Abstract
Background
This study aimed to investigate the association between levels of serum amyloid A (SAA) and the activity of systemic lupus erythematosus (SLE).
Material/Methods
The study included 135 patients with SLE, including 52 patients with active SLE and 83 patients with inactive SLE and 149 healthy controls. The degree of activity of SLE was assessed using the SLE Disease Activity Index 2000 (SLEDAI-2K). Serum SAA levels were measured using a Cobas 8000 c702 modular analyzer.
Results
The levels of SAA were significantly increased in patients with active SLE compared with patients with inactive SLE (median IQR, 16.65 mg/L; range, 9.35–39.68 mg/L, and median IQR, 2.30 mg/L, range, 1.30–4.80 mg/L) (p<0.001). Levels of SAA were significantly correlated with the SLEDAI-2K scores, the erythrocyte sedimentation rate (ESR), and hypersensitive C-reactive protein (Hs-CRP) in patients with SLE (r=0.726, p<0.001; r=0.631, p<0.001; r=0.774, p<0.001, respectively). Multivariate logistic regression analysis showed that the SAA values were independently associated with active SLE when controlled for white blood cell (WBC) count, red blood cell distribution width (RDW), ESR, and Hs-CRP (OR=1.772; p=0.01; 95% CI, 1.101–2.851). Receiver operating characteristic (ROC) curve analysis for SAA was used to identify patients with active SLE with an area under the curve of 0.971, a sensitivity of 90.4%, and a specificity of 94.0%.
Conclusions
SAA levels were significantly correlated with disease activity in patients with SLE.
MeSH Keywords: Biological Markers; Lupus Vasculitis, Central Nervous System; Serum Amyloid A Protein
Background
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease, which results in multiple organ dysfunction [1,2]. SLE is characterized by the loss of immune tolerance to nuclear and cytoplasmic autoantigens, and is a common disease with an incidence of 1.4–24.0 per 100,000 person-years worldwide and predominantly affects women [1,2]. The evaluation of disease activity in patients with SLE mainly depends on laboratory testing, which is used to evaluate disease progression and the prognosis of patients with SLE. Previous studies have shown an association between the activity of SLE and some laboratory parameters, including interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), C-reactive protein (CRP), and the erythrocyte sedimentation rate (ESR) [3–5]. Recently, Hu et al. reported that increased red blood cell distribution width (RDW) was strongly correlated with SLE disease activity, indicating that RDW may be a useful inflammatory biomarker [6]. Also, a relationship between procalcitonin levels and the activity of SLE has previously been reported [7,8].
Serum amyloid A (SAA) has recently been identified as a novel inflammatory indicator that is significantly increased in patients with conditions including idiopathic pulmonary fibrosis, Familial Mediterranean Fever, and neonatal septicemia [9–11]. Also, increased levels of SAA have been reported in lung disorders that include chronic obstructive pulmonary disease (COPD), radiation pneumonitis, obstructive sleep apnea (OSA) syndrome, and lung cancer [12]. These previous findings indicate that this SAA may be an acute-phase protein with a role as a prognosis indicator [12]. Increased serum concentrations of SAA have been reported to be associated with rheumatoid arthritis, indicating that SAA may be an important biomarker in assessing the degree of inflammation in rheumatoid arthritis [13]. However, an association between SAA and SLE disease activity has not been previously determined. Therefore, this study aimed to investigate the association between levels of SAA and the activity of SLE.
Material and Methods
Study participants and study design
A total of 135 patients with systemic lupus erythematosus (SLE) and 149 healthy individuals were enrolled in this study. This study was approved by the Ethics Committee of the Affiliated Hospital of Guilin Medical University. Informed consent was obtained from the study participants. The SLE Disease Activity Index 2000 (SLEDAI-2k) scores were used to evaluate the activity of SLE in the patients [14]. The American College of Rheumatology (ACR) criteria were used to diagnose all patients with SLE [15]. Patients with co-morbidities, such as diabetes, hypertension, known liver disease, end-stage renal disease, hematological disorders, malignant tumors, and other systemic autoimmune diseases, were excluded from the study.
