SUMMARY
We describe a case of life-threatening disseminated coccidioidomycosis in a previously healthy child. Like most patients with disseminated coccidioidomycosis, this child had no genomic evidence of any known, rare immune disease. However, comprehensive immunologic testing showed exaggerated production of interleukin-4 and reduced production of interferon-γ. Supplementation of antifungal agents with interferon-γ treatment slowed disease progression, and the addition of interleukin-4 and interleukin-13 blockade with dupilumab resulted in rapid resolution of the patient’s clinical symptoms. This report shows that blocking of type 2 immune responses can treat infection. This immunomodulatory approach could be used to enhance immune clearance of refractory fungal, mycobacterial, and viral infections.
INFECTION WITH COCCIDIOIDES FUNGI IS ENDEMIC IN THE SOUTHWESTERN United States, with an estimated incidence of more than 20,000 reported cases per year.1 Most infections are asymptomatic or cause minor respiratory disease (“Valley fever”). However, approximately 1% of infections progress to disseminated coccidioidomycosis, defined as spread beyond the lungs and often involving the bones, central nervous system, and skin. Disseminated coccidioidomycosis causes serious illness with a prolonged disease course, permanent tissue damage, and a fatality rate exceeding 40% despite modern medical and surgical treatments.2 Treatment of disseminated coccidioidomycosis often requires lifelong receipt of antifungal agents, since infections may be chronic or incompletely cleared.3,4 Therefore, there is an urgent need for new treatments.
Disease outcomes in coccidioidomycosis depend on cellular immunity, but the precise elements of that response have not been fully characterized. Resolution of infection is associated with robust interferon-γ-mediated, type 1 immune responses, which require the cytokine interleukin-12 for initiation. Accordingly, patients with monogenic defects along the interleukin-12-interferon-γ axis are susceptible to disseminated coccidioidomycosis.2 On the other hand, type 2 immune responses may be deleterious in disseminated coccidioidomycosis, since eosinophilia and high IgE levels are associated with a worse prognosis.5 The evidence is less conclusive regarding the role of other types of helper T-cell immunity in protection against disease. Studies have suggested that type 17 helper T (Th17) cells and regulatory T cells may also be important for promoting and hindering, respectively, resistance to coccidioides in mice and humans.6,7
CASE PRESENTATION
A previously healthy 4-year-old boy presented with fever and a 3-week history of enlarging subcutaneous nodules on his forehead. The physical examination was notable for three tender masses, each 3 to 5 cm in diameter, on the forehead and scalp, a scaly plaque on the posterior neck, and tenderness in the right wrist and ankle. He had no history of recurrent or severe infections and no family history of immune deficiency or autoimmunity. He lived in a coccidioides-endemic region in California.
Imaging showed a focal consolidation in the right lung, lymphadenopathy, and multiple osteolytic lesions in his skull, vertebral bodies, ribs, right radius, and right tibia (Fig. 1A). Examination of surgical specimens from the skull lesions revealed fungal spherules (Fig. 1B) that were confirmed by polymerase chain reaction (PCR) to be coccidioides. Serologic tests showed coccidioides-specific IgG and IgM, which were absent from the cerebrospinal fluid. Coccidioides complement-fixation titers were suggestive of disseminated disease, with activity detectable at a 1:32 dilution. The patient was treated with fluconazole and liposomal amphotericin B and underwent surgical debridement of the most prominent osseous lesions (Fig. 1C). The spinal and radial lesions worsened as new soft-tissue lesions developed, which prompted additional debridement and escalation of antifungal therapy to posaconazole and high-dose liposomal amphotericin B (7.5 mg per kilogram of body weight). Sertraline was also added to the treatment regimen because of its putative antifungal activity.8 Despite these treatments, complement-fixation titers remained elevated, with activity detectable at 1:256.
Figure 1. A Case of Disseminated Coccidioidomycosis Characterized by Defective Interleukin-12 Signaling and Th1 Response.
