Abstract
The corona virus disease of 2019 pandemic caused by the SARS-CoV-2 virus continues to inflict significant morbidity and mortality around the globe. A variety of cardiovascular presentations of SARS-CoV-2 infection have been described so far. However, the impact of SARS-CoV-2 on the right ventricle is largely unknown. Due to its pathophysiologic relevance, the right ventricle finds itself in the eye of the storm of corona virus disease of 2019, placing it at higher risk of failure. Increased afterload from acute respiratory distress syndrome and pulmonary embolism, negative inotropic effects of cytokines, and direct angiotensin converting enzyme 2-mediated cardiac injury from SARS-CoV-2 are potential mechanisms of right ventricle dysfunction in corona virus disease of 2019. Early detection and treatment of right ventricle dysfunction may lead to decreased mortality and improved patient outcomes in corona virus disease of 2019.
Keywords: acute respiratory distress syndromes (ARDSs) and acute lung injury, COVID-19, pulmonary embolism, pulmonary hypertension, right ventricle function and dysfunction
Introduction
The novel corona virus disease of 2019 (COVID-19) has resulted in significant morbidity and mortality across the globe. The mortality from SARS-CoV-2 infection is mainly attributed to acute respiratory distress syndrome (ARDS) and fatal cardiovascular complications. In fact, mortality is markedly higher in COVID-19 patients with myocardial injury than those without (59.6% vs. 8.9%).1 Clinically, there are a variety of cardiovascular presentations of SARS-CoV-2 infection including cardiac ischemia, decompensated heart failure, arrhythmias, and cardiogenic shock.2,3 It is not clear whether these cardiovascular presentations are provoked directly by SARS-CoV-2 or indirectly by cytokine- or stress-induced myocardial dysfunction. Interestingly, a retrospective study of COVID-19 patients with evidence of respiratory distress showed signs of pulmonary hypertension (PH) with mild decrease in Tricuspid annular plane systolic excursion, though no significant difference in right ventricle (RV) size was noted.4 However, a recent study did show that non-survivors of COVID-19 without known cardiomyopathy had RV dilation, diminished RV function, and elevated pulmonary arterial systolic pressure.5 This study demonstrated that evaluation of RV function should be considered as a predictor of mortality in COVID-19 patients.5 Given the physiological relationship between RV and pulmonary vasculature, we suspect that RV dysfunction and failure is contributing to the rapid hemodynamic deterioration, arrhythmias, and sudden cardiac death in patients with COVID-19. Here, we identified potential causes of acute RV dysfunction in the setting of SARS-CoV-2 infection.
Acute respiratory distress syndrome
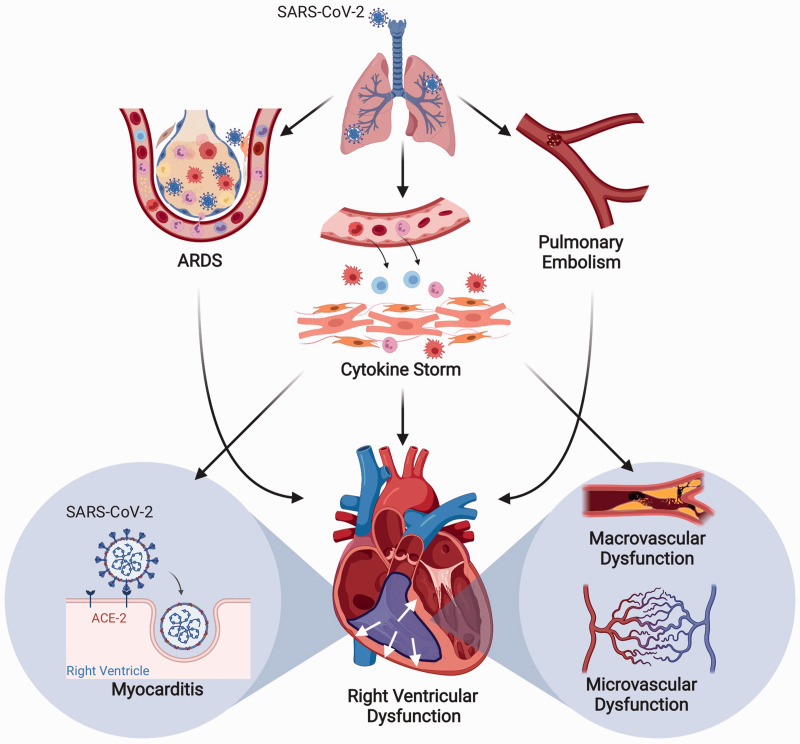
