 | 18 | 54 | 39 | 65 | 18 | 99 | |
| 2015 | 2767 | 52 | 17 | 53 | 38 | 64 | 18 | 97 | |
| 2016 | 2439 | 53 | 18 | 54 | 39 | 66 | 18 | 98 | |
| (c) Patients without hypothyroidism | 2008 | 2785 | 56 | 17 | 56 | 44 | 68 | 18 | 98 |
| 2009 | 1994 | 54 | 17 | 55 | 41 | 66 | 18 | 100 | |
| 2010 | 1344 | 55 | 17 | 55 | 43 | 66 | 18 | 110 | |
| 2011 | 2458 | 56 | 17 | 56 | 45 | 68 | 18 | 99 | |
| 2012 | 2175 | 57 | 17 | 58 | 45 | 69 | 18 | 104 | |
| 2013 | 2041 | 60 | 19 | 60 | 47 | 75 | 18 | 104 | |
| 2014 | 1651 | 58 | 19 | 59 | 45 | 72 | 18 | 114 | |
| 2015 | 1311 | 57 | 18 | 58 | 44 | 69 | 18 | 97 | |
| 2016 | 798 | 56 | 18 | 57 | 42 | 69 | 18 | 104 | |
pctl, percentile
Table 4.
Number of patients with first initiation of a levothyroxine prescription, divided according to age deciles, with patients divided according to whether they carried a diagnosis of hypothyroidism or not.
| Patient group | Age decile | Number of patients with prescription initiation per year |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year (mean age of population) |
||||||||||
| 2008 (49) | 2009 (49) | 2010 (49) | 2011 (49) | 2012 (50) | 2013 (50) | 2014 (50) | 2015 (50) | 2016 (51) | ||
| (a) All patients | 18–29 | 340 | 344 | 248 | 364 | 422 | 383 | 439 | 407 | 325 |
| 30–39 | 584 | 512 | 398 | 579 | 627 | 552 | 612 | 589 | 464 | |
| 40–49 | 746 | 612 | 526 | 865 | 806 | 743 | 686 | 612 | 520 | |
| 50–59 | 973 | 816 | 680 | 1130 | 1134 | 1083 | 1010 | 869 | 635 | |
| 60–69 | 823 | 679 | 524 | 999 | 996 | 896 | 876 | 825 | 633 | |
| 70–79 | 505 | 367 | 287 | 530 | 646 | 635 | 518 | 446 | 386 | |
| 80+ | 405 | 309 | 242 | 444 | 468 | 675 | 493 | 330 | 274 | |
| (b) Patients with hypothyroidism | 18–29 | 164 | 165 | 155 | 204 | 269 | 250 | 311 | 294 | 255 |
| 30–39 | 235 | 239 | 233 | 327 | 394 | 363 | 441 | 443 | 358 | |
| 40–49 | 297 | 301 | 302 | 459 | 481 | 476 | 470 | 436 | 406 | |
| 50–59 | 364 | 349 | 346 | 549 | 649 | 684 | 667 | 593 | 484 | |
| 60–69 | 270 | 304 | 257 | 472 | 533 | 524 | 564 | 547 | 464 | |
| 70–79 | 149 | 158 | 149 | 245 | 371 | 349 | 292 | 275 | 280 | |
| 80+ | 112 | 129 | 119 | 197 | 227 | 280 | 238 | 179 | 192 | |
| (c) Patients without hypothyroidism | 18–29 | 176 | 179 | 93 | 160 | 153 | 133 | 128 | 113 | 70 |
| 30–39 | 349 | 273 | 165 | 252 | 233 | 189 | 171 | 146 | 106 | |
| 40–49 | 449 | 311 | 224 | 406 | 325 | 267 | 216 | 176 | 114 | |
| 50–59 | 609 | 467 | 334 | 581 | 485 | 399 | 343 | 276 | 151 | |
| 60–69 | 553 | 375 | 267 | 527 | 463 | 372 | 312 | 278 | 169 | |
| 70–79 | 356 | 209 | 138 | 285 | 275 | 286 | 226 | 171 | 106 | |
| 80+ | 293 | 180 | 123 | 247 | 241 | 395 | 255 | 151 | 82 | |
Figure 3.
Age at levothyroxine (LT4) initiation divided by age decile and displayed on an annual basis.
