Abstract
Objective
To examine the accuracy of noninvasive inflammatory markers in predicting liver fibrosis stage in patients with autoimmune hepatitis (AIH).
Patients and methods
We enrolled 55 patients with AIH and 60 healthy controls in this study, and divided them into three groups: F0 (control); F1–F3 (noncirrhotic fibrosis); and F4 (cirrhosis). The following markers were analyzed for all participants: lymphocyte-to-neutrophil ratio (LNR); lymphocyte-to-platelet ratio (LPR); lymphocyte-to-monocyte ratio (LMR); immunoglobulin-to-platelet ratio (IGPR); aminotransferase-to-platelet ratio index (APRI); aspartate aminotransferase-to-alanine aminotransferase ratio (AAR); and fibrosis-4 score (FIB-4). The predictive accuracy of these noninvasive markers was assessed using area under the receiver operating characteristic curve. Multivariate ordinal logistic regression models were used to analyze associations between the noninvasive markers and liver fibrosis stage.
Results
AAR, LPR, LMR, IGPR, APRI, and FIB-4 were linked to liver fibrosis-stage (P < 0.05), with correlation indices of − 0.219, 0.258, − 0.149, 0.647, 0.841, and 0.704, respectively, but not LNR (P = 0.093). area under the receiver operating characteristic curves of LPR, IGPR, AAR, LMR, APRI, and FIB-4 for detecting cirrhosis (F4 vs. F0–F3) were 0.936 (95% confidence interval: 0.870–1.000, P < 0.001), 0.939 (0.875–1.000, P < 0.001), 0.528 (0.319–0.738, P = 0.768), 0.555 (0.409–0.700, P = 0.568), 0.798 (0.694–0.902, P = 0.002), and 0.881 (0.796–0.967, P < 0.001). Our multivariate ordinal regression analysis showed that LPR and IGPR were associated independently with liver fibrosis stage, with a coefficient of 0.385 (95% confidence interval: 0.103–0.667, P = 0.007) and 14.903 (2.091–27.786, P = 0.023), respectively.
Conclusion
LPR and IGPR were associated independently with liver fibrosis stage in treatment-naive AIH, and were superior to APRI and FIB-4 in detecting cirrhosis.
Keywords: autoimmune hepatitis, cirrhosis, liver biopsy, liver fibrosis, noninvasive markers
Introduction
Autoimmune hepatitis (AIH) is a considerably rare and heterogeneous disease. It is defined by chronic autoimmune inflammation of the liver, and generally characterized by female predilection, elevated levels of aminotransferases, hypergammaglobulinemia or elevated levels of immunoglobulin G (IgG), positivity for specific autoimmune antibodies, and presence of interface hepatitis on biopsy [1,2]. The possible etiology includes failure of immune tolerance mechanisms, environmental triggers, and genetic predisposition, all of which may collaborate to induce an autoimmune attack on the liver [3–5]. About 70–80% of AIH patients have established chronic disease, and ~ 33% of them have cirrhosis at the time of diagnosis [1,6].
Liver biopsy remains the gold standard for the diagnosis of AIH and assessment of liver inflammation and fibrosis [1,2]. In addition, biopsy is useful for the management of AIH, providing meaningful clinical findings that are unmatched by the available biochemical tests [3,7]. However, the use of liver biopsy is often limited by its expense, complications, and poor patient compliance, particularly in the follow-up period [8]. It is therefore important to develop noninvasive and convenient markers capable of accurately evaluating the grade of inflammation and fibrosis stage in AIH patients. Indeed, several markers have been developed and validated for assessing liver fibrosis versus no-fibrosis; these include the fibrosis-4 score (FIB-4) and the aminotransferase-to-platelet ratio index (APRI) [9–12]. Other markers with promising results in clinical use are the aspartate aminotransferase (AST)-to-alanine aminotransferase (ALT) aspartate aminotransferase-to-alanine aminotransferase ratio (AAR) [13], the platelet (PLT) count-to-spleen diameter ratio [14], and red blood cell distribution width [15]. However, to our knowledge, no studies have identified the ordinal or quantitative association of markers with fibrosis stage in AIH patients.
The aim of this study was therefore to assess the value of available noninvasive inflammatory markers in predicting liver fibrosis stage in treatment-naive AIH patients.
Patients and methods
Patients
In this retrospective study, we reviewed the medical records of AIH patients who underwent percutaneous liver biopsy between January 2010 and December 2017 in the Department of Infectious Disease of the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China. The AIH diagnosis was made on the basis of the criteria defined by the practice guidelines of the American Association for the Study of Liver Diseases [1] and the International Autoimmune Hepatitis Group [16]. Only the patients with definite AIH diagnosis (International Autoimmune Hepatitis Grouprevised original score of ≥ 16) were included. According to the medical records, none of the patients had received any immunosuppressive treatment before the liver biopsy.
The exclusion criteria were chronic viral hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, alcoholic or nonalcoholic fatty liver disease, drug-induced liver disease, hepatobiliary parasitic infection, hereditary metabolic liver disease, co-existence of any other autoimmune disease, and severe systemic disease. In addition, patients with severe liver damage, highly elevated level of ALT ( > 1000 U/l), and severe hyperbilirubinemia (total bilirubin > 100 μmol/l) were excluded, as were patients diagnosed with decompensated cirrhosis with ascites, hepatic encephalopathy, esophageal and/or gastric bleeding, or hepatic carcinoma.
A total of 55 patients fulfilled the diagnostic and exclusion criteria. Each of these AIH patients had signed the consent form to undergo liver biopsy. For the study, an additional 60 individuals were selected randomly as age-matched and sex-matched healthy controls. These healthy controls had normal liver function, liver ultrasound, and transient elastography (Fibroscan; Echosens, Paris, France). On the basis of ethical considerations, no liver biopsies were performed on healthy controls, but the normal results of Fibroscan and other tests confirmed that no liver fibrosis was present in any of the healthy controls. The study protocol was approved by the Ethics Committee of Shanghai Ninth People’s Hospital.