ARDS is one of the major complications in hospitalized patients with COVID-19 (Fig. 1). A recent retrospective study showed development of ARDS in 23.3% of hospitalized patients, of which 58.5% had underlying cardiac injury.6 RV dysfunction is frequently associated with moderate to severe ARDS and is one of the major determinants of mortality.7 In fact, RV dysfunction was found to be an independent predictor of mortality in moderate to severe ARDS patients.7–9 The development of RV failure in the setting of ARDS is mainly attributed to the increased pulmonary vascular resistance (PVR) from increased vasoactive mediators, vascular remodeling, hypoxic pulmonary vasoconstriction, intravascular thrombosis, and vascular compression from atelectasis and edema.6 Since most ARDS patients require positive pressure ventilation (PPV), the uncoupling between the RV and pulmonary circulation under PPV can also contribute to RV failure. Interestingly, recent autopsy reports from COVID-19 patients with severe ARDS showed evidence of RV dilatation.10,11 Thus, it is plausible that the thin-walled RV is particularly susceptible to ischemia and dysfunction in response to sudden increases in afterload and/or coronary occlusion by microthrombi (further discussed below), which in turn may compromise left ventricular function.
Fig. 1.
Plausible mechanisms of development of right ventricular dysfunction in COVID-19. Schematic showing development of right ventricular dysfunction from COVID-19-associated acute respiratory distress syndrome (ARDS), pulmonary embolism, cytokine storm, micro- and macro-vascular dysfunction, and direct angiotensin-converting enzyme 2 (ACE2)-mediated effects of SARS-CoV-2 virus on the right ventricle.
Note: Figure created with BioRender.com
Pulmonary embolism (PE)
Recent reports have demonstrated an increased incidence of pulmonary thromboembolism (PE) in COVID-19 patients,12 predisposing them to acute RV failure13 (Fig. 1). The hemodynamic response of the RV to acute PE depends on the size of embolus, degree of obstruction, physiologic response to released vasoactive compounds, and baseline cardiopulmonary status. RV failure is considered the principal determinant of early mortality in acute PE, and thus early detection of RV dysfunction and myocardial injury is paramount. The mechanism of RV dysfunction in the setting of PE involves increased afterload from acutely elevated PVR secondary to localized hypoxic pulmonary vasoconstriction and release of vasoactive substances.14,15 In regards to COVID-19 patients, there are increasing reports showing marked dysregulation of coagulation pathways as evidenced by elevated d-dimer and fibrin degradation products, prolonged prothrombin time, and thrombocytopenia in COVID-19 patients.16,17 In fact, a recent autopsy study showed higher incidence of deep vein thrombosis and pulmonary embolism in COVID-19 patients.18 Similar thrombotic complications have also been reported in patients with SARS-CoV and Middle East Respiratory Syndrome infection.19 Lung autopsies from patients with SARS-CoV infection revealed thromboemboli in pulmonary, bronchial, and small lung veins.19 In addition, the severe inflammatory response in ARDS can lead to diffuse microthrombi formation within pulmonary vasculature.20,21 Emerging evidence in severe COVID-19 patients with ARDS identified a pattern of tissue damage consistent with complement-mediated thrombotic microvascular injury in the lung.22 Thus, we suspect that the formation of microthrombi within the pulmonary vasculature due to the imbalance of coagulation and inflammatory pathways in ARDS increases the risk of thromboembolic complications and subsequent RV dysfunction in COVID-19 patients.