Age of all patients with LT4 prescriptions
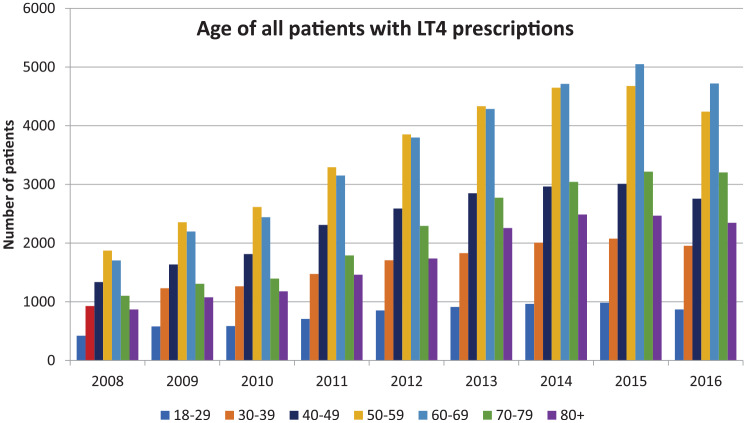
When the age of patients being prescribed LT4, including both existing and new prescriptions, was examined, the mean age of patients ranged from 57 to 62 years of age [Table 5(a)–(c)]. The age of patients being prescribed LT4 with a documented diagnosis of hypothyroidism tended to be a little younger (55–58 years) than those without this diagnosis (58–62 years) [see Table 5(b) and (c)]. When displayed graphically (Figure 4), more patients in the deciles of 50–59 years and 60–69 years had LT4 prescriptions, even though the mean age of the population only increased from 49 years to 51 years over the time period from 2008 to 2016 (see Tables 4 and 6).
Table 5.
Age of all patients with a levothyroxine prescription on an annual basis, with patients divided according to whether they carried a diagnosis of hypothyroidism or not.
| Patient group | Year | Patients n |
Age at prescription |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | 25th pctl | 75th pctl | Minimum | Maximum | |||
| (a) All patients | 2008 | 8229 | 57 | 16 | 57 | 45 | 69 | 18 | 99 |
| 2009 | 10,379 | 57 | 17 | 57 | 45 | 68 | 18 | 100 | |
| 2010 | 11,289 | 57 | 17 | 57 | 45 | 68 | 18 | 110 | |
| 2011 | 14,182 | 57 | 16 | 57 | 46 | 68 | 18 | 111 | |
| 2012 | 16,825 | 58 | 17 | 58 | 46 | 69 | 18 | 104 | |
| 2013 | 19,234 | 59 | 17 | 59 | 47 | 70 | 18 | 109 | |
| 2014 | 20,823 | 59 | 17 | 59 | 47 | 70 | 18 | 114 | |
| 2015 | 21,475 | 59 | 17 | 59 | 48 | 70 | 18 | 115 | |
| 2016 | 20,088 | 59 | 17 | 60 | 48 | 71 | 18 | 105 | |
| (b) Patients with hypothyroidism | 2008 | 3489 | 55 | 16 | 55 | 43 | 66 | 18 | 99 |
| 2009 | 5007 | 55 | 17 | 55 | 43 | 66 | 18 | 99 | |
| 2010 | 6108 | 55 | 16 | 56 | 43 | 66 | 18 | 109 | |
| 2011 | 7827 | 56 | 16 | 56 | 44 | 67 | 18 | 101 | |
| 2012 | 9954 | 56 | 16 | 57 | 44 | 68 | 18 | 102 | |
| 2013 | 12,001 | 57 | 16 | 57 | 45 | 68 | 18 | 109 | |
| 2014 | 13,603 | 57 | 16 | 58 | 46 | 68 | 18 | 110 | |
| 2015 | 14,584 | 57 | 16 | 58 | 46 | 69 | 18 | 104 | |
| 2016 | 14,426 | 58 | 16 | 59 | 47 | 69 | 18 | 105 | |
| (c) Patients without hypothyroidism | 2008 | 4740 | 59 | 16 | 59 | 47 | 71 | 18 | 99 |
| 2009 | 5372 | 58 | 17 | 59 | 47 | 70 | 18 | 100 | |
| 2010 | 5181 | 59 | 17 | 59 | 48 | 71 | 18 | 110 | |
| 2011 | 6355 | 59 | 16 | 59 | 48 | 71 | 18 | 111 | |
| 2012 | 6871 | 60 | 16 | 60 | 49 | 71 | 18 | 104 | |
| 2013 | 7233 | 61 | 17 | 62 | 50 | 73 | 18 | 104 | |
| 2014 | 7220 | 62 | 17 | 62 | 51 | 73 | 18 | 114 | |
| 2015 | 6891 | 62 | 17 | 63 | 51 | 73 | 18 | 115 | |
| 2016 | 5662 | 62 | 17 | 63 | 52 | 74 | 19 | 104 | |
pctl, percentile
Figure 4.