Laboratory investigations
The white blood cell (WBC) count, the platelet count, the red blood cell (RBC) count, hemoglobin level, hypersensitive C-reactive protein (Hs-CRP), red blood cell distribution width (RDW), erythrocyte sedimentation rate (ESR), and the serum amyloid A (SAA) levels of patients with SLE were retrospectively acquired from the electronic medical record. Complete blood counts were measured by using a Sysmex XN900 automated hematology analyzer (Sysmex, Kobe, Hyogo, Japan).
Statistical analysis
The normality of the distribution of continuous variables was analyzed using the Shapiro-Wilk test. Categorical variables were presented as counts and were compared using the chi-squared (χ2) test. Continuous variables with a normal distribution were expressed as the mean±standard deviation (SD) and were compared using Student’s t-test. Continuous variables with a non-normal distribution were presented as the median and interquartile range (IQR) and were compared using the Mann-Whitney U test. Spearman’s rank correlation coefficient was used for correlation analysis. The least absolute shrinkage and selection operator (LASSO) method was utilized to select the key variables from the patients with SLE. Variables with non-zero coefficients, which were selected from the LASSO regression model, were entered into binary logistic regression analysis. The performance of SAA was evaluated by receiver operating characteristics (ROC) curve analysis. Statistical analysis was performed using SPSS version 16.0 (IBM, Chicago, IL, USA), MedCalc version 15.0, and R software version 3.6.1 (https://www.R-project.org). P-values <0.05 indicated statistical significance.
Results
Patients with systemic lupus erythematosus (SLE) and healthy controls
A comparison of the demographic characteristics and clinical laboratory parameters between patients with SLE and healthy individuals is shown in Table 1. There were several significant differences between the two groups. The red blood cell (RBC) and platelet counts and the hemoglobin levels were significantly lower in the patients with SLE compared with the controls. In contrast with the controls, the levels of the red blood cell distribution width (RDW) and hypersensitive C-reactive protein (Hs-CRP) were higher in patients with SLE. The other laboratory parameters were not significantly different between patients with SLE and healthy controls.
Table 1.
Demographic characteristics and laboratory parameters of patients with systemic lupus erythematosus (SLE) and healthy individuals.
| Patients with SLE | Healthy Controls | P-value | |
|---|---|---|---|
| n=135 | n=149 | ||
| Gender (Male/Female) | 14/121 | 22/127 | 0.266 |
| Age (years) | 38.0 (29.0–47.0) | 34.0 (26.0–44.0) | 0.085 |
| WBC (109/L) | 5.90 (3.98–9.24) | 6.68 (5.56–7.74) | 0.059 |
| RBC (1012/L) | 4.10±0.67 | 4.54±0.37 | <0.001 |
| Platelet count (109/L) | 224.56±91.09 | 250.35±37.89 | 0.003 |
| Hemoglobin (g/L) | 116.0 (105.0–125.0) | 133.0 (128.0–140.0) | <0.001 |
| RDW (%) | 13.80 (12.90–15.90) | 12.20 (11.85–12.60) | <0.001 |
| Hs-CRP (mg/L) | 2.35 (0.44–7.71) | 0.67 (0.58–0.99) | <0.001 |
| ESR (mm/h) | 18.0 (11.0–35.0) | – | – |
| SAA (mg/L) | 4.90 (1.90–12.00) | – | – |
| SLEDAI-2k scores | 7.0 (5.0–15.0) | – | – |
WBC – white blood cell count; RBC – red blood cell; RDW – red blood cell distribution width; Hs-CRP – hypersensitive C-reactive protein; ESR – erythrocyte sedimentation rate; SAA – serum amyloid A; SLEDAI-2K – SLE Disease Activity Index 2000.