Histological assessment
Ultrasound-guided liver biopsy was carried using a 16 G disposable needle for all AIH patients, under local anesthesia. The liver pathology diagnosis required a liver specimen of at least 1.0 cm and containing a minimum of 10 portal tracts. Each obtained specimen was fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin–eosin and Masson–trichrome. The histological analysis was carried out by a single experienced pathologist who was blinded to the clinical data. The liver fibrosis stages were assessed according to the Metavir scoring system [17] as follows: F0, no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with few septa; F3, numerous septa and without cirrhosis; and F4, cirrhosis.
Clinical measurements
The medical records of all included patients diagnosed with AIH were reviewed. Demographic data (age and sex) and laboratory results were collected and analyzed. For all patients, the samples for blood routine and biochemistry tests had been collected on the same day within 1 week before the liver biopsy. Blood samples were analyzed using the Sysmex XE-5000 Automated Hematology System (Sysmex Corp., Kobe, Japan) and associated reagents, providing counts for white blood cells (WBCs), neutrophils, lymphocytes, monocytes, and PLTs. Liver function markers were detected using the AU5811 Chemistry Analyzer (Beckman Coulter Inc., Brea, California, USA) with associated reagents. IgG was measured using the BN ProSpec System (Siemens, Munich, Germany). Antibodies were detected by linear immunoassay using the IMTEC-LIVER LIAS (Human Gesellschaft fur Biochemica und Diagnostica GmbH, Wiesbaden, Germany). The lymphocyte-to-neutrophil ratio (LNR), lymphocyte-to-platelet ratio (LPR), lymphocyte-to-monocyte ratio (LMR) [18,19], AAR [13,14], immunoglobulin-to-platelet ratio (IGPR) [15,20,21], APRI [15,22,23], FIB-4 [15,22,24] and model for end-stage liver disease score (MELD) [13] were calculated as follows:
Statistical analysis
All data were analyzed using SPSS software (IBM SPSS statistics for Windows, version 20.0; IBM Corp., Armonk, New York, USA). The Kruskal–Wallis nonparametric test was used for multiple comparisons, without a normal distribution. The Mann–Whitney U-test was used to evaluate the differences between two groups, without a normal distribution. The correlation between noninvasive markers and liver fibrosis stage was determined using Spearman’s rank correlation test. The area under the receiver operating characteristic (AUROC) curve was used to assess the predictive value of selective variables. Optimal cut-off values between fibrosis and cirrhosis were identified at the maximum of total sensitivity and specificity.
All participants were divided into three groups according to their Metavir scores as follows: F0, control; F1–F3, fibrosis/noncirrhosis; and F4, cirrhosis [17]. The demographic and biological parameters of the three groups were compared using univariate and multivariate ordinal logistic regression analyses. We included age, counts of WBC, neutrophils, lymphocytes, monocytes and PLT, and measures of total bilirubin, ALT, AST, γ-glutamyltransferase, alkaline phosphatase, albumin, and globulin as covariates in the multivariate analyses, with one inflammatory marker as the main variable of interest at a time. A P value of less than 0.05 was considered statistically significant.
Ethical statement
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Characteristics of the participants
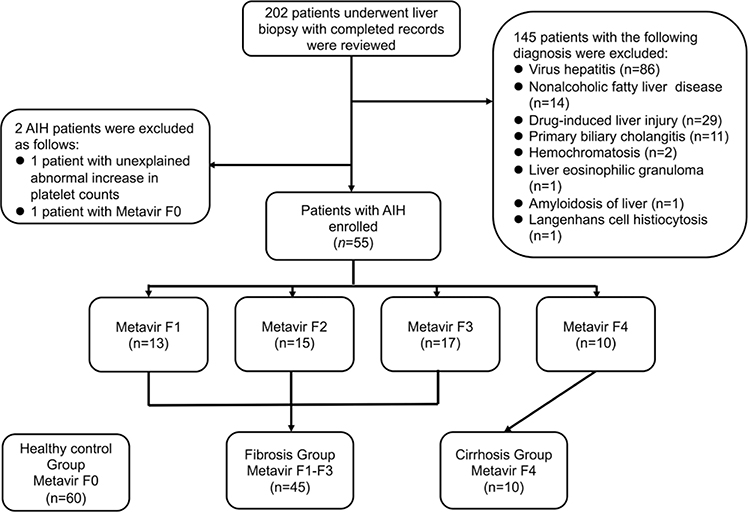
The medical records of 202 patients with liver biopsy were reviewed. We excluded 145 patients according to the exclusion criteria. In addition, one of the AIH patients with an unexplained abnormal increase in PLT counts above the upper limit and another patient with hepatic pathological stage of F0 were excluded. Finally, 55 AIH patients were enrolled in our study (Fig. 1). The overall study population included 115 participants, who were divided into the control group (F0, 52.17%), the noncirrhotic fibrosis group (F1–F3, 39.13%), and the cirrhosis group (F4, 8.7%). We found no difference in the distribution of age or sex among the three groups. In the fibrosis group, the numbers of patients representing each fibrosis stage were 13 for F1, 15 for F2, and 17 for F3. The prevalence of single-positive or multipositive autoantibodies, including smooth muscle antibodies, liver kidney microsomal antibodies, soluble liver antigen/liver pancreas antibodies, liver cytosolic antibodies type 1, and antinuclear antibodies [1,25], was 95.56% in the fibrosis group and 90% in the cirrhosis group. The general characteristics of the study participants are summarized in Table 1.