Age of all patients with levothyroxine (LT4) prescriptions divided by decile and displayed on an annual basis.
Table 6.
Number of all patients with a levothyroxine prescription, divided according to age deciles, with patients divided according to whether they carried a diagnosis of hypothyroidism or not.
| Patient group | Age decile | Number of patients with prescriptions per year |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year (mean age of population) |
||||||||||
| 2008 (49) | 2009 (49) | 2010 (49) | 2011 (49) | 2012 (50) | 2013 (50) | 2014 (50) | 2015 (50) | 2016 (51) | ||
| (a) All patients | 18–29 | 420 | 579 | 584 | 707 | 852 | 910 | 962 | 979 | 867 |
| 30–39 | 927 | 1229 | 1264 | 1472 | 1706 | 1826 | 2002 | 2072 | 1952 | |
| 40–49 | 1337 | 1636 | 1812 | 2305 | 2583 | 2843 | 2960 | 3000 | 2750 | |
| 50–59 | 1871 | 2354 | 2614 | 3287 | 3848 | 4328 | 4639 | 4667 | 4228 | |
| 60–69 | 1704 | 2195 | 2439 | 3151 | 3795 | 4281 | 4707 | 5042 | 4710 | |
| 70–79 | 1100 | 1306 | 1394 | 1788 | 2286 | 2767 | 3036 | 3209 | 3194 | |
| 80+ | 867 | 1076 | 1176 | 1458 | 1734 | 2254 | 2481 | 2462 | 2337 | |
| (b) Patients with hypothyroidism | 18–29 | 225 | 325 | 371 | 436 | 560 | 630 | 691 | 734 | 692 |
| 30–39 | 440 | 663 | 779 | 921 | 1140 | 1276 | 1459 | 1578 | 1544 | |
| 40–49 | 617 | 878 | 1091 | 1397 | 1657 | 1926 | 2107 | 2198 | 2114 | |
| 50–59 | 817 | 1147 | 1424 | 1840 | 2329 | 2811 | 3152 | 3284 | 3149 | |
| 60–69 | 690 | 1035 | 1264 | 1673 | 2129 | 2600 | 3028 | 3392 | 3367 | |
| 70–79 | 395 | 535 | 651 | 897 | 1273 | 1611 | 1835 | 2006 | 2128 | |
| 80+ | 305 | 424 | 526 | 662 | 866 | 1146 | 1330 | 1391 | 1431 | |
| (c) Patients without hypothyroidism | 18–29 | 195 | 254 | 213 | 271 | 292 | 280 | 271 | 245 | 175 |
| 30–39 | 487 | 566 | 485 | 551 | 566 | 550 | 543 | 494 | 408 | |
| 40–49 | 720 | 758 | 721 | 908 | 926 | 917 | 853 | 802 | 636 | |
| 50–59 | 1054 | 1207 | 1190 | 1447 | 1519 | 1517 | 1487 | 1383 | 1079 | |
| 60–69 | 1014 | 1160 | 1175 | 1478 | 1666 | 1681 | 1679 | 1650 | 1343 | |
| 70–79 | 705 | 771 | 743 | 891 | 1013 | 1156 | 1201 | 1203 | 1066 | |
| 80+ | 562 | 652 | 650 | 796 | 868 | 1108 | 1151 | 1071 | 906 | |
Sex of patients with LT4 prescriptions
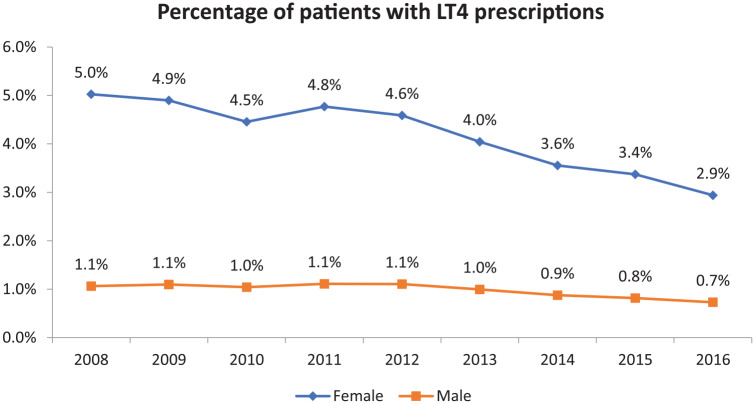
The percentage of male patients being prescribed LT4 stayed steady over the observation period at approximately 1% of patients. However, the percentage of female patients being prescribed LT4 decreased significantly from 5% in 2008 to 2.9% in 2016 (see Figure 5).
