Abstract
The Scedosporium apiospermum complex is a group of emerging opportunistic fungal pathogens that affect both immunocompromised and immunocompetent individuals, most commonly via lung infection. Although they are resistant to many antifungal agents, this group of pathogens has a favorable susceptibility profile to azoles, especially voriconazole. Here, we describe the management of S. apiospermum infection in an otherwise healthy 44-year-old woman. She had exhibited intermittent hemoptysis for 2 years before admission to our hospital. Computed tomography revealed a thin-walled and well-circumscribed cavitary lesion in the left upper lobe; the lesion was filled with consolidative opacities. Fungal culture of bronchoalveolar lavage fluid specimens revealed grayish-white mold; lactophenol cotton blue staining revealed acute angle branched septate hyaline cylindrical hyphae, characteristic of S. apiospermum. Despite voriconazole 200 mg twice daily for 8 weeks, the patient showed no improvement; thus, her left upper lobe was removed via thoracoscopic surgery. Her symptoms immediately improved and chest radiography after surgical resection showed no evidence of radiological progression or reoccurrence. This report demonstrates that S. apiospermum lung infection may not respond well to voriconazole alone in immunocompetent hosts; thus, surgery could be curative for these patients.
Keywords: Scedosporium apiospermum, pulmonary fungal infection, immunocompetent, surgery, voriconazole, antifungal therapy
Introduction
Scedosporium apiospermum complex is a group of emerging fungal pathogens present in soil, sewage, polluted waters, and human-impacted areas (e.g., farming land, where it may affect farmers, gardeners, and agricultural workers). According to the European Confederation of Medical Mycology/International Society for Human and Animal Mycology, this complex comprises five closely related filamentous fungal species: S. apiospermum sensus stricto, Scedosporium boydii (Pseudallescheria boydii), Scedosporium aurantiacum, Scedosporium dehoogii, and Scedosporium minutispora.1–4 Because of the wide spectrum of clinical infections reported thus far, the S. apiospermum complex is currently regarded as one of the most common molds that can cause infection in humans. S. apiospermum complex causes opportunistic infections in immunocompromised patients. However, infections have also been encountered in immunocompetent individuals.5–8
S. apiospermum infection can comprise superficial and localized skin and soft tissue infections with extensions to tendons, ligaments, bones, and internal organs; it can also manifest as disseminated (systemic) infection.9 The lungs and foot are the most commonly encountered sites of nonopportunistic S. apiospermum infection.5 Several types of S. apiospermum infections involve the lungs: transient local colonization of the respiratory tract, bronchopulmonary saprophytic involvement of abnormal airways, allergic bronchopulmonary reaction, fungus ball formation (pseudallescherioma/scedosporioma), and occasional invasive pseudallescheriasis (Pseudallescheria pneumonia).6 Generally, a distinctive clinical syndrome of pulmonary infection in previously healthy, immunocompetent individuals has been associated with near drowning in polluted waters.6–8 Lung infections caused by S. apiospermum complex in otherwise healthy individuals without near drowning events remain rare.
In vitro analyses of S. apiospermum have demonstrated its susceptibility to several antifungal drugs: voriconazole, miconazole, albaconazole, posaconazole, and itraconazole.5,10 Voriconazole is indicated as a first-line antifungal agent for treatment of S. apiospermum infections.11 Here, we describe pulmonary S. apiospermum infection in an otherwise healthy and immunocompetent woman, which was resolved by surgical resection after voriconazole monotherapy had been ineffective.
Case report
Medical history
A 44-year-old woman, who had previously worked at a vegetable plantation, was admitted to West China Hospital of Sichuan University (Chengdu, China) in April 2018, with the complaint of intermittent hemoptysis for 2 years. She reported the following symptoms: productive cough with blood-streaked sputum, night sweats, weight loss, and appetite suppression. However, she denied breathlessness, dizziness, fevers, chest pain, nausea, or vomiting. She had no history of smoking and no history of unusual food or animal exposures. Notably, she had no medical history of notable pulmonary disorders. She had previously undergone medical evaluations at several institutions, which had not resulted in a clear diagnosis. Empirical antibiotic therapies with third-generation cephalosporin (cefdinir), macrolides (erythromycin), and quinolones (moxifloxacin) had been ineffective. Chest computed tomography (CT) scans had revealed an irregular and enhancing cavitary mass, which consisted of a cavity and consolidation within a single lesion in the left upper lobe; there had been no signs of emphysema or bullae. The patient underwent extensive investigations for pulmonary tuberculosis, with multiple hematological and sputum examinations. Her anti-tuberculosis antibody titer was high, but other tuberculosis-related parameters had not shown notable findings. The patient had received treatment for presumed pulmonary tuberculosis for 6 months. No clinical or imaging improvements had been observed; thus, empirical anti-tuberculosis treatment had been discontinued in October 2017. The patient had experienced frequent mild to moderate hemoptysis before admission to our hospital.