Fig. 1.
Flowchart of the study showing the enrollment, exclusion, and grouping of the participants. AIH, autoimmune hepatitis.
Table 1.
Baseline characteristics of the healthy controls and patients with treatment-naive autoimmune hepatitis
| Characteristics | Control (F0) | Noncirrhotic fibrosis (F1–F3) | Cirrhosis (F4) | P |
|---|---|---|---|---|
| Age (years) | 56.17 (55.59–56.75) | 54.24 (52.46–56.03) | 59.10 (56.42–61.78) | 0.184 |
| Sex (n) | 60 | 45 (13/15/17) | 10 | 0.836 |
| Male [n (%)] | 12 (20.00) | 7 (15.56) | 2 (20.00) | |
| Female [n (%)] | 48 (80.00) | 38 (84.44) | 8 (80.00) | |
| Autoantibody positive rate (%) | NA | 95.56 | 90.00 | |
| Routine blood test | ||||
| WBC (×109/l) | 5.57 (5.42–5.71) | 5.11 (4.82–5.40) | 4.22 (3.69–4.75) | 0.011 |
| Neutrophil (×109/l) | 3.01 (2.89–3.12) | 2.56 (2.36–2.76) | 1.94 (1.63–2.26) | <0.001 |
| Lymphocyte (×109/l) | 2.05 (1.99–2.11) | 1.79 (1.68–1.9) | 1.82 (1.52–2.12) | 0.058 |
| Monocyte (×109/l) | 0.36 (0.35–0.37) | 0.5 (0.47–0.53) | 0.35 (0.3–0.41) | <0.001 |
| Platelet (×109/l) | 222.57 (215.14–229.99) | 182.91 (173.19–192.63) | 101.1 (82.38–119.82) | <0.001 |
| INR | NA | 0.99 (0.97–1.01) | 1.02 (0.99–1.04) | 0.24 |
| Liver function test | ||||
| Total bilirubin (μmol/l) | 12.66 (12.18–13.14) | 39.49 (32.66–46.31) | 26.25 (21.41–31.09) | <0.001 |
| ALT (U/l) | 19.02 (17.72–20.31) | 205.82 (167.28–244.37) | 83.8 (50.13–117.47) | <0.001 |
| AST (U/l) | 21.38 (20.74–22.03) | 147.53 (124.47–170.6) | 89.5 (59.04–119.96) | <0.001 |
| GGT (U/l) | 19.9 (18.38–21.42) | 254.18 (212.55–295.81) | 177.6 (105.4–249.8) | <0.001 |
| ALP (U/l) | 82.9 (80.55–85.25) | 247.18 (204.11–290.25) | 179.7 (125.43–233.97) | <0.001 |
| Albumin (g/l) | 44.2 (43.89–44.51) | 38.35 (37.44–39.27) | 36.11 (34.35–37.87) | <0.001 |
| Immune globulin (g/l) | 28.22 (27.81–28.62) | 33.44 (32.12–34.76) | 36.81 (32.29–41.33) | <0.001 |
| Serum IgG (g/l) | NA | 16.33 (15.45–17.21) | 19.78 (16.07–23.48) | 0.125 |
| Noninvasive inflammatory markers | ||||
| AAR | 1.25 (1.19–1.3) | 0.98 (0.87–1.08) | 1.36 (0.96–1.76) | <0.001 |
| LNR | 0.72 (0.69–0.76) | 0.77 (0.72–0.83) | 1.03 (0.87–1.2) | 0.043 |
| LPR (×10−3) | 9.63(9.21–10.06) | 10.30(9.55–11.05) | 18.88(17.05–20.7) | <0.001 |
| LMR | 6.04 (5.75–6.32) | 3.84 (3.55–4.13) | 5.2 (4.72–5.67) | <0.001 |
| IGPR | 0.13 (0.13–0.14) | 0.2 (0.19–0.21) | 0.44 (0.35–0.54) | <0.001 |
| APRI | 0.26 (0.24–0.27) | 2.59 (2.14–3.03) | 3.12 (1.84–4.41) | <0.001 |
| FIB-4 | 1.34 (1.27–1.41) | 3.58 (3.09–4.07) | 8.3 (4.71–11.89) | <0.001 |
| MELD | NA | 4.05 (3.38–4.72) | 3.81 (2.36–5.26) | 0.458 |
The data are all shown as mean (quartiles), except n.
AAR, aspartate aminotransferase-to-alanine aminotransferase ratio; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APRI, aminotransferase-to-platelet ratio index; AST, aspartate aminotransferase; Control, healthy individuals without any known disease, abnormal liver ultrasound findings, and abnormal lab tests; FIB-4, fibrosis-4 score; GGT, γ-glutamyltransferase; IGPR, immunoglobulin-to-platelet ratio; LMR, lymphocyte-to-monocyte ratio; LNR, lymphocyte-to-neutrophil ratio; LPR, lymphocyte-to-platelet ratio; MELD, model for end-stage liver disease; NA, not applicable; WBC, white blood cell.