Figure 5.
Annual percentage of patients with levothyroxine (LT4) prescriptions divided according to patient sex.
TSH values at time of LT4 prescription initiation
When examining the TSH values available in the patient’s EMR prior to initiation of a new LT4 prescription, TSH elevations were noted to be relatively modest with mean TSH values on an annual basis ranging between 7.51 and 13.78 mIU/L (see Table 7), with values being stable at approximately 10 mIU/L between 2014 and 2016.
Table 7.
Mean and median TSH value for patients prior to new LT4 prescription initiation.
| TSH values prior to new LT4 prescription initiation | ||||||
|---|---|---|---|---|---|---|
| Year | Values n |
Mean | SD | Median | 25th pctl | 75th pctl |
| 2008 | 1159 | 11.04 | 29.40 | 3.64 | 1.62 | 7.28 |
| 2009 | 1209 | 10.68 | 26.78 | 4.57 | 1.87 | 7.35 |
| 2010 | 1156 | 13.78 | 41.95 | 5.08 | 2.23 | 8.04 |
| 2011 | 2007 | 7.51 | 16.07 | 4.26 | 1.99 | 6.39 |
| 2012 | 2096 | 8.39 | 18.42 | 4.54 | 1.98 | 6.77 |
| 2013 | 2163 | 7.82 | 16.25 | 4.69 | 2.22 | 6.93 |
| 2014 | 2035 | 10.09 | 24.18 | 4.90 | 2.17 | 7.64 |
| 2015 | 1824 | 10.63 | 25.57 | 4.77 | 1.99 | 7.81 |
| 2016 | 1510 | 10.72 | 25.89 | 4.78 | 2.17 | 7.50 |
LT4, levothyroxine; pctl, percentile; TSH, thyroid stimulating hormone
Discussion
In contrast to other studies or analyses which show increases in LT4 prescribing,8,16,17,19 our study shows that LT4 being provided for a diagnosis of hypothyroidism remained relatively stable from 2008 to 2016. Moreover, the number of LT4 prescriptions being given to patients without a diagnosis of hypothyroidism declined over the same period. If hypothyroidism is accurately documented in the EMR of these patients, this could suggest that LT4 is being given less frequently for non-specific symptoms. The decline in LT4 prescriptions seemed to occur mostly in women. It is indeed surprising that so many LT4 prescriptions were being written for patients without a documented diagnosis of hypothyroidism. It is suspected that such patients may have been prescribed LT4 for conditions or symptoms such as obesity, tiredness, and depression.
Our analysis confirms other studies that show that LT4 prescriptions are being written for patients with relatively mild TSH elevations.7,12,13 This is perhaps illustrated by the pattern of the mean and median TSH values shown in Table 7. Although the mean TSH is mildly elevated, the median TSH is in the high end of the normal range. This could indicate that there are some individuals in the dataset that have high TSH values, possibly meriting treatment, leading to the mean TSH being above the normal range, but that there are a substantial number of patients who only have “high-normal” TSH values, thus accounting for the median TSH actually being in the higher part of the normal range. The mean TSH values prior to prescription initiation during the last 3 years of observation in our study remained steady at 10 mIU/L. It is important to note that often such mild TSH elevations have a tendency to normalize themselves, even without LT4 being initiated, as has been shown in prior studies.20,21 Our study also confirms other studies that have demonstrated that LT4 initiation is prevalent in older individuals.14 In the present study LT4 was most frequently initiated in those in the age groups of 50–59 years and 60–69 years but was also initiated in those of 70–79 years and in those over 80 years. Similar patterns were seen for all LT4 prescription (new and existing).