Serum amyloid A (SAA) and the activity of SLE
All patients with SLE were classified into an active SLE group and an inactive SLE group, as shown in Table 2. There were no significant differences between the active SLE group and the inactive SLE group in terms of gender, age, white blood cell (WBC) counts, RBC counts, and platelet counts. The SAA levels were significantly higher in patients with active SLE compared with the patients with inactive SLE (Figure 1). Also, the RDW, Hs-CRP, and ESR levels were significantly higher in patients with active SLE compared with patients with inactive SLE. The hemoglobin levels in patients with active SLE were significantly lower than those in patients with inactive SLE. The results of the correlation analysis showed that the SAA level was significantly correlated with WBC, RDW, ESR, and Hs-CRP in patients with SLE (r=0.230, p=0.007, r=0.280, p=0.001, and r=0.631, p<0.001 and r=0.774, p<0.001, respectively). SAA levels were significantly correlated with the SLE Disease Activity Index 2000 (SLEDAI-2K) scores in patients with SLE (r=0.726, p<0.001).
Table 2.
Demographic and laboratory parameters in patients with systemic lupus erythematosus (SLE) with active disease and inactive disease.
| Patients with active SLE (n=52) | Patients with inactive SLE (n=83) | P-value | |
|---|---|---|---|
| Gender (Male/Female) | 6/46 | 8/75 | 0.725 |
| Age (years) | 38.1±13.9 | 38.2±13.0 | 0.978 |
| WBC (109/L) | 6.62 (4.33–10.22) | 5.31 (3.82–9.00) | 0.132 |
| RBC (1012/L) | 4.01±0.65 | 4.16±0.68 | 0.210 |
| Platelet count (109/L) | 218.00 (153.50–302.50) | 225.00 (166.00–274.00) | 0.917 |
| Hemoglobin (g/L) | 111.0 (97.8–119.8) | 119.0 (109.0–128.0) | 0.019 |
| RDW (%) | 14.25 (13.20–17.08) | 13.20 (12.60–14.80) | 0.001 |
| Hs-CRP (mg/L) | 9.31 (6.14–24.04) | 0.74 (0.27–1.82) | <0.001 |
| ESR (mm/h) | 49.0 (29.3–74.3) | 12.0 (8.0–17.0) | <0.001 |
| SAA (mg/L) | 16.65 (9.35–39.68) | 2.3 0 (1.30–4.80) | <0.001 |
WBC – white blood cell count; RBC – red blood cell; RDW – red blood cell distribution width; Hs-CRP – hypersensitive C-reactive protein; ESR – erythrocyte sedimentation rate; SAA – serum amyloid A.
Figure 1.
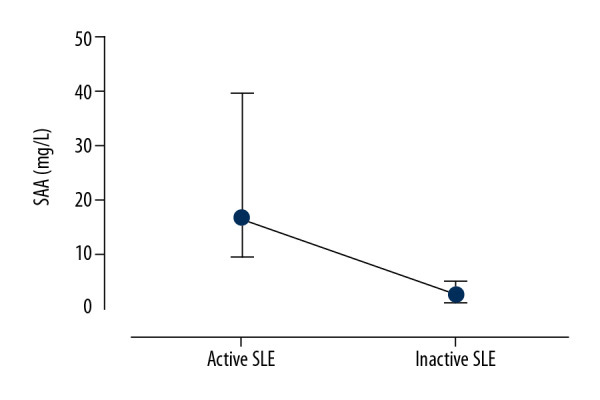
Serum amyloid A (SAA) in patients with active systemic lupus erythematosus (SLE) and patients with inactive SLE. P<0.001, Mann-Whitney U test.