Relationships between noninvasive inflammatory markers and liver fibrosis stage
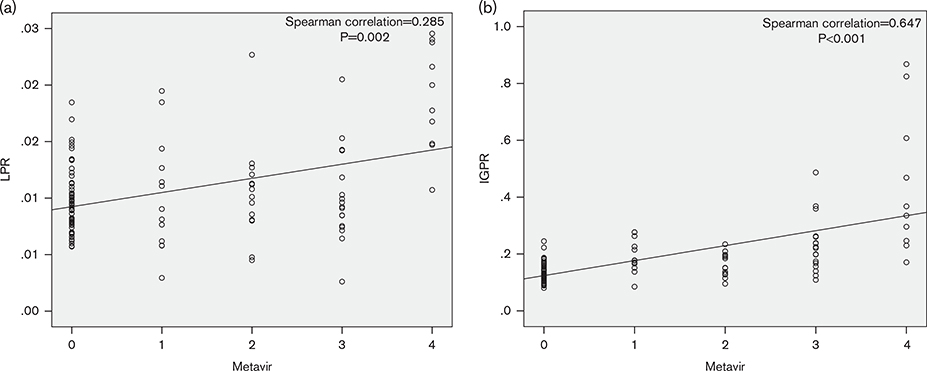
The noninvasive inflammatory markers AAR, LPR, LMR, IGPR, APRI, and FIB-4 correlated significantly with fibrosis stage (P < 0.05), with correlation indices of − 0.249, 0.285, − 0.149, 0.647, 0.841, and 0.704, respectively. Only LNR did not show a correlation (correlation index of 0.158, P = 0.093). The statistical relationships between LPR and IGPR and liver fibrosis stage are shown in Fig. 2.
Fig. 2.
Relationships between LPR and IGPR and liver fibrosis stage. (a) Metavir score system with LPR; (b) Metavir score system with IGPR. IGPR, immunoglobulin-to-platelet ratio; LPR, lymphocyte-to-platelet ratio.
Predictive accuracy between noninvasive inflammatory markers and cirrhosis
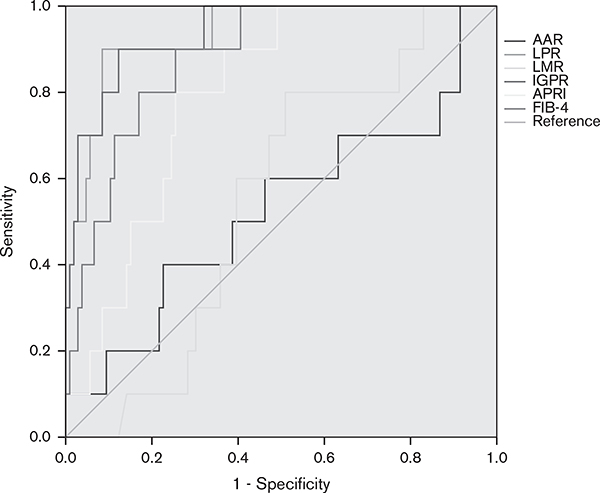
The AUROCs of LPR and IGPR for detecting cirrhosis (F4 vs. F0–F3) were 0.936 [95% confidence interval (CI): 0.870–1.000, P < 0.001] and 0.939 (95% CI: 0.875–1.000, P < 0.001). The AUROCs of AAR, LMR, APRI, and FIB-4 for detecting cirrhosis (F4 vs. F0–F3) were 0.528 (95% CI: 0.319–0.738, P = 0.768), 0.555 (95% CI: 0.409–0.700, P = 0.568), 0.798 (95% CI: 0.694–0.902, P = 0.002), and 0.881 (95% CI: 0.796–0.967, P < 0.001), respectively. The sensitivity and specificity of LPR were 90 and 91.5% according to the optimal cut-off value of 14.67 × 10−3 for predicting cirrhosis. The optimal cut-off value of IGPR was 0.23, with a sensitivity of 90% and a specificity of 87.7%. Both LPR and IGPR were superior to the other noninvasive inflammatory markers tested in predicting cirrhosis (Fig. 3).
Fig. 3.
The receiver operator characteristic curves of noninvasive inflammatory markers for predicting cirrhosis (F4 vs. F0–F3). AAR, aspartate aminotransferase-to-alanine aminotransferase ratio; APRI, aminotransferase-to-platelet ratio index; FIB-4, fibrosis-4 score; IGPR, immunoglobulin-to-platelet ratio; LMR, lymphocyte-to-monocyte ratio; LPR, lymphocyte-to-platelet ratio.
Predictive accuracy between noninvasive inflammatory markers and liver fibrosis
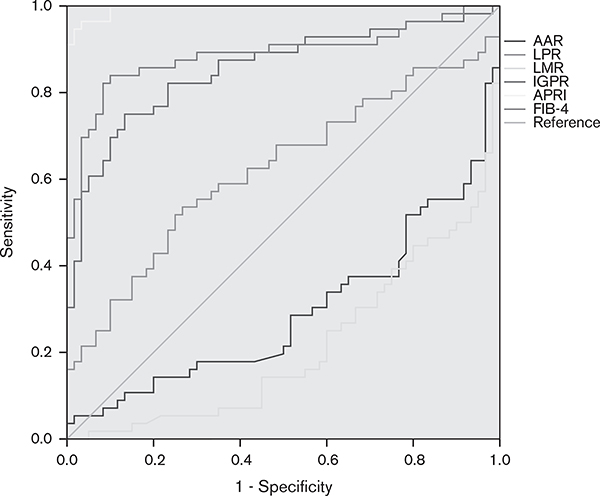
The AUROCs of APRI and FIB-4 for detecting fibrosis (F1–F4 vs. F0) were 0.995 (95% CI: 0.989–1.000, P < 0.001) and 0.889 (95% CI: 0.823–0.955, P < 0.001). The AUROCs of AAR, LPR, LMR, and IGPR for detecting fibrosis (F1–F4 vs. F0) were 0.294 (95% CI: 0.197–0.390, P < 0.001), 0.623 (95% CI: 0.519–0.728, P = 0.022), 0.211 (95% CI: 0.130–0.293, P < 0.001), and 0.854 (95% CI: 0.782–0.926, P < 0.001), respectively. Compared with the other noninvasive inflammatory markers tested, the APRI achieved the highest AUROC, with a sensitivity of 92.9% and a specificity of 98.3% for the detection of liver fibrosis. The sensitivity and specificity of FIB-4 were 82.1 and 91.7%, respectively. The optimal cut-off values of APRI and FIB-4 were 0.4991 and 1.8596, respectively. Both APRI and FIB-4 were better than AAR, LPR, LMR, and IGPR for predicting liver fibrosis (Fig. 4).
