Sterols are essential membrane components in eukaryotes, and sterol synthesis inhibitors can have potent effects against pathogenic fungi and trypanosomatids. Understanding the roles of sterols will facilitate the development of new drugs and counter drug resistance. LSO is required for the formation of the C-5–C-6 double bond in the sterol core structure in mammals, fungi, protozoans, plants, and algae. Functions of this C-5–C-6 double bond are not well understood. In this study, we generated and characterized a lathosterol oxidase-null mutant in Leishmania major. Our data suggest that LSO is vital for the structure and membrane-stabilizing functions of leishmanial sterols. In addition, our results imply that while mutations in lathosterol oxidase can confer resistance to amphotericin B, an important antifungal and antiprotozoal agent, the alteration in sterol structure leads to significant defects in stress response that could be exploited for drug development.
KEYWORDS: Leishmania, amphotericin, drug resistance mechanisms, lipid synthesis, sterols, stress response
ABSTRACT
Lathosterol oxidase (LSO) catalyzes the formation of the C-5–C-6 double bond in the synthesis of various types of sterols in mammals, fungi, plants, and protozoa. In Leishmania parasites, mutations in LSO or other sterol biosynthetic genes are associated with amphotericin B resistance. To investigate the biological roles of sterol C-5–C-6 desaturation, we generated an LSO-null mutant line (lso−) in Leishmania major, the causative agent for cutaneous leishmaniasis. lso− parasites lacked the ergostane-based sterols commonly found in wild-type L. major and instead accumulated equivalent sterol species without the C-5–C-6 double bond. These mutant parasites were replicative in culture and displayed heightened resistance to amphotericin B. However, they survived poorly after reaching the maximal density and were highly vulnerable to the membrane-disrupting detergent Triton X-100. In addition, lso− mutants showed defects in regulating intracellular pH and were hypersensitive to acidic conditions. They also had potential alterations in the carbohydrate composition of lipophosphoglycan, a membrane-bound virulence factor in Leishmania. All these defects in lso− were corrected upon the restoration of LSO expression. Together, these findings suggest that the C-5–C-6 double bond is vital for the structure of the sterol core, and while the loss of LSO can lead to amphotericin B resistance, it also makes Leishmania parasites vulnerable to biologically relevant stress.
IMPORTANCE Sterols are essential membrane components in eukaryotes, and sterol synthesis inhibitors can have potent effects against pathogenic fungi and trypanosomatids. Understanding the roles of sterols will facilitate the development of new drugs and counter drug resistance. LSO is required for the formation of the C-5–C-6 double bond in the sterol core structure in mammals, fungi, protozoans, plants, and algae. Functions of this C-5–C-6 double bond are not well understood. In this study, we generated and characterized a lathosterol oxidase-null mutant in Leishmania major. Our data suggest that LSO is vital for the structure and membrane-stabilizing functions of leishmanial sterols. In addition, our results imply that while mutations in lathosterol oxidase can confer resistance to amphotericin B, an important antifungal and antiprotozoal agent, the alteration in sterol structure leads to significant defects in stress response that could be exploited for drug development.
INTRODUCTION
Leishmaniasis is the second most deadly parasitic disease, after malaria, with more than 12 million people infected worldwide (1). The causative agents belong to a group of trypanosomatid protozoans known as Leishmania. In the sandfly vector, Leishmania parasites are flagellated, extracellular promastigotes, whereas in the mammalian host, they are nonflagellated, intracellular amastigotes (2). Current treatments are limited by toxic side effects, and resistance is on the rise (3). Without a safe vaccine, it is necessary to identify new drug targets, develop new treatments, and decipher the mechanism of drug resistance in Leishmania (4).
The biosynthesis of sterol is an important pathway for most eukaryotes. In mammals, the dominant type of sterol is cholesterol, a vital membrane component that is also the precursor of steroid hormones (5). In fungi and trypanosomatids, ergostane-based sterols, such as ergosterol and 5-dehydroepisterol, are synthesized in high abundance and play roles equivalent to those of cholesterol in cellular membranes (6, 7). Ergosterol differs from cholesterol in the presence of two more double bonds: one at C-7–C-8 on the B ring and the other at C-22–C-23 on the side chain (see Fig. S1 in the supplemental material) (8). In addition, ergosterol has an extra methyl group at the C-24 position (Fig. S1). These structural differences make sterol biosynthesis a desirable source for antifungal and antitrypanosomatid drug targets.
Sterol biosynthesis pathway in L. major WT and lso− parasites. I to VII represent sterol intermediates or final products (underlined). I, lanosterol; II, zymosterol; III, fecosterol; IV, episterol; V, 5-dehydroepisterol; VI, ergosterol; VII, ergosta-7,22-dienol. C14DM, sterol C14α demethylase. SMT, Sterol C24-methyl transferase. LSO, lathosterol oxidase. Download FIG S1, PDF file, 0.4 MB (402.8KB, pdf) .
Copyright © 2020 Ning et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Amphotericin B (Amp B) is a polyene antibiotic that binds to ergostane-based sterols on the plasma membrane of pathogenic fungi or Leishmania, leading to pore formation and the accumulation of reactive oxygen species (ROS) (9–11). It has been used successfully to treat antimony-resistant leishmaniasis and in patients coinfected with Leishmania spp. and human immunodeficiency virus (11, 12). However, resistance to Amp B has been reported both in the laboratory and in clinical isolates (13–16). Multiple Amp B-resistant Leishmania lines show altered sterol composition and mutations in sterol biosynthetic enzymes, such as the sterol C-24-methyltransferase (SMT; EC 2.1.1.41) and sterol C-14-alpha-demethylase (C14DM; EC 1.14.13.70) (13–16).
To interrogate the roles of these enzymes in L. major, we generated null mutants of C14DM (c14dm−) and SMT (smt−) using the targeted gene deletion approach (17). Both c14dm− and smt− mutants lack ergostane-based sterols but are viable in culture and highly resistant to Amp B (18, 19). C14dm− mutants are extremely sensitive to heat and highly attenuated in virulence (19). They also display altered morphology, cytokinesis defects, and increased plasma membrane fluidity (19). In comparison, defects exhibited by smt− mutants, including elevated mitochondrial membrane potential and superoxide level, are less drastic (18). Interestingly, both c14dm− and smt− mutants show altered expression of lipophosphoglycan (LPG), a glycosylphosphatidylinositol (GPI)-anchored virulence factor (20). Compared to L. major wild-type (WT) parasites, the cellular level of LPG appears to be much lower in c14dm− but higher in smt− mutants (18, 19). These findings suggest that loss-of-function mutations in C14DM and SMT can lead to Amp B resistance but result in significant defects in stress response and virulence.