Clinical examination and diagnosis
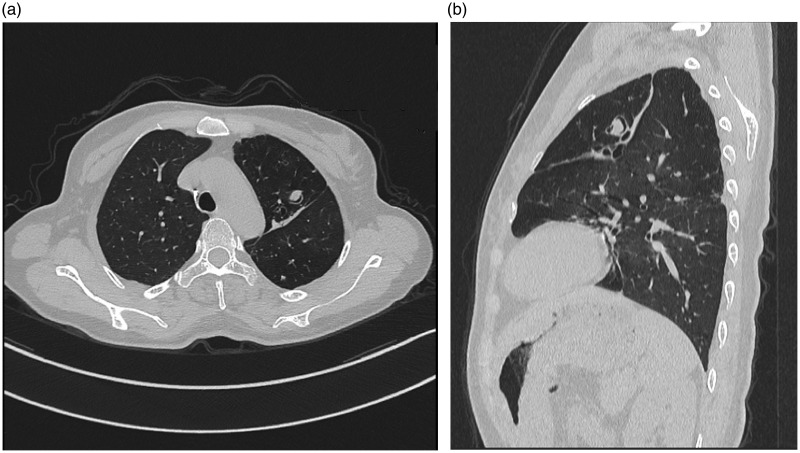
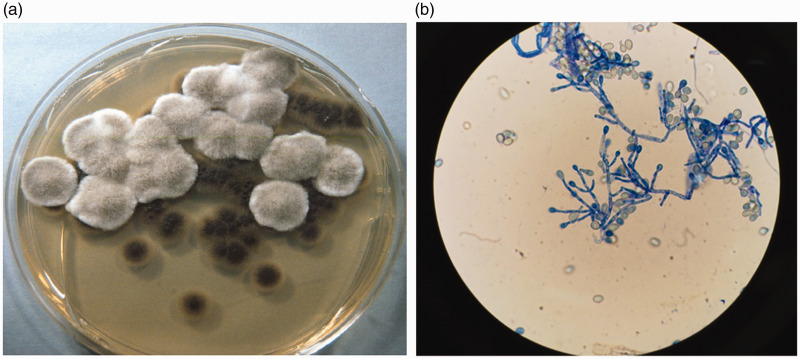
At admission, the patient was not taking any medication and her vital signs were normal. Physical findings were unremarkable. Serology results were negative for human immunodeficiency virus, hepatitis B virus, and hepatitis C virus. Blood examination results were normal. T lymphocyte subsets showed normal CD3+, CD4+, and CD8+ counts, as well as a normal CD4+/CD8+ ratio. IgG, IgA, and IgM levels were within the corresponding normal ranges. Comprehensive gastrointestinal examination revealed no source of bleeding. A CT pulmonary angiogram on the third day after admission excluded pulmonary embolism and pulmonary vascular malformation. A chest CT scan revealed a thin-walled and well-circumscribed cavitary lesion in the left upper lobe; the lesion was filled with consolidative opacities and was accompanied by both minor bronchial dilation and bilateral inflammatory shadows (Figure 1). All other CT findings were unremarkable. Bronchoscopy was then performed; transbronchial biopsies and bronchoalveolar lavage fluid (BALF) were collected. The smear for acid-fast bacilli and cultures for mycobacteria revealed negative results in both sputum and BALF. No bacteria were observed on Gram stain analysis. Cytological analysis of both sputum and BALF revealed no evidence of malignancy. Repeated microscopic examinations of BALF stained with lactophenol cotton blue revealed acute angle branched septate hyaline cylindrical hyphae, characteristic of S. apiospermum (Figure 2a). Fungal culture of BALF specimens subsequently revealed a grayish-white colony and grayish-black reverse (i.e., underside of the agar) after 1 week of incubation in Sabouraud glucose agar (Figure 2b).