Myocarditis
Myocarditis has been reported as a possible cause of acute myocardial injury and heart failure in COVID-19 patients,23 although the underlying mechanism is still unclear. Autopsy reports, albeit limited, have shown either biopsy-proven lymphocytic myocarditis in the absence of pulmonary symptoms,24 scattered myocyte necrosis with no significant lymphocytic infiltration in myocardium of COVID-19 patients,10 or mononuclear infiltrates consisting of lymphocytes in the RV of a COVID-19 patient.18 In addition, a recent study identified pericytes as the site of SARS-CoV-2 infection, which may lead to capillary endothelial cell and microvascular dysfunction.25 Altogether, these findings suggest direct injury to cardiomyocytes and pericytes in a fraction of COVID-19 patients may lead to acute myocarditis. Acute myocarditis is a known cause of new onset RV failure through either direct involvement of RV and/or LV via elevated PVR. Currently, it is unclear whether the inflammatory infiltrates and/or myocyte degeneration/necrosis involves specifically the RV and/or LV given the limited biopsies.
Angiotensin converting enzyme 2 (ACE2) has been implicated in the direct cardiac effects of SARS-CoV-2 and is highly expressed in different heart cells (pericytes, cardiomyocytes, fibroblasts, endothelial cells).25 ACE2 is known to convert angiotensin II (Ang-II) into Ang (1–7), which is considered a counter-regulatory axis of the renin angiotensin aldosterone system (RAAS) that can attenuate vasoconstriction, cell proliferation, fibrosis, and inflammation. Recent studies have demonstrated ACE2 as the key determinant of SARS-CoV-2 viral entry. SARS-CoV-2 likely leads to a loss of ACE2-mediated anti-inflammatory and protective effects in the heart.26 In fact, the transmembrane protease serine 2-primed SARS-CoV-2, after entering into cardiac cells via ACE2, may induce tumor necrosis factor (TNF)-α converting enzyme (TACE/A Disintegrin and Metalloproteinase-17 (ADAM-17))-mediated shedding of ACE2 to its soluble form, resulting in a shift toward increased ACE/Ang-II-mediated pro-inflammatory effects in the RV.
Micro- and macro-vascular dysfunction
The combination of endothelial/pericyte dysfunction and ACE/Ang-II pro-inflammatory effects may possibly explain the increased incidence of COVID-19-associated coagulopathy that appears to mimic disseminated intravascular coagulation without adverse bleeding.27 A recent study identified septal capillary injury accompanied by extensive deposition of complement C5b-9, C4d, and mannan-binding lectin serine protease-2 in the lung.22 Another study noted endothelial cell involvement across multiple vascular beds in COVID-19 patients.11 Since endothelial cells and pericytes express ACE2, it is possible that COVID-19 can lead to complement-mediated endothelial and microvascular injury in the heart. If so, this could lead to accumulation of inflammation that may result in vasculitis and/or disruption of vascular homeostasis by increasing vessel hyper-permeability, vasospasms, perfusion defects, and subsequent ischemia, thus, resulting in an imbalance between oxygen supply and demand, which is a known precipitant of RV dysfunction.
The involvement of SARS-CoV-2 in macrovascular diseases is unclear. Pneumonia and influenza infection have been associated with an increased risk of acute myocardial infarction (MI), especially in patients with pre-existing cardiovascular disease.28–30 Previous studies have demonstrated a significant increase in inflammatory cells in atherosclerotic coronary arteries during an acute systemic infection.31–33 The increase in circulating inflammatory cytokines causes an activation of inflammatory cells embedded in atherosclerotic plaques, which leads to coronary plaque destabilization and subsequent myocardial ischemia. It is possible that direct SARS-CoV-2 infection of endothelial cells and/or indirect release of circulating cytokines could lead to coronary plaque destabilization. Thus, patients with history of coronary artery disease and/or risk factors for atherosclerotic cardiovascular disease could possibly be at a higher risk for developing coronary ischemia in the setting of SARS-CoV-2 infection. Even though Guo et al. reported no evidence of acute MI on admission from COVID-19 patients,1 the combination of high fever or hypoxia due to lung disease with coronary plaque instability may still result in type II myocardial ischemia. Interestingly, a case study from a patient without known cardiovascular risk factors developed an acute MI from an in-situ thrombotic stenosis of the proximal right coronary artery.34