Parameter selection and binary logistic regression analysis
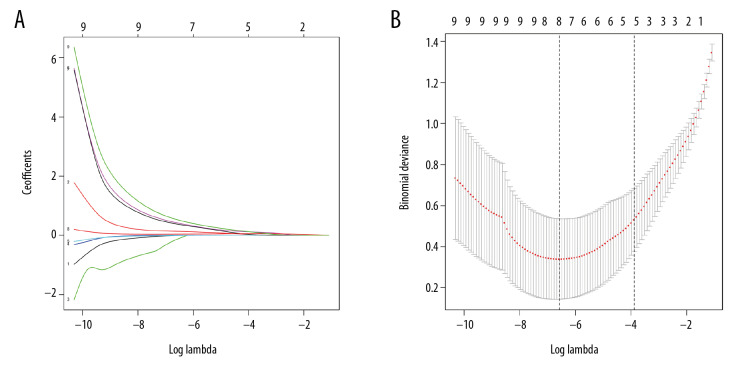
Based on the parameters with non-zero coefficients in the least absolute shrinkage and selection operator (LASSO) regression model, nine variables were reduced to five potential predictors of disease activity (Figure 2A, 2B). All potential predictors, including WBC, RDW, Hs-CRP, ESR, and SAA, were further analyzed using binary logistic regression analysis. The results of the explanatory covariables for the logistic regression analysis of WBC, RDW, Hs-CRP, ESR, and SAA are shown in Table 3. SAA was independently associated with active SLE when logistic regression analysis was conducted to detect potential variables associated with active SLE (OR=1.772; 95% CI, 1.101–2.851; p=0.01). The receiver operating characteristic (ROC) curve analysis of the SAA levels was performed to identify patients with active SLE, and the cut-off value for SAA was 7.2, with a sensitivity of 90.4% and a specificity of 94.0%. The area under the ROC curve (AUC) for SAA was calculated as 0.971 (95% CI, 0.926–0.992; p<0.001) (Figure 3).
Figure 2.
Selection of laboratory parameters by the least absolute shrinkage and selection operator (LASSO) binary logistic regression model. (A) The optimal parameter was selected based on fivefold cross-validation in the LASSO model. (B) LASSO coefficient profiles of the nine features. A coefficient profile plot was produced against the log (lambda) sequence. Fivefold cross-validation was used to draw a vertical line at the selected value, where optimal lambda identified five variables with non-zero coefficients. LASSO, least absolute shrinkage, and selection operator.
Table 3.
The potential factors associated with active systemic lupus erythematosus (SLE) evaluated by binary logistic regression analysis.
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
| WBC (109/L) | 1.006 | 0.651–1.555 | 0.980 |
| RDW (%) | 1.396 | 0.956–2.032 | 0.082 |
| ESR (mm/h) | 1.051 | 1.003–1.102 | 0.037 |
| Hs-CRP (mg/L) | 1.689 | 1.062–2.688 | 0.027 |
| SAA (mg/L) | 1.772 | 1.101–2.851 | 0.01 |
OR – odds ratio; CI – confidence interval; WBC – white blood cell; RDW – red blood cell distribution width; ESR – erythrocyte sedimentation rate; Hs-CRP – hypersensitive C-reactive protein; SAA – serum amyloid A.
Figure 3.

The receiver operating characteristics (ROC) curve analysis of serum amyloid A (SAA) to identify patients with active systemic lupus erythematosus (SLE). The sensitivity of SAA levels for the detection of the activity of SLE was 90.4%, and the specificity was 94.0%, with the area under the ROC curve (AUC) of 0.971(95% CI, 0.926–0.992; p<0.001).
Discussion
The aim of this study was to investigate the association between levels of serum amyloid A (SAA) and the activity of systemic lupus erythematosus (SLE). The findings showed that SAA values increased in patients with active SLE compared with inactive SLE patients. Also, SAA was positively correlated with the SLE Disease Activity Index 2000 (SLEDAI-2K) scores in patients with SLE. Multivariate logistic regression analysis showed that the serum levels of SAA were independently associated with SLE activity.
The rapid, sensitive, and specific evaluation of disease activity in patients with SLE are important for short-term and long-term treatment planning. Previous studies have shown that inflammatory factors, including tumor-necrosis factor-α (TNF-α), complement C3, complement C4, the mean platelet volume, the neutrophil to lymphocyte ratio, IL-6, and IF-34, were associated with the severity of SLE [3,16–19]. The findings from these previous studies support that inflammatory cytokines play an essential role in the pathogenesis and etiology of SLE.