Figure 1.
Initial chest computed tomography scans. (a) Axial and (b) sagittal planes show a thin-walled and well-circumscribed cavitary lesion filled with consolidative opacities on the left upper lobe, accompanied by minor bronchial dilation and bilateral inflammatory shadows.
Figure 2.
Fungal culture and staining findings. (a) Sabouraud dextrose agar culture shows growth of grayish-white colonies and grayish-black reverse (i.e., underside of agar). (b) Lactophenol cotton blue preparation shows hyaline branching septate hyphae with single conidia present on tips of erect conidiophores (×400 magnification).
Treatment and resolution
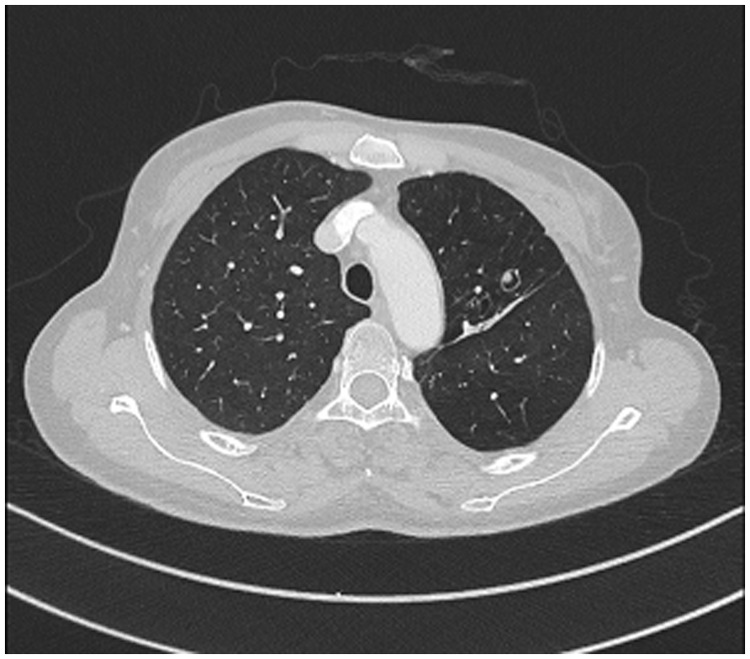
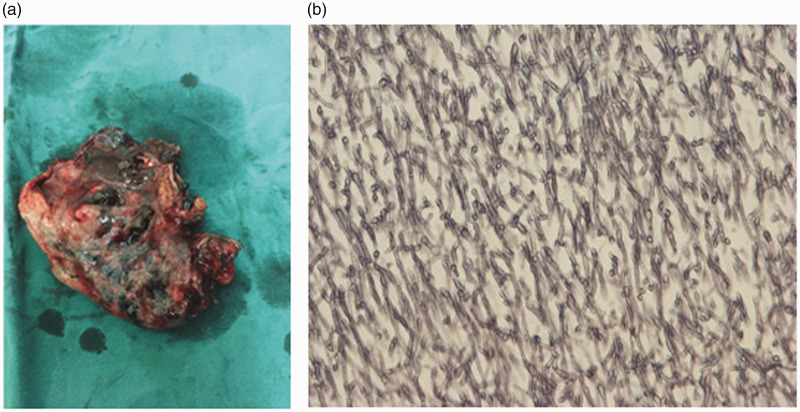
The patient began treatment with intravenous voriconazole 200 mg twice daily for 7 days, then switched to oral dosing for 7 weeks. Although the symptoms of night sweats, weight loss, and appetite suppression were dramatically relieved, the patient exhibited persistent hemoptysis and cough. Chest CT revealed shrinkage of the consolidation in the left upper lung lobe; however, a residual lesion remained (Figure 3). Therefore, the patient was referred to the thoracic surgery clinic in June 2018 and the left upper lobe was removed via video-assisted thoracoscopic surgery (Figure 4a). The excised mass was oval-shaped (11.5 mm × 7 mm × 2.5 mm) and contained brown material; histopathological analysis confirmed the presence of pulmonary scedosporiosis (Figure 4b). After surgery, the patient continued treatment with oral voriconazole 200 mg twice daily for 6 months. Chest CT imaging performed at 3 (Figure 5a), 6 (Figure 5b), and 12 (Figure 5c) months after surgical resection did not show any signs of radiological progression or recurrence. Thus far, the patient has remained asymptomatic for 1.5 years after surgery. The patient provided written informed consent for the publication of this case, in accordance with the guidelines of the Clinical Research Ethics Committee of the West China Hospital of Sichuan University.