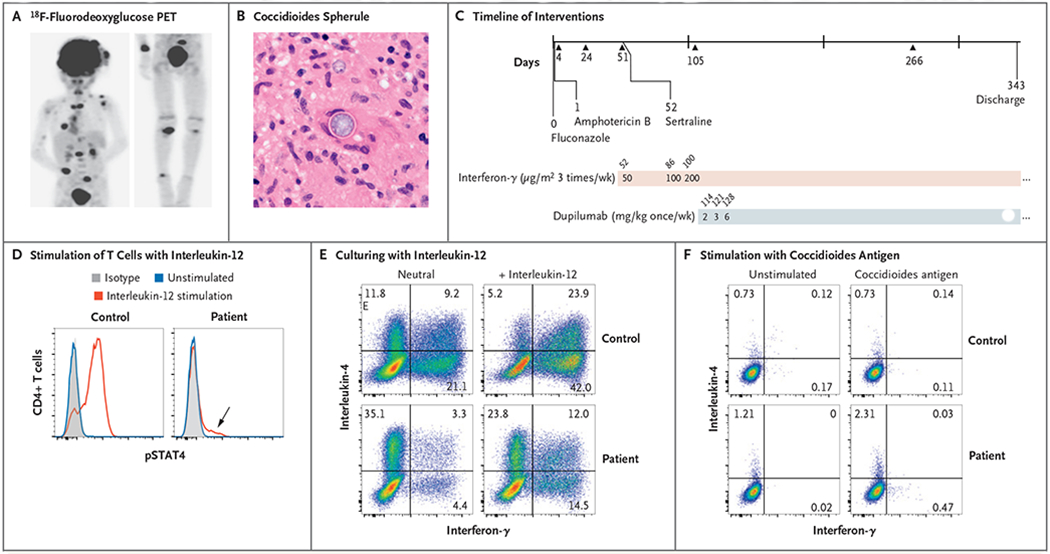
Panel A shows an 18F-fluorodeoxyglucose positron-emission tomographic scan showing disseminated infection with multiple lesions of the spine, clavicle, ribs, paratracheal lymph nodes, right distal radius, and right leg. Panel B shows a coccidioides spherule obtained from surgical biopsy of a scalp lesion. Panel C shows the timeline of interventions in our patient. Initial treatment included fluconazole and liposomal amphotericin B, and sertraline was added at day 52 after admission. Treatment with subcutaneous interferon-γ was also started on day 52, and treatment with dupilumab was started on day 114. Triangles represent major debridement surgical procedures. Doses of interferon-γ and dupilumab are indicated in the shaded bars; numbers above the bars are days after admission. Panel D shows stimulation of helper T cells with interleukin-12, which led to a poor phosphorylated STAT4 (pSTAT4) response; however, the loss of function was not absolute (arrow). Panel E shows intracellular cytokine staining of CD4+ T-cell effectors generated in neutral conditions and stimulated with phorbol myristate acetate (PMA) and ionomycin. Interleukin-4 production was greatly enhanced relative to interferon-γ production in the patient as compared with a control. A normal response was only partially restored by culturing in type 1 helper T (Th1) cell conditions (i.e., with interleukin-12). Panel F shows stimulation of peripheral-blood mononuclear cells with T27K coccidioidal antigen, which led to increased production of interleukin-4 over interferon-γ in helper T cells.
The rapid dissemination of the patient’s infection and his young age prompted further investigation for an underlying immune defect. An initial workup ruled out human immunodeficiency virus (HIV) infection and showed appropriate lymphocyte numbers, normal mitogen-induced lymphocyte proliferation, a normal level of IgM, and elevated levels of IgG, IgA, and IgE (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). We considered that cases of interferon-γ receptor deficiency, STAT1 gain of function, STAT3 deficiency, and interleukin-12 receptor deficiency have been described as contributing to monogenic susceptibility to coccidioidomycosis.2 To evaluate these possibilities, we stimulated monocytes and T cells with interferon-α and interferon-γ, which showed normal phosphorylation and, over time, dephosphorylation of STAT1 (Fig. S1A). STAT3 phosphorylation in response to interleukin-21 stimulation was also intact (Fig. S1B). These results ruled out defects in the interferon-γ receptor, STAT1, or STAT3.