We also present new data about LT4 doses in individuals being prescribed LT4 and the effect of having or not having a diagnosis of hypothyroidism on prescribing patterns. With respect to LT4 doses, the fact that the 50 µg dose was the one most frequently prescribed, irrespective of whether or not a diagnosis of hypothyroidism had been definitely documented, further illustrates that mild degrees of hypothyroidism are being treated, or possibly that hypothyroidism was not present at all. Although the authors do not have data regarding the percentage of patients who had low TSH values following initiation of LT4, it could be hypothesized that in some patients treatment simply lowered their TSH values from the upper to the lower end of the normal range. A full replacement dose of LT4 is approximately 100 µg in someone of about 70 kg with minimal residual endogenous thyroid function. This dose was prescribed in 13.6–15.6% of those with a documented diagnosis of hypothyroidism, and 14.7–16.2% of those with no documentation of hypothyroidism.
Surprisingly, in addition to the pattern of LT4 dosage use not differing on the basis of whether a diagnosis of hypothyroidism was present or not, the ages of patients receiving LT4 did not seem to differ depending on whether a diagnosis of hypothyroidism had been formally documented or not. The mean age of initiation of LT4 prescriptions was similar regardless of whether the patient’s EMR contained a diagnosis of hypothyroidism or not. Prescription initiation seemed to be prevalent across all age groups, although the number of prescriptions rose on an annual basis most notably in those of 50–69 years of age. The second most frequent age range in which LT4 was initiated was in those of 60–69 years, with substantial initiation occurring in older age groups also. This is concerning, as there are fewer data about the benefits of LT4 therapy in older age groups,22 but clear risks of over-replacement.23 In addition, based on age-specific reference ranges for TSH values,24 it is possible that many of these older individuals may not actually have hypothyroidism. Although mild TSH elevation may be associated with cardiac dysfunction,25 there may not be benefits in older individuals,26 who also have additional considerations because of frailty.27
Our study has several limitations. Our data are limited by any inherent inaccuracy of documentation present within the MedStar database. If patients actually did have a diagnosis of hypothyroidism, but this diagnosis was not documented, we would have incorrectly classified them as not having a diagnosis of hypothyroidism. Also, patients moving from a geographic area or physician not covered by the MedStar system might appear to have a new diagnosis of hypothyroidism with a normal serum TSH. Despite the limitations of using a health care database such as this, it is likely that the data generated are relevant and generalizable. However, the strengths of our data include the likelihood that these data are accurate: although prescriptions written by providers outside of the MedStar system would not be captured, “hand-written” prescriptions would be extremely rare due to the lack of availability of prescription pads. Due to the diverse demographics of the MedStar hospitals, we believe there is a high likelihood that these data are representative of the United States in general, with the exception of rural areas.
In summary, we have shown that although the percentage of patients being prescribed LT4 is stable or slightly declining, with most decrement in those without a diagnosis of hypothyroidism, there is nevertheless continued initiation of LT4 in those with mild degrees of TSH elevation, and in those of older age, raising concerns both about unnecessary treatment and about iatrogenic thyrotoxicosis. Such data suggest the need for great consideration of both the degree of thyroid stimulating hormone elevation and the patient context when considering whether treatment of an elevated thyroid stimulating hormone, versus ongoing monitoring, is indicated.
Acknowledgments
SD is a Senior Biostatistician for MedStar Health Research Institute.
Footnotes
Author contribution(s): Jacqueline Jonklaas: Conceptualization; Data curation; Funding acquisition; Methodology; Project administration; Resources; Supervision; Writing-original draft; Writing-review & editing.
Sameer DeSale: Data curation; Formal analysis; Methodology; Resources; Software; Validation; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JJ is supported by NIH grants R01DE025822 and UL1TR001409 Research reported in this publication was supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001409. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
ORCID iDs: Jacqueline Jonklaas  https://orcid.org/0000-0002-2238-2666
https://orcid.org/0000-0002-2238-2666
Contributor Information
Jacqueline Jonklaas, Division of Endocrinology, Georgetown University, 4000 Reservoir Rd, NW, Bldg D Suite 230, Washington, DC, USA.
Sameer DeSale, Department of Biostatistics and Biomedical Informatics, MedStar Health Research Institute, Washington, DC, USA.