In addition to C14DM and SMT, mutations in the gene encoding lathosterol oxidase (LSO) are also implicated in Amp B resistance in Leishmania and Candida spp. (15, 21, 22). LSO (also called sterol C-5-desaturase) catalyzes the formation of the C-5–C-6 double bond in the B ring of sterol intermediates, a late step in sterol synthesis (Fig. S1) (23). Orthologs of LSO have been identified in mammals, yeast, protozoans, plants, and algae (15, 23–26). In Saccharomyces cerevisiae, the activity of LSO (encoded by ERG3) is sensitive to cyanide and requires iron, NAD(P)H, and molecular oxygen (27). LSOs from yeast and Tetrahymena thermophila exhibit dependence on cytochrome b5 and cytochrome b5 reductase, suggesting that this desaturation reaction shares similarity to the sterol C-4- and C-14-demethylation steps (27–30) (Fig. S1).
In S. cerevisiae, deletion or inactivation of ERG3/LSO results in the accumulation of episterol and depletion of ergosterol, and the null mutants fail to grow in the absence of heme synthesis (23, 31) (Fig. S1). In addition, ERG3/LSO mutants are unable to utilize respiratory substrates, such as glycerol, acetate, and ethanol (25). Furthermore, LSO expression contributes to tolerance to high temperature and acidic pH in fission yeast (32). These studies allude to the functions of LSO in regulating respiration and stress response in fungi, although the mechanism of action is not well understood.
While LSO mutations were associated with Amp B resistance in fungi (21, 22), knockout mutants of LSO/Erg3 failed to display such resistance in Candida albicans and Aspergillus fumigatus (33, 34), questioning whether LSO-null mutation alone is sufficient to cause Amp B resistance. Studies on LSO in trypanosomatids are scarce. In light of its potential involvement in the development of Amp B resistance (15), it is necessary to characterize LSO in Leishmania and determine whether it is essential in the promastigote and amastigote stages and whether it is required for the sensitivity of Leishmania to sterol synthesis inhibitors or Amp B. To address these questions, we generated LSO-null mutants in L. major. Our results indicate that LSO deletion confers resistance to Amp B, but the mutants show poor survival and reduced growth rate under the acidic condition due to their inability to maintain intracellular pH. In addition, LSO deletion altered the structure of LPG and reduced parasite virulence in mice. These findings shed new light on the roles of sterol synthesis in Leishmania stress response and reveal the fitness costs associated with the development of drug resistance.
RESULTS
Genetic deletion and cellular localization of L. major LSO.
The L. major LSO gene (TriTrypDB entry LmjF.23.1300) is located on chromosome 23 with 40% identity to S. cerevisiae ERG3p (gene ID 850745) and 38% identity to human sterol C-5-desaturase (GenBank accession no. BAA33729.1). The predicted open reading frame (ORF) contains 302 amino acids with four transmembrane helices and no obvious signal peptide, and it is expected to catalyze the formation of a double bond between C-5 and C-6 in the B ring of sterol intermediates (see Fig. S1 in the supplemental material).
To investigate the roles of the sterol C-5–C-6 desaturation reaction in L. major, we replaced the endogenous LSO alleles with nourseothricin (SAT) and blasticidin (BSD) resistance genes using the homologous recombination approach (17). The resulting LSO-null (lso−) mutants were verified by Southern blotting with an ORF probe and a 5′-flanking sequence probe (Fig. 1A). To complement the null mutants, we introduced an LSO-expressing plasmid (pXG-LSO) into the lso− mutant to generate the lso−/+LSO mutant (the add-back strain). To examine the cellular localization of LSO, the C terminus of LSO was fused to green fluorescent protein (GFP) and introduced into the lso− mutant (lso−/+LSO-GFP) (Fig. S2A). In immunofluorescence microscopy, LSO-GFP showed a distribution similar (∼72% overlap) to that of BiP, an endoplasmic reticulum (ER) marker (35) (Fig. 1B to F), suggesting that LSO is primarily located at the ER. This result is similar to the localizations of C14DM and SMT in Leishmania (18, 19) as well as LSO in T. thermophila (28).
FIG 1.
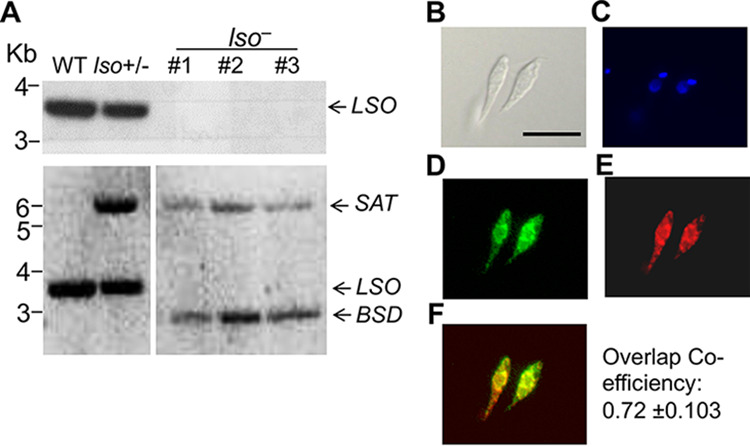
Genetic knockout and cellular localization of LSO. (A) Genomic DNA samples from L. major LV39 WT, LSO+/− (heterozygous knockout), and lso− (homozygous knockout clones 1 to 3) parasites were processed for Southern blot analyses, using radioactive probes from the open reading frame (top) or upstream flanking region (bottom) of LSO. Bands corresponding to LSO and drug resistance genes (BSD/SAT) are indicated. (B to F) Immunofluorescence microscopy of lso−/+LSO-GFP promastigotes. (B) Differential interference contrast (scale bar, 10 μm). (C) DNA staining with Hoechst. (D) GFP fluorescence. (E) Anti-BiP (an ER marker) staining followed by goat anti-rabbit IgG-Texas Red. (F) Merge of panels D and E. Overlap between GFP and ER was calculated using JACoP Image J analysis from 30 cells.
LSO-GFP expression complements lso− mutants. (A) Whole-cell lysates from log-phase promastigotes of lso−/+LSO-GFP (clones 1 and 2). c14dm−/+C14DM-GFP and WT were analyzed by Western blotting (upper, anti-GFP; lower, anti-α-tubulin). (B to E) Partial GC-MS spectra of lipids from WT (B), lso− (C), lso−/+LSO (D), and lso−/+LSO-GFP (E) promastigotes. The blue dashed lines indicate the shift of peaks in lso− mutants compared to WT and add-backs. Download FIG S2, PDF file, 0.7 MB (736.7KB, pdf) .
Copyright © 2020 Ning et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
lso− promastigotes have altered sterol composition.