Recently, SAA has been shown to be a novel inflammatory factor found in several diseases, including rheumatoid arthritis, neonatal septicemia, and diabetic kidney disease [11,13,20]. Hwang et al. [21] reported that an increased SAA level was associated with disease activity in patients with rheumatoid arthritis and was a better indicator of disease activity than C-reactive protein (CRP). A previous study identified SAA as a key biomarker in proinflammatory and proatherogenic disease activity and involved in atherosclerosis development [22]. In another study, the level of SAA was found to be significantly higher in women with polycystic ovary syndrome (PCOS) than in controls [23]. In a case-control study undertaken by Dev et al. [24], serum SAA levels were significantly increased in patients with juvenile idiopathic arthritis compared with healthy controls. Also, increased SAA levels have been associated with pulmonary infections in patients with SLE [25]. Consistent with these previous findings, the findings from the present study showed that SAA values were significantly higher in patients with active SLE than in patients with inactive SLE. The level of SAA was independently associated with disease activity in patients with SLE. In other studies, a strong association between increased SAA values and CRP, ESR, and the percentage of peripheral CD4+ lymphocytes has been shown in patients with polycystic ovary syndrome (PCOS), juvenile idiopathic arthritis, and lung transplantation with acute rejection [23,24,26]. The findings from the present study also showed that increased SAA levels were significantly associated with ESR, Hs-CRP, and SLEDAI-2k scores in patients with SLE.
However, this study had several limitations. This study included a small sample size, especially for patients with active SLE. Second, the SAA concentrations were not evaluated in patients with SLE undergoing treatment with anti-inflammatory drugs. The degree of SLE activity was assessed using the SLE Disease Activity Index 2000 (SLEDAI-2K) in this study, but other inflammatory cytokines, such as IF-34, TNF-α, and IL-6, should also be considered in assessing disease activity in future studies.
Conclusions
This study aimed to investigate the association between levels of serum amyloid A (SAA) and the activity of systemic lupus erythematosus (SLE). SAA levels were significantly correlated with disease activity in patients with SLE, with SAA levels significantly associated with active SLE at a sensitivity of 90.4% and a specificity of 94.0%. Therefore, the measurement of SAA may be a useful and cost-effective biomarker to evaluate disease activity in patients with SLE.
Footnotes
Conflict of interest
None.
Source of support: This study was funded by the Research Project Funded by the Health Committee of Guangxi Zhuang Autonomous Region (No. Z20190303)
References
- 1.Rees F, Doherty M, Grainge MJ, et al. The worldwide incidence and prevalence of systemic lupus erythematosus: A systematic review of epidemiological studies. Rheumatology (Oxford) 2017;56(11):1945–61. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- 2.Stojan G, Petri M. Epidemiology of systemic lupus erythematosus: An update. Curr Opin Rheumatol. 2018;30(2):144–50. doi: 10.1097/BOR.0000000000000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fayad A, Hassan E, Salem T. Melatonin receptor 1beta gene polymorphism rs10830963, serum melatonin, TNF-alpha, IL-6, IL-1beta, in Egyptian patients with systemic lupus erythematosus. Egypt J Immunol. 2019;26(1):101–12. [PubMed] [Google Scholar]
- 4.Wojdasiewicz P, Wajda A, Haladyj E, et al. IL-35, TNF-alpha, BAFF, and VEGF serum levels in patients with different rheumatic diseases. Reumatologia. 2019;57(3):145–50. doi: 10.5114/reum.2019.86424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Che Y, Yang L. Relationship of serum inflammatory cytokines with anemia and vascular endothelial function in children with systemic lupus erythematosus. Clin Hemorheol Microcirc. 2019;73(2):299–306. doi: 10.3233/CH-180492. [DOI] [PubMed] [Google Scholar]