Figure 3.
Chest computed tomography scans before surgery.
Figure 4.
Gross anatomical and histopathological findings. (a) Mass removed by video-assisted thoracoscopic surgery. (b) Histopathological staining of resected lung tissue shows thin, delicate, septate hyphae branching at acute angles (Gomori-methenamine silver stain, ×400 magnification).
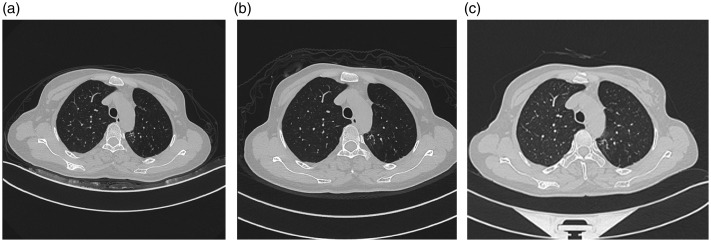
Figure 5.
Chest computed tomography scans show no radiological progressions or reoccurrence at 3 (a), 6 (b), and 12 (c) months after surgery.
Discussion
In our patient, S. apiospermum infection in the lung manifested as a saprophytic process, resulting in the development of a fungus ball in an otherwise healthy and immunocompetent host without obvious lower respiratory tract abnormities. Notably, S. apiospermum lung infection did not respond to voriconazole monotherapy and surgical resection was curative for our patient.
S. apiospermum conidia enter the respiratory tract via inhalation. In a process similar to that of pulmonary aspergillosis, S. apiospermum conidia may be cleared mechanically by the mucociliary escalator mechanism or by pulmonary alveolar macrophages. If macrophages are unable to destroy these conidia, polymorphonuclear leukocytes and mononuclear lymphocytes are then necessary to control the conidia.5 However, immunocompromised hosts may be unable to mount such a response due to the presence of various immune defects. In immunocompetent hosts, S. apiospermum colonization in airways may also be permanent. In most affected patients, saprophytic involvement in the lower respiratory tract occurs in the context of mucosal dysfunction or presence of preformed cavities (e.g., bronchiectasis, chronic obstructive bronchopulmonary disease, tuberculosis, cystic fibrosis, or Job’s syndrome).5 Furthermore, S. apiospermum exhibits variable susceptibility to phagocytosis by macrophages, which may be related to differences in pathogenicity among S. apiospermum isolates.12 The wide range of minimum inhibitory concentrations needed to inhibit the growth of 50% of S. apiospermum isolates illustrates these differences in pathogenicity.5 Moreover, potential mediators released from S. apiospermum mycelia are capable of degrading human fibrinogen and laminin, thereby offering a possible escape mechanism from host effector cells; this may allow invasive fungal cells to migrate into deeper adjacent tissues.13,14 S. apiospermum formed a “fungus ball” in our patient. This “fungus ball” is considered to develop from necrotic host tissue, due to fungal invasion and vascular thrombosis in the lungs.15 Often, cavitary or cystic lung disease (e.g., tuberculosis, sarcoidosis, and previous bacterial infections) may herald the formation of a fungal ball. Serial CT scans in our patient revealed mild bronchiectasis in the left upper lobe, which presumably resulted from the fungal infection rather than preformed dilated bronchi; no bronchiectasis was observed radiographically during the current admission, nor was it reported in the patient’s medical history. Because the patient had no obvious immunodeficiency or pre-existing lung structural damages, the potent pathogenicity of this S. apiospermum isolate may have caused saprobic infection and formation of the “fungal ball.”