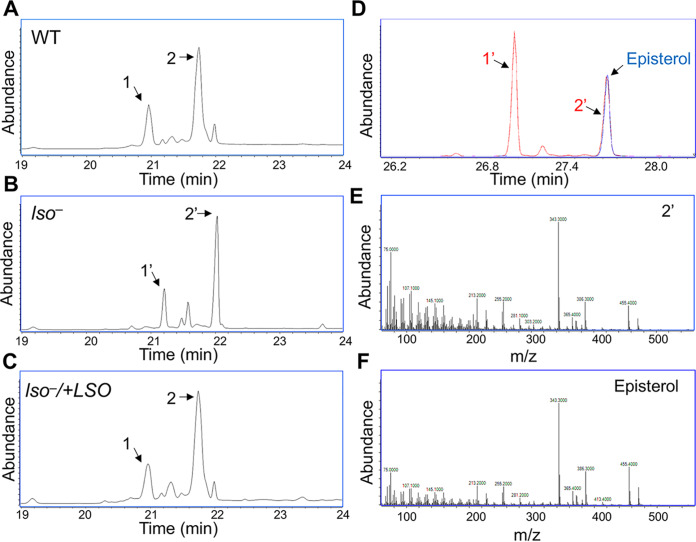
Sterols from promastigotes were converted into trimethylsilyl (TMS) derivatives, followed by electron ionization (EI) gas chromatography-mass spectrometry (GC-MS) analysis. Sterol molecules were identified based on their molecular weights, retention times, and EI spectra. Consistent with the findings we previously reported, L. major WT promastigotes contained two main sterols, i.e., ergosterol and 5-dehydroepisterol, represented by peaks 1 and 2, respectively, in Fig. 2A (18, 19). Interestingly, all the sterols from lso− promastigotes were shifted to the right in the GC chromatogram, including two dominant peaks, 1′ and 2′ (Fig. 2B and Fig. S2B and C). While ergosterol and 5-dehydroepisterol had the expected molecular weight of 468.5 as TMS derivatives, the dominant sterols from the lso− mutant (1′ and 2′) had the molecular weight of 470.5 as TMS derivatives (Fig. 2D). By library search, peak 1′ and peak 2′ matched ergosta-7,22-dien-3-ol, (3β,22E)- and episterol-TMS derivatives, respectively. While we did not have pure ergosta-7,22-dien-3-ol (3β,22E) (not available commercially) to confirm the structure of peak 1′, the retention time and the EI mass spectrum of peak 2′ in lso− were identical to those of the episterol standard (Fig. 2D to F). These findings are consistent with the role of LSO in catalyzing the C-5–C-6 desaturation reaction to form 5-dehydroepisterol (Fig. S1). GC-MS analysis on the area ratios of internal standard peak and leishmanial sterol peaks revealed no significant difference in total cellular sterol abundance between WT and lso− promastigotes. Importantly, the introduction of LSO or LSO-GFP into the lso− strain restored the sterol profile to WT-like composition (Fig. 2C and Fig. S2). Taken together, these results support the identity of L. major LSO as a sterol C-5-desaturase.
FIG 2.
GC-MS analyses of sterol TMS derivatives show altered sterol profile in lso− mutants. (A to C) Total ion current chromatograms plotted from 19.0- to 24.0-min scans (mass range, m/z 50 to 550) of the sterol TMS derivatives of LV39 WT (A), lso− (B), and lso−/+LSO (C) strains. In panels A and C, peaks 1 and 2 represent ergosterol and 5-dehydroepisterol, respectively. In panel B, the peaks including 1′ and 2′ are shifted to the right. (D) The reconstructed ion chromatogram of the M+ ion (m/z 470.5) from full GC-MS scans (m/z 50 to 550) of the lso− sample (trace in red) and from the episterol standard (trace in blue). In addition to the perfect match of the retention time of peak 2′ with the episterol standard, the full-scan EI mass spectra (70 eV) plotted from peak 2′ (E) and the episterol standard (F) are also identical, confirming that peak 2′ is episterol.
lso− mutants are replicative in culture but show poor survival in stationary phase.
The LSO-null mutants were fully viable in culture, with a doubling time of ∼8 h during the log phase, and could reach a maximal density of 2 × 107 to 3 × 107 cells/ml, similar to WT and add-back parasites (Fig. 3A). However, after reaching maximal density, lso− promastigotes showed significantly reduced viability in the stationary phase. First, we measured the percentage of cells whose long axis was less than twice the length of the short axis. Such a round shape was indicative of cells under duress. In early stationary phase (stationary days 1 to 2), 18 to 32% of lso− promastigotes were round, whereas only 2 to 9% of WT and add-back cells were round (Fig. 3B). The difference became less pronounced in late stationary stage (stationary days 3 to 4) when the percentages of round cells increased among WT and add-back parasites (Fig. 3B). Similarly, we observed a much higher percentage of dead cells in lso− mutants (28 to 38%) than in WT and add-back parasites (3 to 14%) from stationary day 2 to day 3 (Fig. 3C). We also examined the ability of lso− mutants to form metacyclics, which are the nondividing and infective form of promastigotes (36). lso− produced 40 to 50% fewer metacyclics than WT and add-back parasites in stationary phase. In conclusion, LSO is not required for the survival or replication of log-phase promastigotes but is important for maintaining viability during the stationary phase.
FIG 3.
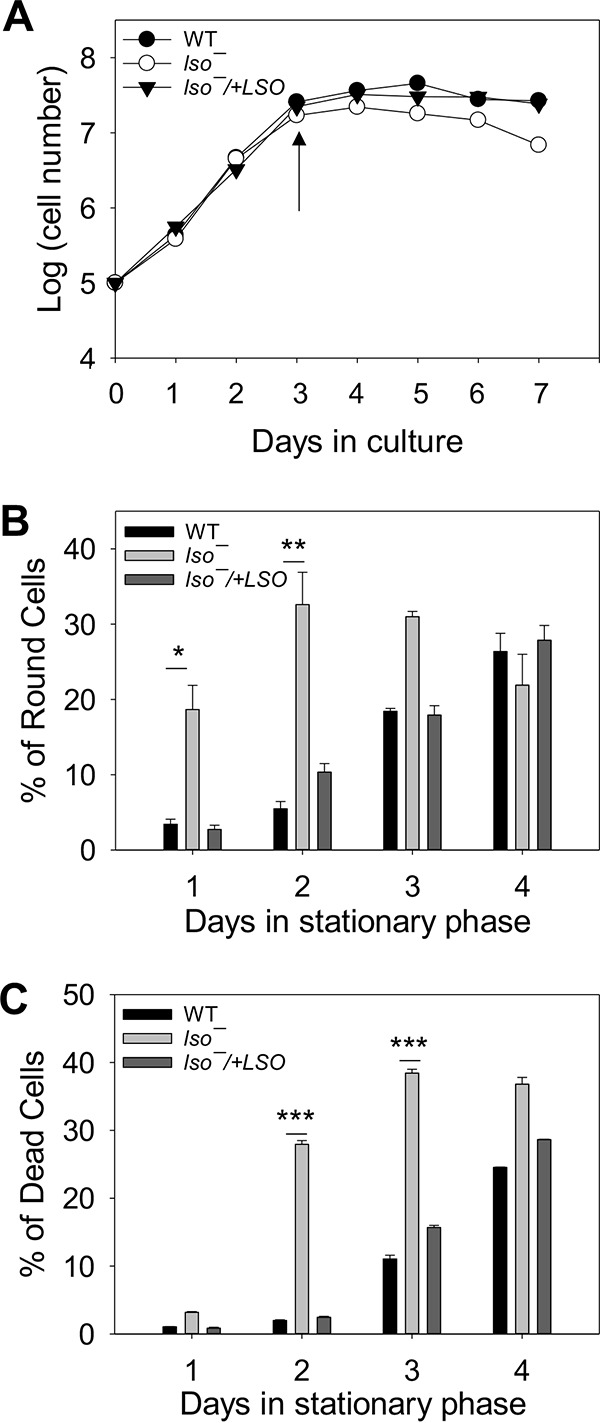
lso− mutants show poor survival in stationary phase. (A) Promastigotes were inoculated in M199 medium at 1 × 105/ml, and cell densities were determined daily. The arrow marks the onset of stationary phase. (B and C) Percentage of round cells (B) and dead cells (C) were measured during days 1 to 4 in stationary phase. Error bars represent standard deviations from 3 experiments (**, P < 0.01; ***, P < 0.001).
lso− promastigotes show increased resistance to Amp B and are hypersensitive to Triton X-100.