To test interleukin-12 receptor function, we stimulated CD4+ T cells from the patient with interleukin-12. As compared with the response in a healthy control, very low numbers of cells were observed to respond to interleukin-12 with STAT4 phosphorylation (83% responding cells in the control, vs. 12% in the patient) (Fig. 1D). This result was not attributable to the absence of the receptor, since staining for interleukin-12 receptor β1 (CD212) was similar to that in a healthy control (Fig. S1C). When CD4+ T cells were cultured under neutral conditions, their in vitro differentiation into interferon-γ-producing type 1 helper T (Th1) cells was severely impaired, and the proportion of interleukin-4-producing type 2 helper T (Th2) cells was much higher than in healthy controls (Fig. 1E). However, culturing under Th1 conditions (with exogenous interleukin-12) increased the proportion of interferon-γ-producing cells by a factor of 3 (Fig. 1E), indicating that the interleukin-12 signaling defect could be overcome. We saw a similar excess of Th2 cells in specific responses to the coccidioides antigen T27K (Fig. 1F) (Th1:Th2 ratio of 0.41, with the background subtracted). In contrast, when stimulated with coccidioides antigen, cells from a patient with a resolved case of Valley fever showed an almost exclusive Th1 response, as expected (Fig. S2).
We next examined the possibility that a previously described monogenic immunodeficiency was the cause of the observed defect in interleukin-12 receptor signaling. Whole-genome sequencing revealed no plausible rare variants in or near IL12RB1, IL12RB2, or TYK2 or in any primary immunodeficiency gene. No relevant structural variation was detected across the genome. Our patient did not have any of the polymorphic “RTR” variants (low-functioning interleukin-12 receptor β alleles) that confer susceptibility to infection.9 Because genome sequencing revealed no plausible rare exonic variants, RNA sequencing was used to look for aberrant splicing as a cause of disease.10 Using this method, we identified the well-known short and long transcriptional isoforms of IL12RB1.11 Surprisingly, we found that 94% of our patient’s transcripts (61 of 65 reads across the exon-exon junctions) were the short isoform, as compared with an average of 67% of the transcripts among healthy controls (Fig. S3A and S3B). We found no variants in or near the five poly-G tracts that promote splicing of the short isoform.12 Our patient thus produced aberrantly high levels of the short, nonfunctional isoform of IL12RB1. leading to impaired interleukin-12 signaling and type 1 immunity.
Because of the patient’s progressive, refractory disease and the reported success of treatment with interferon-γ in a few patients with disseminated coccidioidomycosis,13,14 treatment with subcutaneous interferon-γ, at a dose of 50 μg per square meter of body-surface area three times per week, was initiated on week 8 of his hospital stay. The treatment did not have substantial adverse effects other than transient fevers, and the patient had a decline in inflammatory markers (Table S2). However, his complement-fixation titers remained elevated, with activity detectable at 1:256. The dose of interferon-γ was gradually increased to 200 μg per square meter three times per week. We reexamined interleukin-12 signaling after treatment with interferon-γ and noted a marked improvement in the interleukin-12 receptor-mediated phosphorylation of STAT4 (Fig. S4A). The patient’s initially defective response to interleukin-12 stimulation was not absolute, indicating that a latent ability to respond to interleukin-12 was awakened by interferon-γ therapy. The proportion of Th1 cells observed in vitro also increased after treatment with interferon-γ (Fig. 2B). The clinical disease, however, continued to progress, albeit at a slower pace, and the patient underwent additional surgical debridement of his radial lesion. The remaining calvarial lesions and the T3 spinal lesion continued to enlarge despite antifungal and interferon-γ therapies (Fig. 2A).
Figure 2. Resolution of Disseminated Coccidioidomycosis after Treatment with Interferon-γ and Dupilumab.