Cytokine storm
Cytokine storm is common in COVID-19 patients and comprises an array of inflammatory cytokines that exert pleiotropic effects on the immune system (Fig. 1). Serum levels of one such cytokine interleukin 6 (IL-6) correlate with respiratory failure, ARDS, and adverse clinical outcomes.25,35,36 SARS-CoV-2 infection in the RV is expected to increase the release of vasoactive peptides and induce RAAS imbalance to promote myocardial recruitment of inflammatory cells and is speculated to activate T cell-mediated adaptive responses.37,38 On the contrary, Li et al. proposed that SARS-CoV-2 could directly infect T-lymphocytes and initiate/promote their death that may eventually lead to lymphopenia and impeded anti-viral responses.39 It is speculated that the sustained and substantial reduction in CD4 + and CD8 + T helper cell subtypes40 and hypoxia-induced excessive intracellular calcium may result in cardiomyocyte apoptosis in COVID-19 patients.41 Cardiac myofibroblasts and cardiomyocytes act as a key source of a wide array of pro-inflammatory cytokines including IL-6 and are highly responsive to IL-6, IL-1β, and TNFα. Furthermore, prolonged/excessive synthesis of IL-6 is reported to induce cardiac hypertrophy, fibrosis, and diastolic dysfunction.37 The plethora of pro-inflammatory cytokines involved in developing the cytokine storm can also contribute to RV dysfunction through their negative inotropic effects on the myocardium.42 Taken together, the depressed RV contractility may prove to be fatal in the face of acutely elevated PVR from ARDS and pulmonary embolism in COVID-19.
Pulmonary hypertension
SARS-CoV-2 may worsen RV function in the presence of pre-existing chronic right heart failure conditions. For example, PH is the most common cause of RV failure. Currently, there are limited reports on SARS-CoV-2 infection in PH patients.43 However, numerous studies have demonstrated the involvement of ACE2 in PH and ARDS. ACE2 is expressed in the lung epithelial cells and pulmonary vascular cells, and recent evidence suggests it's critical role in maintaining pulmonary function.26,44 The current evidence includes, but is not limited to the following: (1) ACE2 knock-out in mice exacerbates ARDS,45 pulmonary congestion and heart failure,46 and chronic hypoxia-induced PH47; (2) lung ACE2 expression is down-regulated in PH48,49 and fibrosis50; (3) increased circulating levels of Ang-II and ACE2, but reduced ACE2 enzymatic activity51 in pulmonary arterial hypertension (PAH) patients; (4) administration of recombinant ACE2 attenuates lipopolysaccharide-induced ARDS52; and (5) overexpression of ACE2 by lentivirus53 and administration of ACE2 agonists attenuates preclinical models of PH.54 These studies among others have supported the fundamental concept that lung ACE2 is protective against various pulmonary diseases, but the role of ACE2 in the RV remains unclear. Previous studies have shown improvement of RV function secondary to PH by modulation of the RAAS system with ACE inhibitors, angiotensin receptor blockers, and mineralocorticoid receptor antagonist.53,55–58 In addition, administration of recombinant ACE2 can improve RV function and diminish RV hypertrophy57 in an experimental PH model.
There is increasing evidence that the immune system plays a pivotal role in the pathogenesis of PH. For instance, patients with idiopathic PAH have substantial increase in perivascular mast cells, macrophages, dendritic cells, and T-cells.59 Inflammatory cytokines and chemokines can contribute to the pathogenesis of PH by recruiting immune cells, activating and proliferating pulmonary arterial smooth muscle cells, and causing endothelial cell dysfunction. Interestingly, direct administration or over-expression of IL-6 can lead to PH,60–62 whereas loss of IL-6 attenuated hypoxia-induced PH and RV hypertrophy.60 Clinically, elevated levels of IL-6 have been reported in idiopathic PAH patients.61 Also, a recent study showed an independent inverse relationship between serum IL-6 levels and RV function in PH patients,63 though the underlying mechanism is unclear.