Amp B is a potent drug that interacts with ergosterol or ergosterol-like sterols on the plasma membrane, resulting in pore formation, oxidant accumulation, and cell death (9–11, 37). Alterations in sterol biosynthesis can confer resistance to Amp B (14, 16). In Candida lusitaniae, the Amp B-resistant clinical isolates showed reduced ERG3 gene expression, suggesting that the C-5–C-6 double bond contributes to the binding of Amp B to membrane sterol (22). In another report, mutations in sterol C-5-desaturase (LSO) were found to be associated with Amp B resistance in Leishmania mexicana (15). Here, we measured the sensitivity of lso− promastigotes to Amp B in liquid culture by growing cells in various concentrations of Amp B for 48 h (Fig. 4A). The effective concentrations to inhibit 25%, 50%, and 90% of growth (EC25, EC50, and EC90, respectively) were determined using cells grown in the absence of Amp B as a control (Table 1). Compared to WT and lso−/+LSO promastigotes, lso− mutants were 2 to 4 times more resistant to Amp B (Fig. 4A and Table 1). The increase in Amp B resistance was close to that of the smt− mutants (18) but not as pronounced as that of the c14dm− mutants (10 to 100 times more resistant than the WT). This result is in agreement with the notion that C-5–C-6 desaturation enhances the binding between membrane sterol and Amp B. Meanwhile, the susceptibility of lso− mutants to itraconazole, an inhibitor of C14DM (19), was similar to that of WT and lso−/+LSO parasites (Fig. 4B).
FIG 4.
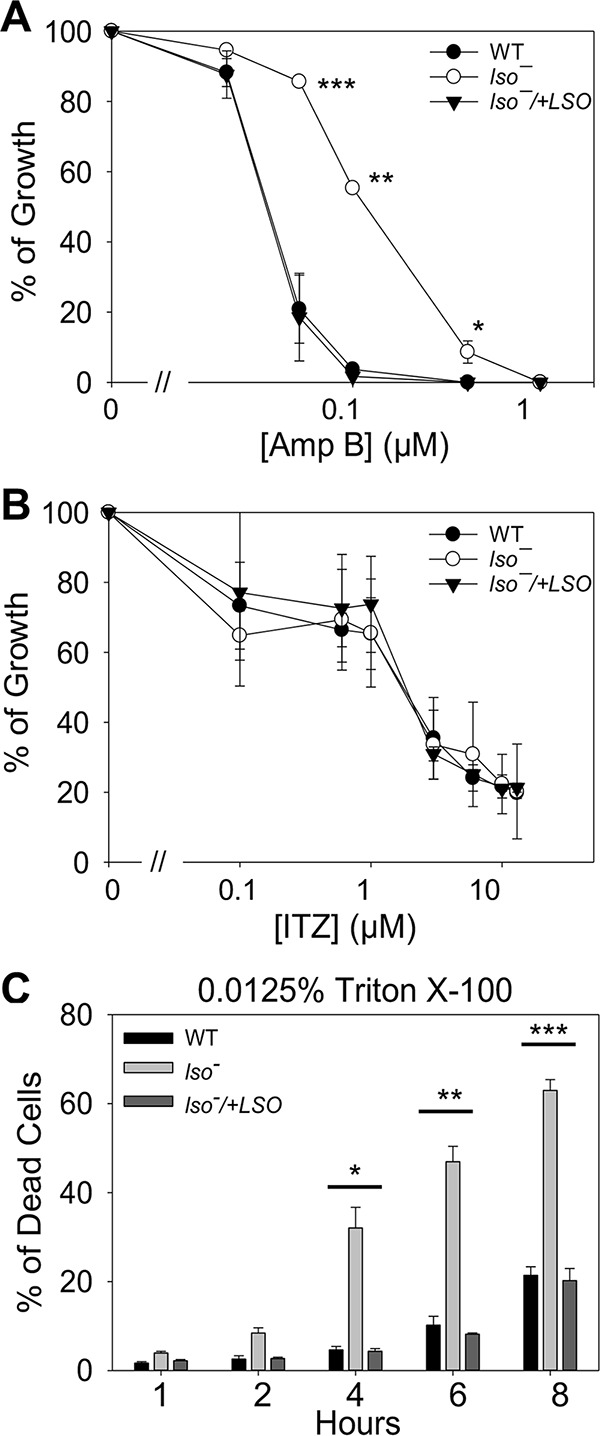
lso− mutants are resistant to Amp B and hypersensitive to Triton X-100. (A and B) Log-phase promastigotes were inoculated into M199 medium with different concentrations of Amp B (A) or ITZ (B). Cells grown in the absence of drugs were used as controls, and percentages of growth were calculated after 48 h. (C) Log-phase promastigotes were incubated in the presence of 0.0125% Triton X-100 for 1 to 8 h. Cell viability was determined by flow cytometry. Error bars represent standard deviations from 3 to 4 experiments (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
TABLE 1.
lso− mutants show reduced sensitivity to Amp Ba
| EC25 (μM ± SD) | EC50 (μM ± SD) | EC90 (μM ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|
| WT | lso− | WT/lso− | WT | lso− | WT/lso− | WT | lso− | WT/lso− |
| 0.034 ± 0.003 | 0.077 ± 0.004 | 1/2.24 | 0.046 ± 0.003 | 0.133 ± 0.005 | 1/2.89 | 0.084 ± 0.005 | 0.303 ± 0.033 | 1/3.61 |
EC25, EC50, and EC90 (averages ± standard deviations from 3 independent experiments) were calculated through comparison to control cells grown in the absence of Amp B.
We also examined whether the change in sterol composition could alter the plasma membrane stability in lso− mutants. Log-phase promastigotes were incubated in regular medium containing 0.0125% Triton X-100, and percentages of dead cells were monitored over time. As shown in Fig. 4C, lso− exhibited hypersensitivity to Triton X-100 after 4 h, and the defect was rescued by the introduction of LSO. This finding resembles our previous observation in the c14dm− mutants, which are unable to form detergent-resistant membrane fractions (19). Therefore, alteration in sterol composition may cause hypersensitivity to detergent-induced plasma membrane disruption in L. major.
LSO is required for promastigote survival and optimal growth under acidic conditions.