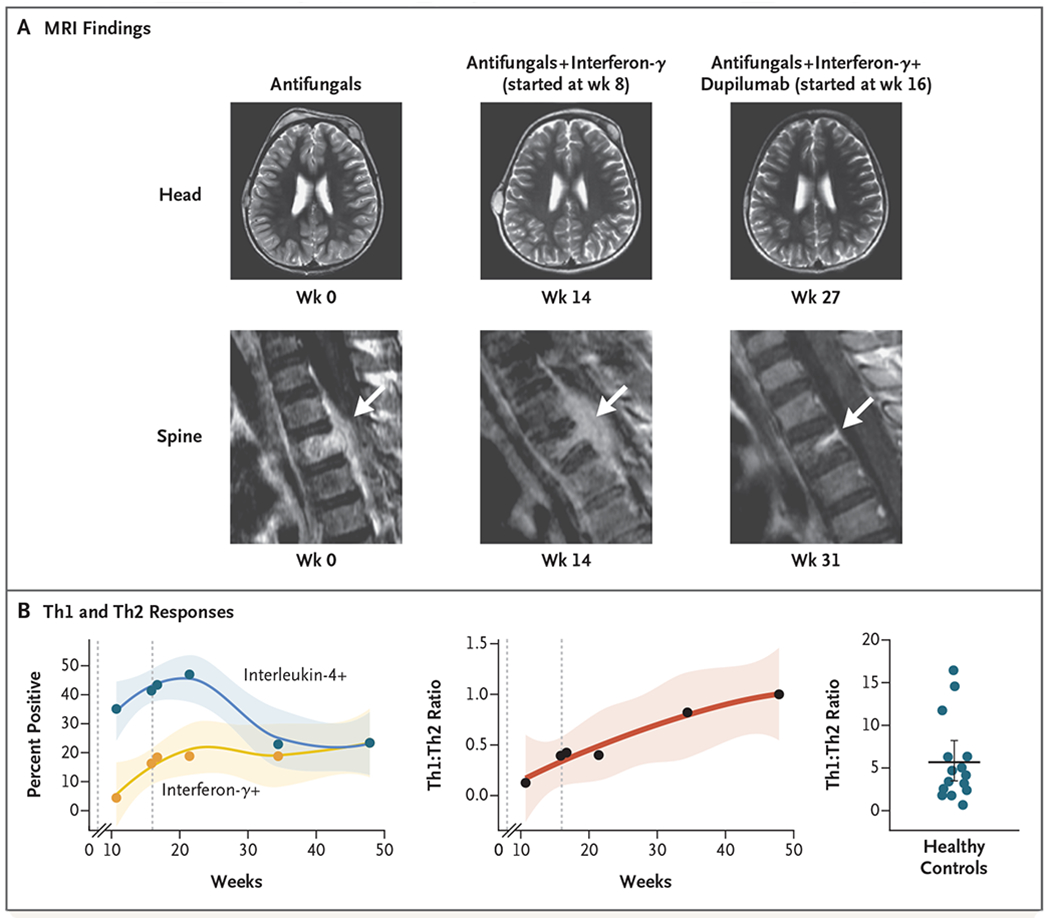
Panel A shows magnetic resonance imaging (MRI) of the head and spine at baseline and during treatment. A spinal lesion is indicated by the arrow. Panel B shows the percentage of CD4+ T cells producing interferon-γ (Th1 cells) or interleukin-4 (type 2 helper T [Th2] cells) (left) and their ratio (center) over time. The ratio does not include doublepositive (i.e., positive for interferon-γ and interleukin-4) cells. The first dashed line represents the initiation of interferon-γ treatment, and the second dashed line represents the initiation of dupilumab treatment. Shading indicates the 95% confidence interval. For comparison, the Th1:Th2 ratio for 15 healthy controls is shown on the right. The horizontal line indicates the bootstrapped mean, and the I bar indicates the 95% confidence interval.
Dupilumab is a monoclonal antibody that blocks the alpha chain common to the interleukin-4 and interleukin-13 receptors. It is indicated for the treatment of severe asthma and atopic dermatitis and has an excellent safety profile. Dupilumab has not typically been used to promote clearance of infections. In vitro incubation of our patient’s T cells with dupilumab resulted in an increase in the ratio of Th1 to Th2 cells (Fig. S4B). In light of this finding and the patient’s refractory disease, dupilumab was added on week 16 of his hospital stay (starting at 2 mg per kilogram of body weight per week and increasing gradually to 6 mg per kilogram per week) without adverse effects. Signaling through the interleukin-4 receptor was completely suppressed under this treatment regimen (Fig. S4C). Over time, the proportion of interleukin-4-producing T cells decreased, resulting in a 1:1 ratio of polyclonal Th1 to Th2 cells (Figs. 2B and S4D), and the Th1:Th2 ratio of coccidioides-specific T cells improved to 0.65 (a 46% increase over baseline). IgE levels also decreased substantially (Table S2). The complement-fixation titers became undetectable, and inflammatory markers normalized. Treatment with dupilumab plus interferon-γ resulted in dramatic clinical improvement, followed by resolution of disease.