Fig. 4.
The receiver operator characteristic curves of noninvasive inflammatory markers for predicting liver fibrosis (F1–F4 vs. F0). AAR, aspartate aminotransferase-to-alanine aminotransferase ratio; APRI, aminotransferase-to-platelet ratio index; FIB-4, fibrosis-4 score; IGPR, immunoglobulin-to-platelet ratio; LMR, lymphocyte-to-monocyte ratio; LPR, lymphocyte-to-platelet ratio.
Univariate and multivariate ordinal logistic regression prediction of liver fibrosis stage
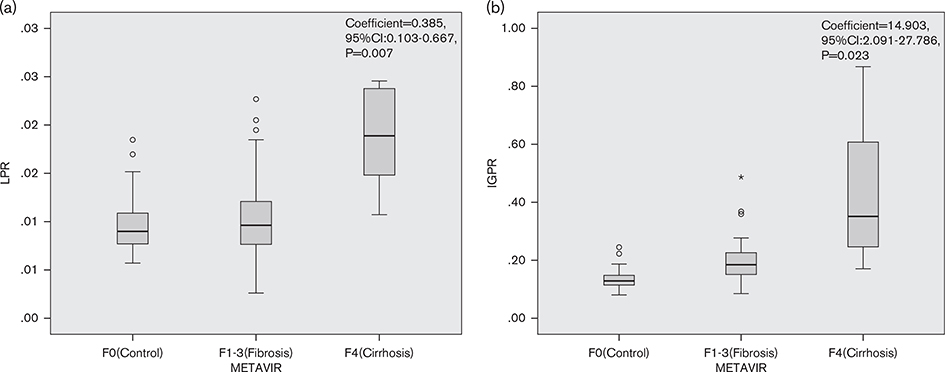
We carried out univariate and multivariate ordinal logistic regression analyses to determine which noninvasive markers were linked to liver fibrosis stage in AIH. Significant associations were found for age, WBC count, neutrophils count, lymphocyte count, monocytes count, PLT count, total bilirubin, ALT level, AST level, γ-glutamyltransferase level, alkaline phosphatase level, albumin level, and globulin level with liver fibrosis stage in the univariate ordinal logistic regression (Supplementary Table, Supplemental digital content 1, http://links.lww.com/EJGH/A411). The LPR and IGPR showed strong independent associations with liver fibrosis stage in the multivariate ordinal logistic regression, with coefficients of 0.385 (95% CI: 0.103–0.667, P = 0.007) and 14.903 (95% CI: 2.091–27.786, P = 0.023), respectively (Fig. 5). We found no independent association of AAR, LMR, APRI, or FIB-4 with liver fibrosis stage in the multivariate analyses (Table 2).
Fig. 5.
Multivariate ordinal logistic regression for predicting severity of liver fibrosis. (a) Metavir score system with LPR; (b) Metavir score system with IGPR. The LPR and IGPR were calculated in F0 (healthy controls), F1–F3 (noncirrhotic fibrosis), and F4 (cirrhosis) based on the results of liver biopsy. CI, confidence interval; IGPR, immunoglobulin-to-platelet ratio; LPR, lymphocyte-to-platelet ratio.
Table 2.
Multivariate ordinal logistic regression analysis on the factors associated with liver fibrosis stage in treatment-naive autoimmune hepatitis patients
| Noninvasive markers | β | 95% confidence intervals | P | |
|---|---|---|---|---|
| AAR | −0.362 | −1.465 | 0.741 | 0.52 |
| LPR (×10−3) | 0.385 | 0.103 | 0.667 | 0.007 |
| LMR | 0.008 | −0.491 | 0.507 | 0.976 |
| IGPR | 14.903 | 2.091 | 27.786 | 0.023 |
| APRI | 0.097 | −0.233 | 0.427 | 0.564 |
| FIB-4 | 0.252 | −0.055 | 0.559 | 0.108 |
The analyses were adjusted for age, white blood cell, neutrophil, lymphocyte, monocyte, platelet, total bilirubin, alanine aminotransferase, aspartate aminotransferase, γ-glutamyltransferase, alkaline phosphatase, albumin, and immune globulin.
AAR, aspartate aminotransferase-to-alanine aminotransferase ratio; APRI, aminotransferase-to-platelet ratio index; FIB-4, fibrosis-4 score; IGPR, immunoglobulin-to-platelet ratio; LMR, lymphocyte-to-monocyte ratio; LPR, lymphocyte-to-platelet ratio.
Discussion
In this retrospective study, we assessed the predictive value of noninvasive inflammatory markers for liver fibrosis, including AAR, LNR, LPR, LMR, APRI, and FIB-4, in a cohort of 55 patients with treatment-naive AIH and 60 healthy controls. We found that the LPR and IGPR were risk factors associated independently with liver fibrosis stage in the AIH patients. The LPR and IGPR were also superior to APRI and FIB-4 in predicting cirrhosis (vs. no-cirrhosis), whereas APRI and FIB-4 were better than LPR and IGPR in predicting liver fibrosis (vs. no-fibrosis).