Our recent review revealed a delay of 5.5 months in the diagnosis of Scedosporium infection in immunocompetent patients;16 this was much longer than the duration reported for patients with a history of near-drowning.17 Diagnostic delays may lead to inappropriate treatment with grave consequences, even in immunocompetent patients. Therefore, a systematic diagnostic approach is needed to accurately identify the infectious agents and ensure both timely and appropriate therapy. Because of the nonspecific clinical presentation and radiographic findings in patients with S. apiospermum complex infections, cytopathological and/or histopathological methods are the main approaches for detection of these infections.18 In our patient, the diagnosis of S. apiospermum infection was confirmed by fungal culture, which revealed floccose, grayish-white colonies; diagnosis was also supported by typical histological manifestations of hyphal structures. The clinical and radiographical manifestations of pulmonary scedosporiosis resemble the manifestations of aspergillosis. Although these fungi share similar hyaline hyphal structures,5,19,20 Aspergillus displays a regular, dichotomous branching pattern in cytology and histopathological sections, while Scedosporium exhibits a more irregular branching pattern and terminal annelloconidia.21
Because of the ubiquity of this group of molds in the environment, caution should be exercised when making a diagnosis of S. apiospermum infection. When these organisms are isolated in clinical specimens, it is important to consider the source of each specimen, the number of times the fungus was isolated (especially in patients with pulmonary infections), and the underlying condition of the patient.5 In our patient, we made the diagnosis of active S. apiospermum infection, rather than fungal colonization or contamination, for the following reasons: (i) S. apiospermum does not appear to frequently colonize humans, and opportunistic colonization by this pathogen is relatively unlikely in immunocompetent hosts;22 (ii) BALF specimens were carefully handled to avoid contamination and positive isolation was confirmed in both sputum and BALF specimens; (iii) S. apiospermum was identified on microscopy in multiple cytologic specimens; (iv) the patient had several episodes of clinical symptoms leading to admission or treatment; and (v) improvements in both CT scans and clinical symptoms were observed after surgical removal of the apparent site of infection.
Because members of the S. apiospermum complex are not commonly encountered in the clinical laboratory and there are no validated methods for determining their resistance to any antifungal agent, antifungal susceptibility testing was not performed for isolates from our patient. Treatment with voriconazole was initiated based on clinical reports, as well as prior in vitro and in vivo susceptibility studies.9 Whereas treatment of infections caused by S. prolificans is often challenging because of its extensive resistance to all currently available antifungal agents, infections caused by S. apiospermum have been reported to exhibit antifungal susceptibility in both patients and animals.9 Amphotericin B, itraconazole, and echinocandins exhibit variable in vitro or in vivo activity against isolates of S. apiospermum.23–25 Voriconazole is a novel broad-spectrum triazole, which remains under investigation; it is indicated as a first-line antifungal medication for the treatment of S. apiospermum with robust in vitro antifungal activity.10,11 Other azoles with weaker antifungal activity include miconazole, albaconazole, posaconazole, and itraconazole.10,11 A recent review identified voriconazole as the most common monotherapy for the treatment of S. apiospermum lung infections in immunocompetent patients, especially in the past 5 years. Whereas outcomes were generally dismal after voriconazole monotherapy in immunocompromised patients with S. apiospermum lung infections, the overall response was 83.3% in immunocompetent patients with S. apiospermum lung infections.16 However, voriconazole is inhibitory, rather than fungicidal.26,27 Voriconazole treatment was ineffective for our patient; thus, surgical resection was necessary.
A recent systematic review revealed that postoperative systemic antifungal treatment was performed in five of 22 immunocompetent patients with pulmonary Scedosporium spp. infection who underwent surgery to ensure treatment efficacy or prevent recurrence.16 Among these five patients, postoperative voriconazole was administered in two; treatment durations were 3 months and 4 months, respectively.28,29 In our patient, a 6-month course of postoperative systemic voriconazole treatment was prescribed; no drug-related side effects were observed. Although the recurrence of S. apiospermum lung infection has not been conclusively established, a high potential for recurrence was reported in patients with eumycetoma who had undergone surgery.30,31 This recurrence may have been related to the presence of undiagnosed subclinical lesions, robust fungal defense against antifungal drugs, or incomplete surgical procedures.32 Importantly, even in patients who exhibited clinical cure after surgery, later exogenous reinfection may occur. Therefore, the relationships of mortality and recurrence with the use of postoperative antifungal therapy should be investigated in future studies.