When promastigotes are transmitted from the sandfly vector to the mammalian host, they encounter elevated temperature, acidic pH, and oxidative bursts (38). Here, we investigated whether LSO is required for parasites to survive under stress conditions. First, promastigotes were inoculated in complete medium at pH 7.4 (the regular pH) or pH 5.0 for 0 to 60 h to examine their tolerance to acidic stress. While WT and lso−/+LSO cells showed good viability at pH 5.0 (<8% death), 25 to 30% of lso− mutants died after 8 to 12 h (Fig. 5A and B). The dead cell percentage in lso− mutants went down after 24 h (although still higher than those of WT and lso−/+LSO strains), which was likely due to the rapid lysis of dead cells (Fig. 5B). Next, we evaluated the ability of lso− promastigotes to withstand heat stress by increasing the culture temperature from 27°C to 37°C. As indicated in Fig. 5C, no significant difference was detected until 48 h into the temperature shift, when lso− mutants showed ∼2 times more dead cells than WT and add-back parasites. We also incubated promastigotes in phosphate-buffered saline (PBS) to assess their resistance to starvation, and results indicated that the lso− mutants responded similarly to WT parasites (Fig. 5D).
FIG 5.
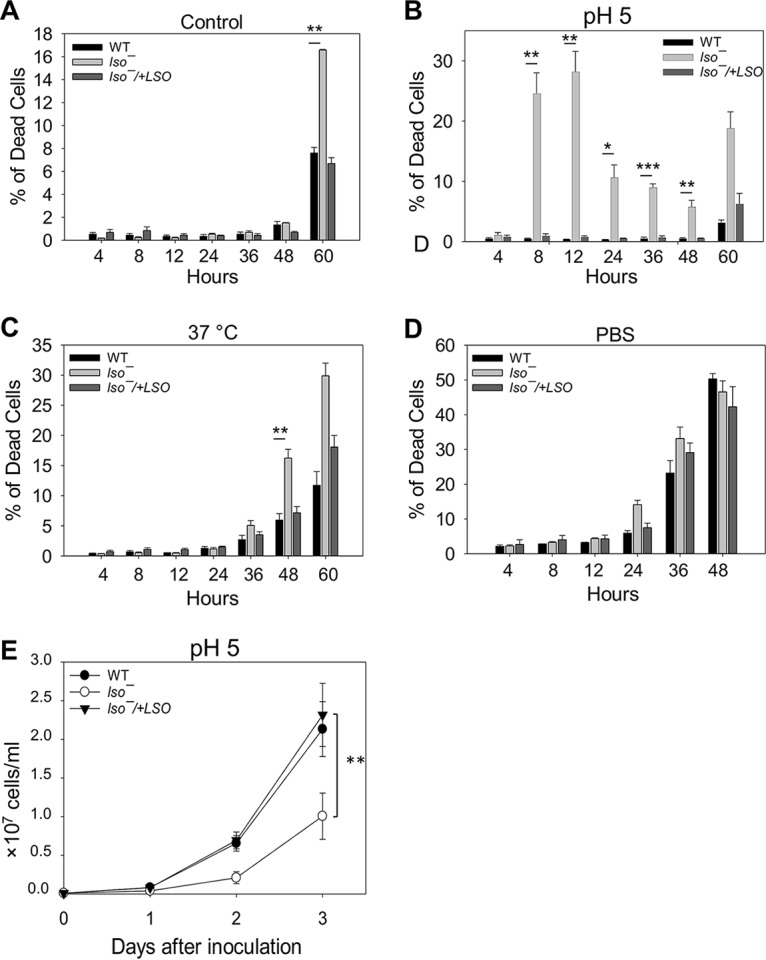
lso− mutants show poor viability under acidic and heat stress. (A to D) Log-phase promastigotes were incubated at 5 × 106 cells/ml in M199 medium (A to C) or PBS (D) under neutral (A, C, and D) or acidic (B) conditions at either 27°C (A, B, and D) or 37°C (C). Percentages of dead cells were determined at the indicated times. (E) Log-phase promastigotes were inoculated into an acidic medium (pH 5.0) at 1 × 105 cells/ml, and culture densities were determined daily. Error bars represent standard deviations from 4 experiments (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Consistent with their hypersensitivity to acidic pH, lso− promastigotes proliferated slowly in the pH 5.0 medium (Fig. 5E). Overall, the mutants’ poor survival and growth delay at pH 5 suggest that LSO is crucial for L. major promastigotes to tolerate acidic stress and affect parasite growth in the phagolysosome (39).
LSO contributes to intracellular pH homeostasis.
Their hypersensitivity to acidic conditions prompted us to examine whether lso− mutants can regulate intracellular pH. When cultivated in the regular medium (pH 7.4), lso− mutants had a slightly lower intracellular pH than WT parasites (7.5 versus 7.8) (Table 2). However, when grown in an acidic medium (pH 5.0), the intracellular pH of the lso− mutant dropped to 7.0, whereas in WT and lso−/+LSO parasites it remained at 7.8 to 7.9 (Table 2). This finding argues that alteration in sterol composition can affect the cytosolic pH homeostasis in L. major.
TABLE 2.
LSO is required for the maintenance of intracellular pHa
| Cell type | Intracellular pH at medium pH of: |
|||
|---|---|---|---|---|
| 7.4 |
5.0 |
|||
| Mean | SD | Mean | SD | |
| WT | 7.76 | 0.265 | 7.90*** | 0.122 |
| lso− | 7.48 | 0.265 | 7.05*** | 0.031 |
| lso−/+LSO | 7.83 | 0.124 | 7.78 | 0.130 |
Promastigotes were cultivated in the regular medium (pH 7.4) or acidic medium (pH 5.0) for 3 days, and intracellular pH values were measured after labeling with 10 μM of BCECF-AM for 30 min. Mean values and standard deviations (SDs) were calculated from 3 experiments (***, P < 0.001).
The plasma membrane is the first barrier against the change of extracellular pH. Hypersensitivity of lso− mutants to Triton X-100 (Fig. 4C) suggests that their plasma membrane is less stable, thereby affecting their ability to control intracellular pH. Besides plasma membrane, certain intracellular organelles and proteins are essential for intracellular pH homeostasis as well (40). Acidocalcisomes are electron-dense acidic organelles rich in divalent cations and polyphosphate first identified in trypanosomatids (41). They play important roles in calcium homeostasis, osmoregulation, and the maintenance of intracellular pH (41, 42). The acidocalcisome has a vacuolar-type H+-pyrophosphatase (VP1) that is involved in the uptake of H+ from the cytosol into acidocalcisomes and regulates intracellular pH homeostasis (43, 44). After labeling stationary-phase or metacyclic-like promastigotes with an anti-T. brucei VP1 antibody, we observed a 40 to 45% reduction in fluorescence intensity in lso− mutants compared to that of WT and add-back parasites (Fig. 6A and B), suggesting that LSO is involved in the expression and/or localization of VP1 at the acidocalcisome. We did not detect any significant difference in acidocalcisome morphology, abundance, or contents in short-chain and long-chain polyphosphate between lso− and WT parasites (Fig. S3A and B and data not shown). From these analyses, we postulate that compromised expression of VP1 along with increased plasma membrane instability in lso− parasites contribute to their inability to maintain intracellular pH when challenged with acidic stress.
FIG 6.