Given the involvement of ACE2 and pro-inflammatory state in PH patients, we suspect that patients with PH are at an increased risk for RV dysfunction and failure in the setting of SARS-CoV-2 infection. It is plausible that RV function can easily decompensate by precipitating factors such as stress of an acute infection (fever, tachycardia) and ARDS in PH patients as a consequence of imbalance between increased metabolic demand and reduced cardiac reserve. The presence of compensatory RV hypertrophy in patients with pre-existing PH is meant to reduce wall stress in a high afterload state. However, the sudden increase in RV afterload with the development of severe ARDS/hypoxemia and/or reduction in effective RV function by direct myocardial injury/infarction may contribute to poor outcomes in these patients. The reported increased in circulating levels of IL-6 in COVID-19 may also contribute to RV dysfunction in PH patients.
Finally, experimental and clinical studies suggest baseline ACE2 deficiency in PH. We speculate that ACE2 down-regulation by SARS-CoV-2 in patients with baseline ACE2 deficiency in the lung may further enhance the ACE/Ang-II-Angiotensin II receptor type I (pro-inflammatory) axis. This can increase expression of TACE/ADAM-17 by Ang-II, promote shedding of membrane-bound ACE2 and reduce its activity, and consequently lead to further loss of ACE2/Ang (1–7) protective effect. The imbalance between ACE/Ang-II and ACE2/Ang (1–7) axis can facilitate the progression of inflammation/cytokine storm, worsening PVR, and hypercoagulable state in pre-existing lung disease. Also, ACE2 is highly expressed by type II pneumocytes in the lung, which produces alveolar surfactant and serve as progenitor cells of type I pneumocytes. Thus, injury to type II pneumocytes from SARS-CoV-2 binding of ACE2 could lead to alveolar collapse and impaired gas exchange, inflammation, and fibrosis.
Long-term consequences from COVID-19 infection
COVID-19 may lead to long-term detrimental effects in the lungs and heart, particularly in critically ill patients. A 12-year longitudinal study of patients who recovered from SARS reported abnormal lipid metabolism (68%), cardiovascular abnormalities (44%), and altered glucose metabolism (60%).64 Also, the development of pulmonary fibrosis in SARS-CoV and ARDS patients during recovery, and animal models of SARS have been reported.65 Given SARS-CoV-2 affinity for lung and heart tissues, it is possible that a cohort of recovered patients may sustain long-lasting injury to heart and lung. Based on these studies, severe lung injury in the setting of SARS-CoV-2 infection may lead to pulmonary fibrosis, elevated pulmonary pressures, cardiovascular disease, and altered metabolism after recovery. These are all risk factors for the development of RV failure. Long-term follow-up of cardiopulmonary function will be necessary for COVID-19 patients, and further investigation of RV dysfunction should be considered.
Current knowledge gaps in RV related research in COVID-19
RV dysfunction and failure are recognized complications of various cardiac and pulmonary disorders, which are associated with poor prognosis. Given the recent evidence of RV involvement in COVID-19 patients, addressing the following knowledge gaps could be crucial in advancing our understanding of RV function in COVID-19 patients: (1) right heart catheterization and echocardiography data from large, multicenter COVID-19 patient cohorts; (2) circulating pro-inflammatory and coagulation system biomarkers and their correlation with right ventricular dysfunction; (3) electrophysiologic evidence for the involvement of right ventricular arrhythmias to COVID-19-related mortality; (4) detailed histopathologic studies of the right ventricular and pulmonary vascular autopsy tissue from COVID-19 patients; and (5) molecular and biochemical analysis of right ventricular tissue and pulmonary vasculature and further characterization of ACE2, ADAM-17, and soluble ACE2 expression and function in COVID-19.
Conclusion
In conclusion, the unique pathophysiology of COVID-19 places the RV at higher risk of failure. Elevated PVR from ARDS and pulmonary embolism, negative inotropic effects of cytokines, microvascular and macrovascular dysfunction, and direct ACE2-mediated cardiac injury from SARS-CoV-2 are potential mechanisms of RV dysfunction in COVID-19. More research is needed to understand the precise molecular mechanisms of RV pathology in COVID-19. Early detection and treatment of RV dysfunction can lead to decreased mortality and improved patient outcomes in COVID-19.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: S.U. is supported by a K08 grant (1K08 HL141995 01A1) from the National Institutes of Health.