Repeat imaging 5 weeks after the addition of dupilumab showed improvement of the calvarial lesions for the first time, with complete resolution 11 weeks later (Fig. 2A). The T3 spinal lesion was found to be resolved 15 weeks after dupilumab was added (Fig. 2A). The patient was discharged and continues to take antifungals plus interferon-γ and dupilumab. The dose of dupilumab was reduced to 4 mg per kilogram per week. At a 1-year follow-up visit, no new foci of infection were discovered. The dose of interferon-γ was decreased to 150 μg per square meter three times per week. Transcriptional analysis of IL12RB1 showed that the patient now had a normal splicing pattern (Fig. S3C).
DISCUSSION
Risk factors for disseminated coccidioidomycosis include pregnancy, immunosuppression, HIV and acquired immunodeficiency syndrome, and monogenic defects of the interleukin-12-interferon-γ axis2 — all states in which type 2 immunity dominates over type 1 immunity. In this report, we showed that treatment with interferon-γ (augmenting type 1 immunity) in combination with dupilumab (suppressing type 2 immunity) resulted in complete resolution of disease in a patient with life-threatening disseminated coccidioidomycosis who had no known monogenic immunodeficiency. These observations imply that a relative insufficiency of type 1 immunity combined with strong type 2 responses confers susceptibility to disseminated coccidioidomycosis. We propose that restoring the balance between type 1 and type 2 immunity enables clinical improvement and that the relative differentiation state of helper T cells may serve as a useful biomarker in this disease.
Th1 cells produce interferon-γ, which augments microbial killing by macrophages and other innate cells. A direct correlation between disease resolution and production of interferon-γ by lymphocytes in response to coccidioides antigen has been found in patients with disseminated coccidioidomycosis.15 Consequently, interferon-γ has been used with success as adjunctive therapy in a few cases of disseminated coccidioidomycosis.13,14 In our patient, however, this approach was insufficient to eliminate disease, despite the improvement of interleukin-12 signaling and some restoration of helper T-cell differentiation.
Type 2 immune responses have been shown to be deleterious in animal models of coccidioidomycosis.16 Interleukin-4 suppresses Th1 development and reduces the antifungal activity of phagocytes and neutrophils.17 We therefore reasoned that inhibiting the Th2 milieu could halt the relentless dissemination of the disease in this patient. Indeed, the addition of dupilumab accelerated clinical improvement, with resolution of bone and soft-tissue lesions. Whether dupilumab exerts disease control by altering T-ceII differentiation and function, phagocyte microbicidal activity, or both remains to be determined.
In our patient, genome sequencing did not identify any plausible rare variants that could explain a susceptibility to disseminated coccidioidomycosis. Pathogenic variants are expected to be rare, because genes required for fitness usually fall under purifying selection, but only when selective pressures are universal. Outside the narrow geographic region in which coccidioides is endemic, selective pressures on genes that confer susceptibiIity to disseminated coccidioidomycosis may be minimal. Thus, a not-so-rare variant may be pathogenic for persons who are exposed to coccidioides.18 Similarly, we know that not-so-rare variants explain susceptibility to tuberculosis,19 another “Th1 disease.”
RNA sequencing from whole blood picked up both the short and the long transcriptional isoforms of IL12RB1. The short isoform cannot respond to cytokines because it lacks its signaling domain and localizes in an intracellular compartment.20 In our patient, the ratio of short to long isoforms was 25:1, whereas in healthy humans the mean ratio is approximately 2:1. We speculate in this case that a nonrare genomic variant, a noncoding or epigenetic change, or a novel immunodeficiency drove aberrant splicing, which was rescued by activation through interferon-γ-STAT1 signaling and thereby promoted expression of the longer isoform.
We found that the combination of interferon-γ and dupilumab successfully controlled a severe case of disseminated coccidioidomycosis. We propose that this immunomodulatory approach may have therapeutic potential for other severe fungal infections, and we speculate it may also be useful in other infections where type 1 immunity is important, including viral and mycobacterial infections.