- 6.Hu ZD, Chen Y, Zhang L, et al. Red blood cell distribution width is a potential index to assess the disease activity of systemic lupus erythematosus. Clin Chim Acta. 2013;425:202–5. doi: 10.1016/j.cca.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Niu R, Jiang L, et al. The diagnostic values of C-reactive protein and procalcitonin in identifying systemic lupus erythematosus infection and disease activity. Medicine. 2019;98(33):e16798. doi: 10.1097/MD.0000000000016798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Serougy E, Zayed HS, Ibrahim NM, Maged LA. Procalcitonin and C-reactive protein as markers of infection in systemic lupus erythematosus: The controversy continues. Lupus. 2019;28(11):1329–36. doi: 10.1177/0961203318777101. [DOI] [PubMed] [Google Scholar]
- 9.Maggio MC, Castiglia M, Corsello G. Familial Mediterranean Fever: An unusual cause of liver disease. Ital J Pediatr. 2019;45(1):121. doi: 10.1186/s13052-019-0712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vietri L, Bennett D, Cameli P, et al. Serum amyloid A in patients with idiopathic pulmonary fibrosis. Respir Investig. 2019;57(5):430–34. doi: 10.1016/j.resinv.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Wu F, Hou XQ, Sun RR, Cui XJ. The predictive value of joint detection of serum amyloid protein A, PCT, and Hs-CRP in the diagnosis and efficacy of neonatal septicemia. Eur Rev Med Pharmacol Sci. 2019;23(13):5904–11. doi: 10.26355/eurrev_201907_18335. [DOI] [PubMed] [Google Scholar]
- 12.Vietri L, Fui A, Bergantini L, et al. Serum amyloid A: A potential biomarker of lung disorders. Respir Investig. 2020;58(1):21–27. doi: 10.1016/j.resinv.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Shen C, Sun XG, Liu N, et al. Increased serum amyloid A and its association with autoantibodies, acute phase reactants and disease activity in patients with rheumatoid arthritis. Mol Med Rep. 2015;11(2):1528–34. doi: 10.3892/mmr.2014.2804. [DOI] [PubMed] [Google Scholar]
- 14.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91. [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 16.Feng M, Zhang SL, Liang ZJ, et al. Peripheral neutrophil CD64 index combined with complement, CRP, WBC count and B cells improves the ability of diagnosing bacterial infection in SLE. Lupus. 2019;28(3):304–16. doi: 10.1177/0961203319827646. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann LT, Alegretti AP, Machado A, et al. Assessment of mean platelet volume in patients with systemic lupus erythematosus. Open Rheumatol J. 2018;12:129–38. doi: 10.2174/1874312901812010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soliman WM, Sherif NM, Ghanima IM, El-Badawy MA. Neutrophil to lymphocyte and platelet to lymphocyte ratios in systemic lupus erythematosus: Relation with disease activity and lupus nephritis. Reumatol Clin. :2018. doi: 10.1016/j.reuma.2018.07.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Cao J, Lai X. Serum interleukin-34 levels are elevated in patients with systemic lupus erythematosus. Molecules. 2016;22(1) doi: 10.3390/molecules22010035. pii: E35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderberg RJ, Meek RL, Hudkins KL, et al. Serum amyloid A and inflammation in diabetic kidney disease and podocytes. Lab Invest. 2015;95(3):250–62. doi: 10.1038/labinvest.2014.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang YG, Balasubramani GK, Metes ID, et al. Differential response of serum amyloid A to different therapies in early rheumatoid arthritis and its potential value as a disease activity biomarker. Arthritis Res Ther. 2016;18(1):108. doi: 10.1186/s13075-016-1009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shridas P, Tannock LR. Role of serum amyloid A in atherosclerosis. Curr Opin in Lipidol. 2019;30(4):320–25. doi: 10.1097/MOL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Chen X, Ouyang B, et al. Serum amyloid A is a novel inflammatory biomarker in polycystic ovary syndrome. Clin Lab. 2019;65(5) doi: 10.7754/Clin.Lab.2018.181038. [DOI] [PubMed] [Google Scholar]
- 24.Dev S, Singh A. Study of role of serum amyloid A (SAA) as a marker of disease activity in juvenile idiopathic arthritis. J Family Med Prim Care. 2019;8(6):2129–33. doi: 10.4103/jfmpc.jfmpc_339_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li MY, Bai YQ, Liu Y. [Matrix metalloproteinase-3 in patients with systemic lupus erythematosus and its significance in differentiating disease activity from pulmonary infections]. Zhonghua Nei Ke Za Zhi. 2020;59(1):58–61. doi: 10.3760/cma.j.issn.0578-1426.2020.01.010. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 26.Vietri L, Fui A, Bergantini L, et al. Serum amyloid A: A potential biomarker of lung disorders. Respir Investig. 2020;58(1):21–27. doi: 10.1016/j.resinv.2019.09.005. [DOI] [PubMed] [Google Scholar]