ORCID iD
Soban Umar https://orcid.org/0000-0001-8036-3079
References
- 1.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. Epub ahead of print 27 March 2020. DOI: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID-19. Circulation. Epub ahead of print 3 April 2020. DOI: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation 2020; 141: 1648–1655. [DOI] [PubMed] [Google Scholar]
- 4.Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. Epub ahead of print 8 April 2020. DOI: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. Epub ahead of print 2020. 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. Epub ahead of print 25 March 2020. DOI: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zochios V, Parhar K, Tunnicliffe W, et al. The right ventricle in ARDS. Chest 2017; 152: 181–193. [DOI] [PubMed] [Google Scholar]
- 8.Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med 2013; 39: 1725–1733. [DOI] [PubMed] [Google Scholar]
- 9.Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 2016; 42: 862–870. [DOI] [PubMed] [Google Scholar]
- 10.Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans | medRxiv, www.medrxiv.org/content/10.1101/2020.04.06.20050575v1 (accessed 21 April 2020).
- 11.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. Epub ahead of print 21 April 2020. DOI: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klok FA, Kruip MJHA, Van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. Epub ahead of print 10 April 2020. DOI: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ullah W, Saeed R, Sarwar U, et al. COVID-19 complicated by acute pulmonary embolism and right-sided heart failure. JACC Case Rep. Epub ahead of print 17 April 2020. DOI: 10.1016/j.jaccas.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev 2008; 4: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. Epub ahead of print 27 March 2020. DOI: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. Epub ahead of print 7 February 2020. DOI: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. Epub ahead of print 6 May 2020. DOI: 10.7326/M20-2003. [DOI] [PubMed] [Google Scholar]
- 19.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 2020; 127: 104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang JC. Acute respiratory distress syndrome as an organ phenotype of vascular microthrombotic disease: based on hemostatic theory and endothelial molecular pathogenesis. Clin Appl Thromb Hemost 2019; 25: 1076029619887437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frantzeskaki F, Armaganidis A, Orfanos SE. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration 2017; 93: 212–225. [DOI] [PubMed] [Google Scholar]
- 22.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. Epub ahead of print 15 April 2020. DOI: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholas SH, Mark HD, Biykem B, et al. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation 2020; 141: 1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. Epub ahead of print 8 April 2020. DOI: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 2020; 116: 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ Res 2020; 126: 1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis 2020; 15: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musher DM, Abers MS, Corrales-Medina VF. Acute infection and myocardial infarction. N Engl J Med 2019; 380: 171–176. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen JL, Yang W, Ito K, et al. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol 2016; 1: 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madjid M, Miller CC, Zarubaev VV, et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34,892 subjects. Eur Heart J 2007; 28: 1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madjid M, Vela D, Khalili-Tabrizi H, et al. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries. Tex Heart Inst J 2007; 34: 11–18. [PMC free article] [PubMed] [Google Scholar]
- 32.Kaynar AM, Yende S, Zhu L, et al. Effects of intra-abdominal sepsis on atherosclerosis in mice. Crit Care 2014; 18: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mauriello A, Sangiorgi G, Fratoni S, et al. Diffuse and active inflammation occurs in both vulnerable and stable plaques of the entire coronary tree: a histopathologic study of patients dying of acute myocardial infarction. J Am Coll Cardiol 2005; 45: 1585–1593. [DOI] [PubMed] [Google Scholar]