Supplementary Material
Acknowledgments
Supported by the Jeffrey Modell Foundation (to Dr. Butte) and by an award (U01HG007703) from the National Institutes of Health (NIH) Common Fund, through the Office of Strategic Coordination and the Office of the NIH Director, to the University of California, Los Angeles (UCLA) (to Drs. Nelson, Lee, and Butte). Deidentified whole blood from healthy controls was provided through a UCLA Center for AIDS Research grant (5P30AI028697) and the UCLA AIDS Institute.
We thank the patient and his family for participating in research studies, Drs. Alex Nobori and Yuna Kang of the UCLA Department of Pathology for histopathological analysis, the UCLA Clinical Genomics Center for help, and the UCLA Department of Pediatrics for support.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis. Clin Infect Dis 2005;41:1217–23. [DOI] [PubMed] [Google Scholar]
- 2.Odio CD, Marciano BE, Galgiani JN, Holland SM. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis 2017;23:308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewsnup DH, Galgiani JN, Graybill JR, et al. Is it ever safe to stop azole therapy for Coccidioides immitis meningitis? Ann Intern Med 1996;124:305–10. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev MicrobioI 2010;36:1–53. [DOI] [PubMed] [Google Scholar]
- 5.Cox RA, Baker BS, Stevens DA. Specificity of immunogIobuIin E in coccidioidomycosis and correIation with disease invoIvement. Infect Immun 1982;37:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wüthrich M, Gern B, Hung CY, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest 2011;121:554–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davini D, Naeem F, Phong A, et al. Elevated regulatory T cells at diagnosis of Coccidioides infection associates with chronicity in pediatric patients. J Allergy Clin Immunol 2018;142(6):1971–1974.e7. [DOI] [PubMed] [Google Scholar]
- 8.Paul S, Mortimer RB, Mitchell M. Sertraline demonstrates fungicidal activity in vitro for Coccidioides immitis. Mycology 2016; 7:99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Vosse E, de Paus RA, van Dissel JT, Ottenhoff THM. Molecular complementation of IL-12Rbeta1 deficiency reveals functional differences between IL-12Rbeta1 alleles including partial IL-12Rbeta1 deficiency. Hum Mol Genet 2005;14:3847–55. [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Huang AY, Wang LK, et al. Diagnostic utility of transcriptome sequencing for rare Mendelian diseases. Genet Med 2020;22:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson RT. IL12R01: the cytokine receptor that we used to know. Cytokine 2015;71:348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeme AE, Claeys TA, Aggarwal P, et al. Human IL12RB1 expression is allele-biased and produces a novel IL12 response regulator. Genes Immun 2019;20:181–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duplessis CA, Tilley D, Bavaro M, Hale B, Holland SM. Two cases illustrating successful adjunctive interferon-y immunotherapy in refractory disseminated coccidioidomycosis. J Infect 2011;63: 223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuberski TT, Servi RJ, Rubin PJ. Successful treatment of a critically ill patient with disseminated coccidioidomycosis, using adjunctive interferon-gamma. Clin Infect Dis 2004;38:910–2. [DOI] [PubMed] [Google Scholar]
- 15.Ampel NM, Nesbit LA, Nguyen CT, et al. Cytokine profiles from antigen-stimulated whole-blood samples among patients with pulmonary or nonmeningeal disseminated coccidioidomycosis. Clin Vaccine Immunol 2015;22:917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magee DM, Cox RA. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect Immun 1995;63:3514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romani L Immunity to fungal infections. Nat Rev Immunol 2011;11:275–88. [DOI] [PubMed] [Google Scholar]
- 18.Hung CY, Hsu AP, Holland SM, Fierer J. A review of innate and adaptive immunity to coccidioidomycosis. Med Mycol 2019;57:Suppl 1:S85–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boisson-Dupuis S, Ramirez-Alejo N, Li Z, et al. Tuberculosis and impaired IL-23-dependent IFN-γ immunity in humans homozygous for a common TYK2 missense variant. Sci Immunol 2018;3(30): eaau8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ford NR, Miller HE, Reeme AE, et al. Inflammatory signals direct expression of human IL12RB1 into multiple distinct isoforms. J Immunol 2012;189:4684–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