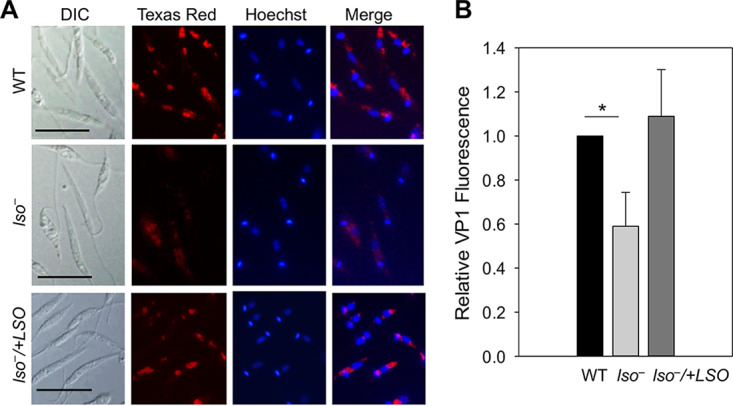
lso− mutants express less vacuolar proton pyrophosphatase (VP1). (A) Day 3 stationary-phase promastigotes were labeled with rabbit anti-TbVP1 antiserum (1:800), followed by anti-rabbit IgG-Texas Red (1:1,000). DNA was stained with Hoechst. DIC, differential interference contrast; merge, the merge of Texas Red and Hoechst images. Scale bar, 10 μm. (B) Relative levels of VP1 staining were determined from 100 metacyclic-like promastigotes for each line. Error bars represent standard deviations from 3 experiments (*, P < 0.05).
Short-chain and long-chain phosphate contents are not changed in lso− mutants. Acidocalcisomes from log phase or day 3 stationary-phase promastigotes of WT, lso−, and lso−/+LSO strains were isolated and adjusted to an equivalence of 1.3 × 109 cells/ml. The concentrations of short-chain polyphosphate (A) and long-chain polyphosphate (B) were determined as previously described. Error bars represent standard deviations from 3 repeats. Download FIG S3, PDF file, 0.3 MB (270.4KB, pdf) .
Copyright © 2020 Ning et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Depletion of LSO alters the expression and structure of LPG.
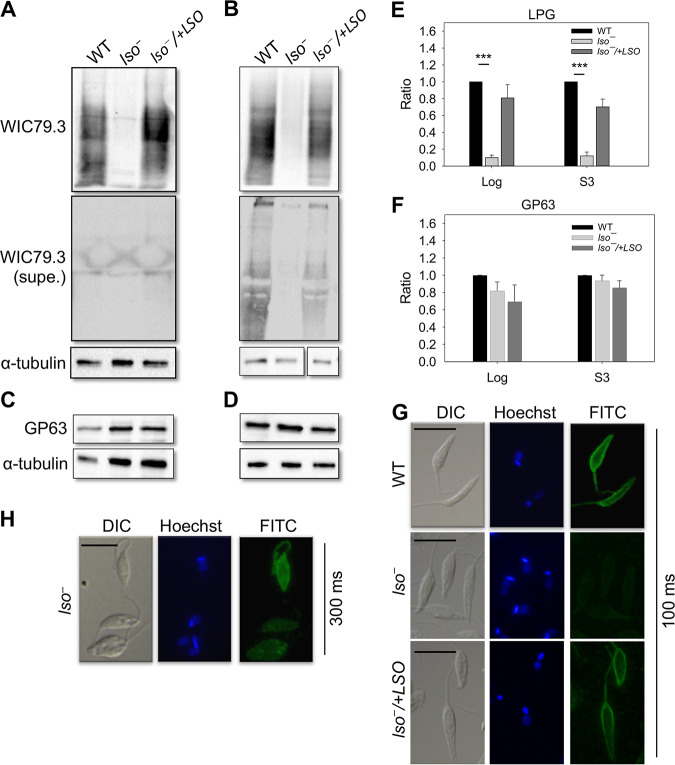
Sterols are enriched in the ordered microdomains (lipid rafts) along with sphingolipids and glycosylphosphatidylinositol (GPI)-anchored proteins on the plasma membrane (45). Previous work on c14dm− and smt− mutants demonstrates that the alteration of sterol synthesis can affect the expression level of membrane-bound GPI-anchored virulence factors, such as LPG and GP63 (a metalloprotease) (18, 19). To determine the role of LSO in the synthesis of GPI-anchored glycoconjugates, we performed Western blot and immunofluorescence microscopy assays using the WIC79.3 monoclonal antibody, which recognizes the terminal Gal (β1,3) subunits on the side chains branching off the Gal (β1,4)-Man (α1)-PO4 repeat units of L. major LPG (46, 47). As illustrated in Fig. 7A and B, whole-cell lysates from log-phase and stationary-phase lso− parasites appeared to have less LPG than WT and lso−/+LSO parasites (10 to 20% of the WT level) (Fig. 7E). The reduction was not due to increased release of LPG from lso− parasites into the culture supernatant (Fig. 7A and B). Immunofluorescence microscopy assay confirmed this finding in lso− mutants (Fig. 7G and H). Using the same WIC79.3 antibody, WT and add-back parasites displayed robust surface labeling at an exposure time of 100 ms (Fig. 7G). In contrast, signals from lso− mutants were only detectable at a longer exposure time showing significant intracellular staining (Fig. 7H). Therefore, the expression of LPG was clearly altered in lso− parasites. Meanwhile, these mutants showed levels of GP63 similar to those of WT and add-back parasites (Fig. 7C, D, and F).
FIG 7.
LSO is required for the synthesis of WT-like LPG. (A to D) Whole-cell lysates or culture supernatants (supe.) from log-phase (A and C) or day 3 stationary-phase (B and D) promastigotes were analyzed by Western blotting using anti-L. major LPG (MAb WIC79.3), anti-GP63, or anti-α-tubulin antibodies. (E and F) The relative abundance of LPG (E) and GP63 (F) in lso− and lso−/+LSO was normalized to the levels in LV39 WT promastigotes. Error bars represent standard deviations from 4 experiments (***, P < 0.001). (G and H) Log-phase promastigotes were labeled with MAb WIC79.3, followed by anti-mouse IgG-FITC. DNA was stained with Hoechst. Exposure times for the FITC channel were 100 ms (G) and 300 ms (H). Scale bar, 10 μm.