Saprobic involvement of S. apiospermum could occur in immunocompetent hosts without obvious pre-existing cavitary lesions, potentially in relation to robust fungal pathogenicity. Antifungal susceptibility testing might aid in pharmacotherapeutic management of S. apiospermum infections; surgery could be curative for affected patients.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520931620 for Management of pulmonary Scedosporium apiospermum infection by thoracoscopic surgery in an immunocompetent woman by Wei Liu, Ruizhi Feng and Hongli Jiang in Journal of International Medical Research
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81700024) and the China Postdoctoral Science Foundation Grant (grant number 2018M643505).
ORCID iD
Hongli Jiang https://orcid.org/0000-0003-4448-5508
References
- 1.Gilgado F, Cano J, Gene J, et al. Molecular and phenotypic data supporting distinct species statuses for Scedosporium apiospermum and Pseudallescheria boydii and the proposed new species Scedosporium dehoogii. J Clin Microbiol 2008; 46: 766–771. DOI: 10.1128/jcm.01122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilgado F, Cano J, Gene J, et al. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J Clin Microbiol 2005; 43: 4930–4942. DOI: 10.1128/jcm.43.10.4930-4942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaela L, Sybren De Hoog G, Liyue Y, et al. Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Divers 2014; 67: 1–10. DOI:10.1007/s13225-13014-10295-13224. [Google Scholar]
- 4.Sandrine G, Jean PB. Scedosporium apiospermum complex: diagnosis and species identification. Curr Fungal Infect Rep 2014; 8: 211–219. DOI:210.1007/s12281-12014-10192-z. [Google Scholar]
- 5.Cortez KJ, Roilides E, Quiroz-Telles F, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev 2008; 21: 157–197. DOI: 10.1128/cmr.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowacs PA, Soares Silvado CE, Monteiro De Almeida S, et al. Infection of the CNS by Scedosporium apiospermum after near drowning. Report of a fatal case and analysis of its confounding factors. J Clin Pathol 2004; 57: 205–207. DOI: 10.1136/jcp.2003.8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messori A, Lanza C, De Nicola M, et al. Mycotic aneurysms as lethal complication of brain pseudallescheriasis in a near-drowned child: a CT demonstration. AJNR Am J Neuroradiol 2002; 23: 1697–1699. [PMC free article] [PubMed] [Google Scholar]
- 8.Mursch K, Trnovec S, Ratz H, et al. Successful treatment of multiple Pseudallescheria boydii brain abscesses and ventriculitis/ependymitis in a 2-year-old child after a near-drowning episode. Childs Nerv Syst 2006; 22: 189–192. DOI: 10.1007/s00381-005-1151-3. [DOI] [PubMed] [Google Scholar]
- 9.Cobo F, Lara-Oya A, Rodriguez-Granger J, et al. Infections caused by Scedosporium/Lomentospora species: clinical and microbiological findings in 21 cases. Med Mycol 2018; 56: 917–925. DOI: 10.1093/mmy/myx147. [DOI] [PubMed] [Google Scholar]
- 10.Meletiadis J, Meis JF, Mouton JW, et al. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob Agents Chemother 2002; 46: 62–68. DOI: 10.1128/aac.46.1.62-68.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaenman JM, DiGiulio DB, Mirels LF, et al. Scedosporium apiospermum soft tissue infection successfully treated with voriconazole: potential pitfalls in the transition from intravenous to oral therapy. J Clin Microbiol 2005; 43: 973–977. DOI: 10.1128/jcm.43.2.973-977.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gil-Lamaignere C, Roilides E, Maloukou A, et al. Amphotericin B lipid complex exerts additive antifungal activity in combination with polymorphonuclear leucocytes against Scedosporium prolificans and Scedosporium apiospermum. J Antimicrob Chemother 2002; 50: 1027–1030. DOI: 10.1093/jac/dkf198. [DOI] [PubMed] [Google Scholar]
- 13.Larcher G, Cimon B, Symoens F, et al. A 33 kDa serine proteinase from Scedosporium apiospermum. Biochem J 1996; 315: 119–126. DOI: 10.1042/bj3150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva BA, Pinto MR, Soares RM, et al. Pseudallescheria boydii releases metallopeptidases capable of cleaving several proteinaceous compounds. Res Microbiol 2006; 157: 425–432. DOI: 10.1016/j.resmic.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz DA. Organ-specific variation in the morphology of the fungomas (fungus balls) of Pseudallescheria boydii. Development within necrotic host tissue. Arch Pathol Lab Med 1989; 113: 476–480. [PubMed] [Google Scholar]