Here, we report early evidence on the ordinal association of LPR and IGPR (as continuous variables) with liver fibrosis stage in AIH patients. This finding is clinically important because these two noninvasive markers may be used in monitoring the progression or stabilization of liver fibrosis in AIH patients. There was also only a nominal difference between the respective abilities of LPR and IGPR in predicting fibrosis stages. The similar performance of these factors is likely because of their shared denominator of PLT number in their formulas [15,20,26,27]. In addition, our head-to-head comparison showed that FIB-4 and APRI – the two well-known markers of liver fibrosis [9–12] – did not correlate independently with liver fibrosis stage in our AIH cohort. These data suggest that these factors may not be suitable for monitoring the progression of liver fibrosis stage in our AIH cohort, despite their high performance in predicting the presence of fibrosis (vs. no-fibrosis). Certainly, more studies are needed to further compare LPR and IGPR, including investigations into whether LPR and IGPR could also predict liver fibrosis stage in other liver diseases.
Our data on the ordinal association of LPR and IGPR with liver fibrosis stage are consistent with most of the previous studies. For example, LPR (shown as 1/PLR by He et al. [28]) was reportedly lower in patients with chronic hepatitis C virus (HCV) and healthy controls than in patients with HCV-related cirrhosis. Meng et al. [27] also reported that patients with either HCV-related cirrhosis or HCV-related hepatocellular carcinoma had significantly lower PLR levels than either patients with HCV-related hepatitis or healthy controls. For IGPR, globulin may be a meaningful marker for predicting liver inflammation in chronic hepatitis patients, along with other markers [15]. There appears to be a strong association of levels of serum globulin, IgG, and the globulin–platelet model with liver fibrosis stage among patients with chronic viral hepatitis B infection [21,29]. From a clinical point of view, hyperglobulinemia is one of the prominent clinical characteristics of AIH patients, and the decrease in IgG is an important aspect of this disease’s control [1]. IGPR was capable of predicting the stage of liver fibrosis in our study population, which is consistent with the clinical characteristics of AIH. However, no studies reported in the literature have shown the relationship between IGPR and liver fibrosis that was caused by alcoholic liver disease, nonalcoholic fatty liver disease, or drug-induced liver injury. Consistent with our findings, APRI and FIB-4 have also been reported to be remarkably good predictors of fibrosis in patients with nonalcoholic fatty liver and primary biliary cholangitis [22,30], as well as those with viral hepatitis [10,12,31,32]. However, in other studies, APRI and FIB-4 do not predict the liver fibrosis stage in chronic liver diseases well by ordinal logistic regression [33–35], which was similar to our outcomes.
It is important to note that our findings on the noninvasive inflammatory markers for liver fibrosis are contrary to the recent study by Zeng et al. [19] Those authors reported that the PLR level (which is reciprocal of LPR) did not differ between AIH patients and healthy controls, or between AIH patients and patients with cirrhosis [19]. The differences between those findings and ours may be attributable to several reasons. First, the statistical modeling approaches were different. Zeng et al. [19] seemed to have included all markers of interest in a single model, compared F1–F3 versus F4, and did not report the P values and odds ratios of the markers that showed no statistically significant effects (P > 0.05). However, we included only one composite marker at a time and compared F0 versus F1–F3 versus F4 (ordinal outcome). Moreover, in their study, the distribution of PLR in the AIH cirrhosis group was fairly wide, suggesting considerable heterogeneity among the patients investigated. Furthermore, the proportion of their AIH cirrhosis patients was half, and fibrosis stages were not specified in the chronic AIH group. Indeed, the distribution of noncirrhotic fibrosis in our AIH patients was likely different from their (noncirrhotic) AIH patients. In addition, the two studies used different testing instruments, and the standard values of the parameters may be different. Finally, the geographic difference of the study participants (Jiangxi province vs. Shanghai city) may also have contributed to the different findings.
Some strengths of our study are noteworthy. First, to our knowledge, our study represents the first to have included and modeled the three-tier liver fibrosis for AIH patients as the outcome, namely, no fibrosis (healthy controls), noncirrhotic fibrosis, and cirrhosis. Most of the previous studies have reported only the comparison for two of the three stages (F0 vs. F1–F4, F1–3 vs. F4, F0–F3 vs. F4, F1–F2 vs. F3–F4, and others) in chronic liver disease [36–38]. Second, we performed head-to-head comparisons of the AUROCs and adjusted odds ratios of several noninvasive inflammatory markers, including AAR, LPR, LMR, IGPR, APRI, and FIB-4. To our knowledge, ours is now one of the only two comprehensive studies [19] on these markers. Third, our multivariate regression analyses included only one of the markers of interest at a time. This approach allowed for elimination of unnecessary interactions among the markers, while adjusting for other potential confounders. Thus, our approach may be superior to that by Zeng et al. [19] Fourth, all of the liver fibrosis cases were staged by an experienced, expert liver pathologist. Finally, all of our patients were treatment naive. Our study, therefore, likely well represents the natural history of AIH and the real-world data on correlations of markers with liver fibrosis stage.
Our works may shed light on the mechanisms of liver fibrogenesis in AIH. Inflammation is known for its association with fibrosis [3,4]. Moreover, T cells and plasma cells are known to play an essential role in the pathogenesis of AIH [3,39–41]; they also likely contribute to the long-term liver injury and ultimate development of liver fibrosis. Immunoglobulin is also known to be critical for the regulation of the immune system as well as development of AIH [41,42]. Although thrombocytopenia is known to result from hypersplenism in cirrhosis, the relationship between PLT number and degree of fibrosis is not clear. PLTs have been shown to be independent predictors of significant fibrosis by multivariable analysis in chronic hepatitis B [43]. Some researchers have also established PLT-related models to predict the degree of liver fibrosis in chronic hepatitis B [43,44]. However, the association between PLTs and the degree of liver fibrosis in AIH is poorly understood. Our data therefore partially fill this knowledge gap. Of course, more studies are warranted to further elucidate the roles of T cells, PLT, and immunoglobulin in AIH fibrosis.