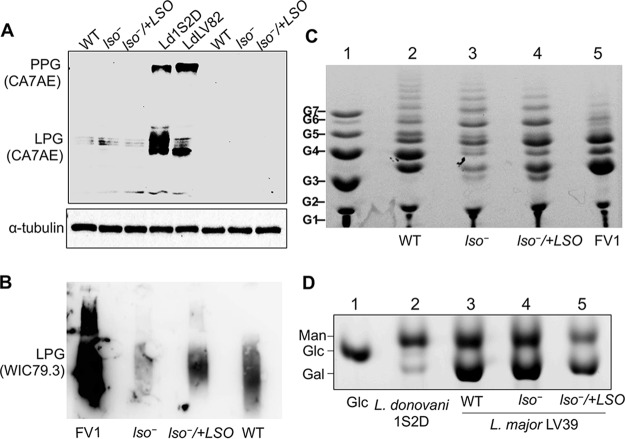
LPG in Leishmania is a complex, polymorphic molecule composed of a lysoalkylphosphatidylinositol lipid anchor, a phosphorylated oligosaccharide core, a phosphoglycan backbone made of Gal (β1,4)-Man (α1)-PO4 repeat units, which may contain side chains, and an oligosaccharide cap (20, 48). The lack of reactivity to WIC79.3 antibody in the lso− mutant could reflect a loss or modification of side chains that branch off the phosphoglycan backbone (49, 50) or deficiencies in the synthesis of lipid anchor, oligosaccharide core, or phosphoglycan backbone (51, 52). To probe the LPG structure in lso− parasites, we first carried out a Western blot analysis using the CA7AE monoclonal antibody, which recognizes the unsubstituted (bare) Gal (β1,4)-Man (α1)-PO4 backbone (53). As shown in Fig. 8A, CA7AE could label the LPG and related proteophosphoglycan from L. donovani (strains lS2D and LV82), which was expected, since their phosphoglycan backbones were devoid of side chains (54). Meanwhile, no significant labeling was detected from L. major WT or lso− parasites with the CA7AE antibody, suggesting that their LPG backbones had side chain modifications (Fig. 8A).
FIG 8.
lso− mutants have fewer short side chain sugar residues on their LPG. (A) Whole-cell lysates were processed for Western blotting with MAb CA7AE or anti-α-tubulin antibody. Log-phase (left three lanes) and day 3 stationary-phase (right three lanes) promastigotes of LV39 WT, lso−, and lso−/+LSO strains were analyzed along with L. donovani strains 1S2D and LV82 (middle two lanes). PPG, proteophosphoglycan. (B) Immunoblotting of purified LPG (5 μg per lane) from L. major strains probed with MAb WIC79.3. (C) FACE analysis of dephosphorylated LPG repeat units from L. major (lanes 2 to 5). Lane 1, malto-oligomer ladder represented by 1 to 7 glucose residues (G1 to G7). (D) Monosaccharide profile of GIPLs. Lane 1, glucose standard; lane 2, type I GIPL from L. donovani 1S2D containing mostly mannose residues and low levels of galactose; lane 3, repeat units of L. major LV39 WT (type II GIPL); lane 4, repeat units of L. major lso−; and lane 5, repeat units of L. major lso−/+LSO. Man, mannose; Glc, glucose; Gal, galactose. Experiments were performed twice, and results from one representative set are shown.
To explore the carbohydrate composition of the LPG side chain from lso− mutants, we purified LPG from WT, lso−, and lso−/+LSO promastigotes as previously described (55). The yield of LPG was similar (150 to 200 μg/1010 cells for all lines), and these LPG samples exhibited reactivity similar to that of the WIC79.3 antibody, as we observed with whole-cell lysates (Fig. 7A and B and 8B). As expected, LPG from the L. major Friedlin V1 strain (positive control) was recognized strongly by this antibody (Fig. 8B) (50).
The LPG samples then were subjected to fluorophore-assisted carbohydrate electrophoresis (FACE) to analyze the sizes of their Gal (β1,4)-Man (α1)-PO4 repeat units. As shown in Fig. 8C, L. major LV39 WT parasites had both short (G3 and G4) and intermediate (G5 to G11) side chains branching off the LPG repeat units, indicative of a mixture of mono-, di- ,and polygalactosylated residues on the side chains (49). Notably, lso− mutants had a profile similar to that of the LV39 WT for the intermediate side chains (G5 to G11), but their short side chains (G3 and G4) were much reduced (Fig. 8C). As expected, L. major FV1 parasites had more short side chains (G3 and G4) than intermediate side chains (G5 to G11), which is consistent with the dominance of Gal1-2 short side chains capped with arabinose in this strain (Fig. 8C) (50, 56, 57).
We next examined if LSO deletion affected the carbohydrate composition of glycoinositolphospholipids (GIPLs), another major glycoconjugate in Leishmania (58). After strong acid hydrolysis, the carbohydrate composition of GIPLs was very similar among WT, lso−, and lso−/+LSO promastigotes, consistent with the galactose- and mannose-rich type II GIPLs (58, 59) (Fig. 8D). This profile was distinct from that of the type I GIPLs in L. donovani, which is highly enriched in mannose (59) (Fig. 8D). Together, these data suggest that LSO deletion does not change the carbohydrate profile of GIPLs but reduces the abundance of short (Gal1-2) side chains on the LPG backbone in L. major.
lso− mutants have minor defects in the mitochondria.
Previous reports indicate that the inhibition of sterol biosynthesis can lead to compromised mitochondrial functions in trypanosomatids (18, 60–62). In S. cerevisiae, LSO/Erg3 is not required for viability in media containing ergosterol, but mutants fail to grow on nonfermentable substrates, such as glycerol and ethanol, suggesting that this enzyme is needed for respiration (31, 63). To assess the role of LSO in mitochondrial functions in L. major, we first examined the mitochondrial membrane potential (ΔΨm) after labeling cells with tetramethylrhodamine ethyl ester (TMRE) (64). Compared to WT and add-back parasites, lso− mutants had 30 to 50% higher ΔΨm in the early stationary stage but did not show significant difference in log phase or late log phase (Fig. S4A). To measure the production of mitochondrial ROS, we labeled cells using a mitochondrion-specific ROS indicator, MitoSox Red. As shown in Fig. S4B, lso− mutants had slightly higher fluorescence signal than the WT and lso−/+LSO parasites, although the difference was not statistically significant, except for that during log phase, suggesting a modest accumulation of ROS in their mitochondria. Next, we used the MitoXpress probe to examine oxygen consumption rate (65) by incubating log-phase promastigotes in a respiration buffer containing sodium pyruvate but no glucose. Under this condition, lso− showed an oxygen consumption rate similar to that of WT and add-back parasites (Fig. S4C). Together, these data indicate that LSO deletion causes minor defects to the mitochondria in L. major.
lso− mutants exhibit mild mitochondrial defects. (A and B) Log-phase and stationary-phase (day 1 to 4) promastigotes were labeled with 100 nM TMRE for 15 min for mitochondrial membrane potential (A) or 5 μM MitoSox Red for 25 min for mitochondrial ROS level (B). Mean fluorescence intensities were determined by flow cytometry. (C) Log-phase promastigotes were resuspended in a respiration buffer (HBSS plus 5 mM 2-deoxyglucose plus 5 mM sodium pyruvate), and oxygen consumption over time was measured after labeling with 1 μM MitoXpress. Error bars represent standard deviations from 3 experiments (*, P < 0.05; **, P < 0.01). Download FIG S4, PDF file, 0.04 MB (40.4KB, pdf) .
Copyright © 2020 Ning et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
lso− mutants show attenuated virulence in a mouse model.