- 16.Liu W, Feng RZ, Jiang HL. Scedosporium spp. lung infection in immunocompetent patients: a systematic review and MOOSE-compliant meta-analysis. Medicine 2019; 98: e17535. DOI: 10.1097/md.0000000000017535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katragkou A, Dotis J, Kotsiou M, et al. Scedosporium apiospermum infection after near-drowning. Mycoses 2007; 50: 412–421. DOI: 10.1111/j.1439-0507.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 18.Husain S, Munoz P, Forrest G, et al. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin Infect Dis 2005; 40: 89–99. DOI: 10.1086/426445. [DOI] [PubMed] [Google Scholar]
- 19.Fessler RG, Brown FD. Superior sagittal sinus infection with Petriellidium boydii: case report. Neurosurgery 1989; 24: 604–607. DOI: 10.1227/00006123-198904000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Hachimi-Idrissi S, Willemsen M, Desprechins B, et al. Pseudallescheria boydii and brain abscesses. Pediatr Infect Dis J 1990; 9: 737–741. DOI: 10.1097/00006454-199010000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Walts AE. Pseudallescheria: an underdiagnosed fungus? Diagn Cytopathol 2001; 25: 153–157. DOI: 10.1002/dc.2027. [DOI] [PubMed] [Google Scholar]
- 22.Beguin H, Nolard H. Mould biodiversity in homes I. Air and surface analysis of 130 dwellings. Aerobiologia (Bologna) 1994; 10: 157–166. DOI:110.1007/BF02459231. [Google Scholar]
- 23.Walsh TJ, Peter J, McGough DA, et al. Activities of amphotericin B and antifungal azoles alone and in combination against Pseudallescheria boydii. Antimicrob Agents Chemother 1995; 39: 1361–1364. DOI: 10.1128/aac.39.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walsh TJ, Groll A, Hiemenz J, et al. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect 2004; 10: 48–66. DOI: 10.1111/j.1470-9465.2004.00839.x. 2004/01/30. [DOI] [PubMed] [Google Scholar]
- 25.Odds FC, Van Gerven F, Espinel-Ingroff A, et al. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob Agents Chemother 1998; 42: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radford SA, Johnson EM, Warnock DW. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrob Agents Chemother 1997; 41: 841–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuenca-Estrella M, Ruiz-Diez B, Martinez-Suarez JV, et al. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J Antimicrob Chemother 1999; 43: 149–151. DOI: 10.1093/jac/43.1.149. [DOI] [PubMed] [Google Scholar]
- 28.Rahman FU, Irfan M, Fasih N, et al. Pulmonary scedosporiosis mimicking aspergilloma in an immunocompetent host: a case report and review of the literature. Infection 2016; 44: 127–132. DOI: 10.1007/s15010-015-0840-4. [DOI] [PubMed] [Google Scholar]
- 29.Masukane S, Kitahara Y, Okumoto J, et al. The effective treatment of lung infection due to Scedosporium prolificans with voriconazole and surgery. Intern Med 2017; 56: 973–977. DOI: 10.2169/internalmedicine.56.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed J, Ditmars DM, Sheppard T, et al. Recurrence of Scedosporium apiospermum infection following renal re-transplantation. Am J Transplant 2004; 4: 1720–1724. DOI: 10.1111/j.1600-6143.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- 31.Sampaio FM, Galhardo MC, Quintella LP, et al. Eumycetoma by Madurella mycetomatis with 30 years of evolution: therapeutic challenge. An Bras Dermatol 2013; 88: 82–84. DOI: 10.1590/abd1806-4841.20132136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampaio FM, Wanke B, Freitas DF, et al. Review of 21 cases of mycetoma from 1991 to 2014 in Rio de Janeiro, Brazil. PLoS Negl Trop Dis 2017; 11: e0005301. DOI: 10.1371/journal.pntd.0005301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060520931620 for Management of pulmonary Scedosporium apiospermum infection by thoracoscopic surgery in an immunocompetent woman by Wei Liu, Ruizhi Feng and Hongli Jiang in Journal of International Medical Research