This study has several limitations that must be considered when interpreting the findings. First, this was a single-center retrospective study, and the results may be biased by measured and unmeasured confounding factors at the patient level. Similarly, all of the liver biopsies were analyzed by a single expert pathologist, although this could provide a benefit in avoidance of observer biases. Second, the patient sample size was small because of the low prevalence of AIH. Third, the unequal sample sizes in each group, especially in the cirrhosis group, which included only 10 cases, may have led to a statistical bias; however, the distribution of F1–F4 liver fibrosis appears to correlate with our clinical experience. Fourth, and related to point three, the small sample size of the cirrhosis group and the different regions from which patients originated may have led to large sample variances. Fifth, we did not include a validation group; the conclusions, therefore, should be confirmed in a prospective multicenter study with a large sample size. Sixth, our study outcome did not differentiate F1–F3, which represent early fibrosis and should be subject to future studies. Finally, sixth, the cross-sectional nature of this study precluded direct examination of the relationship between markers and the progression of liver fibrosis stage during individual patient follow-up.
Conclusion
The LPR and IGPR were identified as independent factors associated with liver fibrosis stage in treatment-naive AIH patients, and may be useful in monitoring the progression of liver fibrosis stage (ordinal order, from F0 to F1–F3 to F4). The LPR and IGPR were also found to be superior to APRI and FIB-4 in detecting cirrhosis versus no-cirrhosis among treatment-naive AIH patients.
Supplementary Material
Footnotes
Conflicts of interest
There are no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.eurojgh.com.
References
- 1.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51:2193–2213. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol 2015; 63:971–1004. [DOI] [PubMed] [Google Scholar]
- 3.Heneghan MA, Yeoman AD, Verma S, Smith AD, Longhi MS. Autoimmune hepatitis. Lancet 2013; 382:1433–1444. [DOI] [PubMed] [Google Scholar]
- 4.Mieli-Vergani G, Vergani D, Czaja AJ, Manns MP, Krawitt EL, Vierling JM, et al. Autoimmune hepatitis. Nat Rev Dis Primers 2018; 4:18017. [DOI] [PubMed] [Google Scholar]
- 5.Krawitt EL. Autoimmune hepatitis. N Engl J Med 2006; 354:54–66. [DOI] [PubMed] [Google Scholar]
- 6.Sahebjam F, Vierling JM. Autoimmune hepatitis. Front Med 2015; 9:187–219. [DOI] [PubMed] [Google Scholar]
- 7.Czaja AJ, Rakela J, Ludwig J. Features reflective of early prognosis in corticosteroid-treated severe autoimmune chronic active hepatitis. Gastroenterology 1988; 95:448–453. [DOI] [PubMed] [Google Scholar]
- 8.Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. J Hepatol 2009; 50:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teshale E, Lu M, Rupp LB, Holmberg SD, Moorman AC, Spradling P, et al. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS). J Viral Hepat 2014; 21:917–920. [DOI] [PubMed] [Google Scholar]
- 10.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 2015; 61:292–302. [DOI] [PubMed] [Google Scholar]
- 11.Yin Z, Zou J, Li Q, Chen L. Diagnostic value of FIB-4 for liver fibrosis in patients with hepatitis B: a meta-analysis of diagnostic test. Oncotarget 2017; 8:22944–22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–36. [DOI] [PubMed] [Google Scholar]
- 13.Su CW, Chan CC, Hung HH, Huo TI, Huang YH, Li CP, et al. Predictive value of aspartate aminotransferase to alanine aminotransferase ratio for hepatic fibrosis and clinical adverse outcomes in patients with primary biliary cirrhosis. J Clin Gastroenterol 2009; 43:876–883. [DOI] [PubMed] [Google Scholar]
- 14.Sheptulina A, Shirokova E, Nekrasova T, Blum H, Ivashkin V. Platelet count to spleen diameter ratio non-invasively identifies severe fibrosis and cirrhosis in patients with autoimmune hepatitis. J Gastroenterol Hepatol 2016; 31:1956–1962. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Xu H, Qu L, Wang X, Wu R, Gao X, et al. Red blood cell distribution width and globulin, noninvasive indicators of fibrosis and inflammation in chronic hepatitis patients. Eur J Gastroenterol Hepatol 2016; 28:997–1002. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999; 31:929–938. [DOI] [PubMed] [Google Scholar]
- 17.Bedossa P, Bioulacsage P, Callard P, Chevallier M. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24:289–293. [DOI] [PubMed] [Google Scholar]
- 18.Du J, Chen S, Shi J, Zhu X, Ying H, Zhang Y, et al. The association between the lymphocyte–monocyte ratio and disease activity in rheumatoid arthritis. Clin Rheumatol 2017; 36:2689–2695. [DOI] [PubMed] [Google Scholar]
- 19.Zeng T, Yu J, Tan L, Wu Y, Tian Y, Wu Q, et al. Noninvasive indices for monitoring disease course in Chinese patients with autoimmune hepatitis. Clin Chim Acta 2018; 486:135–141. [DOI] [PubMed] [Google Scholar]
- 20.Liu XD, Wu JL, Liang J, Zhang T, Sheng QS. Globulin-platelet model predicts minimal fibrosis and cirrhosis in chronic hepatitis B virus infected patients. World J Gastroenterol 2012; 18:2784–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Lu C, Li W, Huang Y, Chen L. Globulin-platelet model predicts significant fibrosis and cirrhosis in CHB patients with high HBV DNA and mildly elevated alanine transaminase levels. Clin Exp Med 2018; 18:71–78. [DOI] [PubMed] [Google Scholar]