- 34.Dominguez-Erquicia P, Dobarro D, Raposeiras-Roubín S, et al. Multivessel coronary thrombosis in a patient with COVID-19 pneumonia. Eur Heart J. Epub ahead of print 6 May 2020. DOI: 10.1093/eurheartj/ehaa393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. Epub ahead of print 3 March 2020. DOI: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore BJB, June CH. Cytokine release syndrome in severe COVID-19. Science. Epub ahead of print 17 April 2020. DOI: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 37.Dewachter L, Dewachter C. Inflammation in right ventricular failure: does it matter?. Front Physiol. Epub ahead of print 20 August 2018. DOI: 10.3389/fphys.2018.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. Epub ahead of print 30 March 2020. DOI: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. Epub ahead of print 17 April 2020. DOI: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Y-Y, Ma Y-T, Zhang J-Y, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol 2020; 17: 259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sydykov A, Mamazhakypov A, Petrovic A, et al. Inflammatory mediators drive adverse right ventricular remodeling and dysfunction and serve as potential biomarkers. Front Physiol. Epub ahead of print 2018. DOI: 10.3389/fphys.2018.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamanian RT, Pollack CV, Gentile MA, et al. Outpatient inhaled nitric oxide in a patient with vasoreactive IPAH and COVID-19 infection. Am J Respir Crit Care Med. Epub ahead of print 5 May 2020. DOI: 10.1164/rccm.202004-0937LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005; 436: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto K, Ohishi M, Katsuya T, et al. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension 2006; 47: 718–726. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Dong J, Martin M, et al. AMP-activated protein kinase phosphorylation of angiotensin-converting enzyme 2 in endothelium mitigates pulmonary hypertension. Am J Respir Crit Care Med 2018; 198: 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan WSD, Liao W, Zhou S, et al. Targeting the renin–angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr Opin Pharmacol 2018; 40: 9–17. [DOI] [PubMed] [Google Scholar]
- 49.Yuan Y-M, Luo L, Guo Z, et al. Activation of renin-angiotensin-aldosterone system (RAAS) in the lung of smoking-induced pulmonary arterial hypertension (PAH) rats. J Renin Angiotensin Aldosterone Syst 2015; 16: 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Molina-Molina M, Abdul-Hafez A, et al. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2008; 295: L178–L185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandoval J, Del Valle-Mondragón L, Masso F, et al. Angiotensin converting enzyme 2 and angiotensin (1-7) axis in pulmonary arterial hypertension. Eur Respir J. Epub ahead of print 2 April 2020. DOI: 10.1183/13993003.02416-2019. [DOI] [PubMed] [Google Scholar]
- 52.Treml B, Neu N, Kleinsasser A, et al. Recombinant angiotensin-converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide-induced lung injury in piglets. Crit Care Med 2010; 38: 596–601. [DOI] [PubMed] [Google Scholar]
- 53.Yamazato Y, Ferreira AJ, Hong K-H, et al. Prevention of pulmonary hypertension by angiotensin converting enzyme 2 gene transfer. Hypertension 2009; 54: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guignabert C, De Man F, Lombès M. ACE2 as therapy for pulmonary arterial hypertension: the good outweighs the bad. Eur Respir J. Epub ahead of print 2018. DOI: 10.1183/13993003.00848-2018. [DOI] [PubMed] [Google Scholar]
- 55.Rouleau JL, Kapuku G, Pelletier S, et al. Cardioprotective effects of ramipril and losartan in right ventricular pressure overload in the rabbit: importance of kinins and influence on angiotensin II type 1 receptor signaling pathway. Circulation 2001; 104: 939–944. [DOI] [PubMed] [Google Scholar]
- 56.Rondelet B, Kerbaul F, Van Beneden R, et al. Prevention of pulmonary vascular remodeling and of decreased BMPR-2 expression by losartan therapy in shunt-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 2005; 289: H2319–H2324. [DOI] [PubMed] [Google Scholar]
- 57.Johnson JA, West J, Maynard KB, et al. ACE2 improves right ventricular function in a pressure overload model. PLoS One 2011; 6: e20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maron BA, Zhang Y-Y, White K, et al. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation 2012; 126: 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savai R, Pullamsetti SS, Kolbe J, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 897–908. [DOI] [PubMed] [Google Scholar]
- 60.Savale L, Tu L, Rideau D, et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res 2009; 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koudstaal T, Boomars KA, Kool M. Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: an immunological perspective. J Clin Med. Epub ahead of print 19 February 2020. DOI: 10.3390/jcm9020561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steiner MK, Syrkina OL, Kolliputi N, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 2009; 104: 236–244. 28p following 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prins KW, Archer SL, Pritzker M, et al. Interleukin-6 is independently associated with right ventricular function in pulmonary arterial hypertension. J Heart Lung Transplant 2018; 37: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Q, Zhou L, Sun X, et al. Altered lipid metabolism in recovered SARS patients twelve years after infection. Sci Rep 2017; 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venkataraman T, Frieman MB. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antiviral Res 2017; 143: 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]