References
- 1. Jonklaas J, Bianco AC, Bauer AJ, et al. ; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 2014; 24: 1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang S, Jonklaas J, Danielsen M. The glucocorticoid agonist activities of mifepristone (RU486) and progesterone are dependent on glucocorticoid receptor levels but not on EC50 values. Steroids 2007; 72: 600–608. [DOI] [PubMed] [Google Scholar]
- 3. Díez JJ, Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab 2004; 89: 4890–4897. [DOI] [PubMed] [Google Scholar]
- 4. Jonklaas J. Persistent hypothyroid symptoms in a patient with a normal thyroid stimulating hormone level. Curr Opin Endocrinol Diabetes Obes 2017; 24: 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Canaris GJ, Steiner JF, Ridgway EC. Do traditional symptoms of hypothyroidism correlate with biochemical disease? J Gen Intern Med 1997; 12: 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carlé A, Pedersen IB, Knudsen N, et al. Hypothyroid symptoms and the likelihood of overt thyroid failure: a population-based case-control study. Eur J Endocrinol 2014; 171: 593–602. [DOI] [PubMed] [Google Scholar]
- 7. Taylor PN, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med 2014; 174: 32–39. [DOI] [PubMed] [Google Scholar]
- 8. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015; 314: 1818–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez-Gutierrez R, Maraka S, Ospina NS, et al. Levothyroxine overuse: time for an about face? Lancet Diabetes Endocrinol 2017; 5: 246–248. [DOI] [PubMed] [Google Scholar]
- 10. Mitchell AL, Hickey B, Hickey JL, et al. Trends in thyroid hormone prescribing and consumption in the UK. BMC Public Health 2009; 9: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frank RA, Wilby KJ, Mamdani MM. Cross-national comparison of levothyroxine utilization in four developed countries. J Health Spec 2014; 2: 152–155. [Google Scholar]
- 12. Delemer B, Aubert JP, Nys P, et al. An observational study of the initial management of hypothyroidism in France: the ORCHIDÉE study. Eur J Endocrinol 2012; 167: 817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medici BB, Nygaard B, la Cour JL, et al. Changes in prescription routines for treating hypothyroidism between 2001 and 2015: an observational study of 929,684 primary care patients in copenhagen. Thyroid 2019; 29: 910–919. [DOI] [PubMed] [Google Scholar]
- 14. Somwaru LL, Arnold AM, Cappola AR. Predictors of thyroid hormone initiation in older adults: results from the cardiovascular health study. J Gerontol A Biol Sci Med Sci 2011; 66: 809–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Livadas S, Bothou C, Androulakis I, et al. Levothyroxine replacement therapy and overuse: a timely diagnostic approach. Thyroid 2018; 28: 1580–1586. [DOI] [PubMed] [Google Scholar]
- 16. The IQVIA Institute. Medicine use and spending in the U.S: a review of 2018 and outlook to 2023, https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023 (2019, accessed 15 April 2020).
- 17. Endocrine Society. Prevalence and Incidence of Hypothyroidism. Endocrine Facts and Figures, 1st ed, https://www.endocrine.org/topics/thyroid-disorders-and-cancer/facts-and-figures (2010, accessed 15 April 2020).
- 18. Census United States Bureau. Age and Sex Composition in the United States: 2016, https://www.census.gov/data/tables/2016/demo/age-and-sex/2016-age-sex-composition.html (accessed 26 May 2020).
- 19. IMS Institute for Healthcare Informatics. Medicines use and spending shifts: a review of the use of medicines in the U.S. in 2014. Parsipanny, NJ: IMS Institute for Healthcare Informatics. [Google Scholar]
- 20. Stott DJ, Rodondi N, Kearney PM, et al. ; TRUST Study Group. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med 2017; 376: 2534–2544. [DOI] [PubMed] [Google Scholar]
- 21. Meyerovitch J, Rotman-Pikielny P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med 2007; 167: 1533–1538. [DOI] [PubMed] [Google Scholar]
- 22. Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: a review. JAMA 2019; 322: 153–160. [DOI] [PubMed] [Google Scholar]
- 23. Somwaru LL, Arnold AM, Joshi N, et al. High frequency of and factors associated with thyroid hormone over-replacement and under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab 2009; 94: 1342–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J 2013; 2: 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gencer B, Collet TH, Virgini V, et al. ; Thyroid Studies Collaboration. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation 2012; 126: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med 2012; 172: 811–817. [DOI] [PubMed] [Google Scholar]
- 27. Calsolaro V, Niccolai F, Pasqualetti G, et al. Overt and subclinical hypothyroidism in the elderly: when to treat? Front Endocrinol (Lausanne) 2019; 10: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]