- 22.Olmez S, Sayar S, Avcioglu U, Tenlik I, Ozaslan E, Koseoglu HT, et al. The relationship between liver histology and noninvasive markers in primary biliary cirrhosis. Eur J Gastroenterol Hepatol 2016; 28:773–776. [DOI] [PubMed] [Google Scholar]
- 23.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–526. [DOI] [PubMed] [Google Scholar]
- 24.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 25.Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis – update 2015. J Hepatol 2015; 62 (Suppl):S100–S111. [DOI] [PubMed] [Google Scholar]
- 26.Alsebaey A, Elhelbawy M, Waked I. Platelets-to-lymphocyte ratio is a good predictor of liver fibrosis and insulin resistance in hepatitis C virus-related liver disease. Eur J Gastroenterol Hepatol 2018; 30:207–211. [DOI] [PubMed] [Google Scholar]
- 27.Meng X, Wei G, Chang Q, Peng R, Shi G, Zheng P, et al. The platelet-to-lymphocyte ratio, superior to the neutrophil-to-lymphocyte ratio, correlates with hepatitis C virus infection. Int J Infect Dis 2016; 45:72–77. [DOI] [PubMed] [Google Scholar]
- 28.He Q, He Q, Qin X, Li S, Li T, Xie L, et al. The relationship between inflammatory marker levels and hepatitis C virus severity. Gastroenterol Res Pract 2016; 2016:2978479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmilovitz-Weiss H, Tovar A, Halpern M, Sulkes J, Braun M, Rotman Y, et al. Predictive value of serum globulin levels for the extent of hepatic fibrosis in patients with chronic hepatitis B infection. J Viral Hepat 2006; 13:671–677. [DOI] [PubMed] [Google Scholar]
- 30.Sebastiani G, Alshaalan R, Wong P, Rubino M, Salman A, Metrakos P, et al. Prognostic value of non-invasive fibrosis and steatosis tools, hepatic venous pressure gradient (HVPG) and histology in nonalcoholic steatohepatitis. PLoS One 2015; 10:e0128774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cepeda JA, Solomon SS, Srikrishnan AK, Nandagopal P, Balakrishnan P, Kumar MS, et al. Serum fibrosis markers for the diagnosis of liver disease among people with chronic hepatitis C in Chennai, India. Open Forum Infect Dis 2016; 3:ofw156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatar B, Kose S, Pala E, Tatar E. Inflammatory biomarkers and liver histopathology in non-uremic and uremic chronic hepatitis C patients. Acta Medica (Hradec Kralove) 2017; 60:71–75. [DOI] [PubMed] [Google Scholar]
- 33.Shire NJ, Rao MB, Succop P, Buncher CR, Andersen JA, Butt AA, et al. Improving noninvasive methods of assessing liver fibrosis in patients with hepatitis C virus/human immunodeficiency virus co-infection. Clin Gastroenterol Hepatol 2009; 7:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu YR, Lin BB, Zeng DW, Zhu YY, Chen J, Zheng Q, et al. Alpha-fetoprotein level as a biomarker of liver fibrosis status: a cross-sectional study of 619 consecutive patients with chronic hepatitis B. BMC Gastroenterol 2014; 14:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mann JP, De Vito R, Mosca A, Alisi A, Armstrong MJ, Raponi M, et al. Portal inflammation is independently associated with fibrosis and metabolic syndrome in pediatric nonalcoholic fatty liver disease. Hepatology 2016; 63:745–753. [DOI] [PubMed] [Google Scholar]
- 36.Leung DH, Khan M, Minard CG, Guffey D, Ramm LE, Clouston AD, et al. Aspartate aminotransferase to platelet ratio and fibrosis-4 as biomarkers in biopsy-validated pediatric cystic fibrosis liver disease. Hepatology 2015; 62:1576–1583. [DOI] [PubMed] [Google Scholar]
- 37.Kayadibi H, Yasar B, Ozkara S, Serdar MA, Kurdas OO, Gonen C. The diagnostic accuracy of the Forns index, platelet count and AST to platelet ratio index derived fibrosis index for the prediction of hepatitis C virus-related significant liver fibrosis and cirrhosis. Scand J Clin Lab Invest 2014; 74:240–247. [DOI] [PubMed] [Google Scholar]
- 38.Wu HM, Sheng L, Wang Q, Bao H, Miao Q, Xiao X, et al. Performance of transient elastography in assessing liver fibrosis in patients with autoimmune hepatitis-primary biliary cholangitis overlap syndrome. World J Gastroenterol 2018; 24:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli-Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol 2004; 41:31–37. [DOI] [PubMed] [Google Scholar]
- 40.Longhi MS, Ma Y, Mitry RR, Bogdanos DP, Heneghan M, Cheeseman P, et al. Effect of CD4+ CD25+ regulatory T-cells on CD8 T-cell function in patients with autoimmune hepatitis. J Autoimmun 2005; 25: 63–71. [DOI] [PubMed] [Google Scholar]
- 41.Liberal R, Krawitt EL, Vierling JM, Manns MP, Mieli-Vergani G, Vergani D. Cutting edge issues in autoimmune hepatitis. J Autoimmun 2016; 75:6–19. [DOI] [PubMed] [Google Scholar]
- 42.Vierling JM. Autoimmune hepatitis and overlap syndromes: diagnosis and management. Clin Gastroenterol Hepatol 2015; 13:2088–2108. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Yan X, Yang Y, Chang H, Jia B, Zhao XA, et al. A novel predictive model using routinely clinical parameters to predict liver fibrosis in patients with chronic hepatitis B. Oncotarget 2017; 8: 59257–59267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu YC, Liu H, Liu XY, Ma LN, Guan YH, Luo X, et al. Value of gamma-glutamyltranspeptidase-to-platelet ratio in diagnosis of hepatic fibrosis in patients with chronic hepatitis B. World J Gastroenterol 2017; 23:7425–7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.