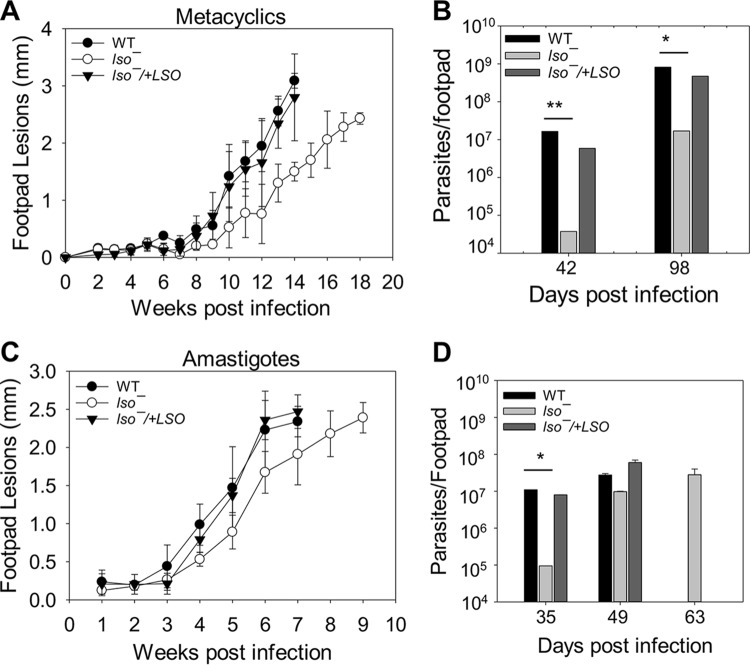
To study the role of LSO in L. major virulence, metacyclics were isolated from stationary-phase cultures and used to infect BALB/c mice in the footpads. Parasite virulence was assessed by measuring the development of footpad lesions over time. Compared to WT and lso−/+LSO parasites, mice infected by lso− mutants showed a 2- to 4-week delay in lesion progression (Fig. 9A), which was consistent with the lower parasite numbers in the infected footpads at weeks 6 and 14 postinfection (Fig. 9B). To explore the virulence of amastigotes, we isolated amastigotes from promastigote-infected footpads and used them to infect naive BALB/c mice. As shown in Fig. 9C and D, lso− amastigotes were slightly attenuated in virulence compared to those of WT and lso−/+LSO amastigotes, but the difference was less pronounced than that of metacyclics. These findings suggest that LSO is important for L. major promastigotes to grow and cause disease in mice.
FIG 9.
lso− mutants show attenuated virulence in mice. BALB/c mice were infected in the footpads with metacyclics (A and B) or amastigotes (C and D). Footpad lesions were measured weekly (A and C), and parasite numbers were determined by the limiting dilution assay (B and D). Error bars represent standard deviations (*, P < 0.05; **, P < 0.01).
DISCUSSION
In this study, we characterized the gene encoding LSO (sterol C-5-desaturase), a sterol biosynthetic enzyme, in the protozoan parasite L. major. LSO catalyzes the formation of a double bond between C-5 and C-6 in the B ring of sterol intermediates (see Fig. S1 in the supplemental material). L. major LSO-null (lso−) mutants were devoid of ergosterol or 5-dehydroepisterol (abundant in WT parasites). Instead, they accumulated C-5–C-6 saturated sterols such as ergosta-7,22-dienol and episterol (Fig. 2 and Fig. S2B to E). While the difference appears to be minor, lso− mutants were 2 to 4 times more resistant to Amp B than WT and lso−/+LSO parasites (Fig. 4A and Table 1). These mutants were fully replicative in culture during the log phase but showed poor viability after entering stationary phase (Fig. 3). LSO deletion also led to hypersensitivity to acidic pH (Fig. 5). These observations are largely in agreement with the phenotypes of the ERG3 mutant in S. cerevisiae (23, 31).
It is interesting that LSO activity appears to contribute (directly or indirectly) to the binding affinity between Amp B and ergostane-based sterols. The C-5–C-6 double bond is conserved among all major sterols, including cholesterol (mammals), ergosterol (fungi and trypanosomatids), and stigmasterol (plants) (15, 23–26). Without this double bond, the A ring could twist/rotate more freely from the B ring, potentially making the sterol core less flat and reducing its binding capacity to Amp B (Fig. S1). Such a change in sterol core conformation could also increase the gap between sterol and phospholipid, making the membrane less stable. This is consistent with the increased sensitivity of lso− mutants to heat and Triton X-100 (Fig. 4C and 5C).
Compared to WT and add-back parasites, lso− mutants had a lower intracellular pH, and the difference became more pronounced when cells were cultivated in a pH 5.0 medium (Table 2). This finding is consistent with their reduced capacity to survive and replicate under the acidic conditions (Fig. 5B and E). Besides affecting the plasma membrane, the loss of LSO may influence the function of intracellular organelles, as sterols are located not only in the plasma membrane but also in the membrane of intracellular organelles. Acidocalcisomes are membrane-enclosed storage organelles involved in osmoregulation, phosphate metabolism, calcium homeostasis, and intracellular pH maintenance in protozoan parasites (41). The expression of VP1, an acidocalcisome-associated vacuolar H+-pyrophosphatase, was lower in lso− parasites during stationary phase (Fig. 6). Since VP1 transports protons from the cytosol into acidocalcisomes (using pyrophosphate hydrolysis as the energy source), the reduced VP1 expression may lead to a more acidic intracellular pH and slower recovery of intracellular pH under acidic conditions (44).
Similar to the C14DM-null mutants, the cellular level of LPG in lso− parasites appeared to be significantly reduced based on Western blot and immunofluorescence microscopy analyses using the WIC79.3 antibody, which recognizes the terminal Gal (β1,3) subunits on the side chain of L. major LPG (46, 62) (Fig. 7 and 8B). However, further analyses suggest that lso− mutants still synthesized bulk glycoconjugates, as their LPG and GIPLs could be extracted and purified to yields similar to those of WT and add-back parasites (LPG, ∼150 μg/1010 cells; GIPLS, ∼60 μg/1010 cells). Lack of reactivity to CA7AE antibody indicates that the LPG backbone in lso− parasites is not bare like that in L. donovani (54) (Fig. 8A). In addition, the FACE result indicates that lso− mutants possess fewer short side chains and similar levels of intermediate side chains compared to those of WT parasites (Fig. 8C). Together, these data imply that the low WIC79.3 reactivity in lso− parasites is not due to a total loss of LPG structure, like several previously characterized LPG-biosynthetic mutants (51, 52, 66). Instead, it is likely caused by the reduced level of terminal Gal (β1,3) subunits on the LPG side chain. In L. major, certain groups of galactosyltransferases and arabinosyltransferases catalyze the attachment of galactose and arabinose, respectively, to the LPG side chains (49, 50). Future studies on the expression of these sugar transferases, along with detailed LPG structure determination in sterol mutants (lso−, c14dm−, and smt−), will help elucidate the molecular mechanism by which sterol synthesis influences LPG production in Leishmania.
Despite these defects, lso− parasites showed only slightly attenuated virulence in BALB/c mice (Fig. 9). While the hypersensitivity to acidic pH and heat likely compromised their ability to survive and replicate in the phagolysosome (39), these defects may be restricted to the promastigote stage and, thus, have only limited impact on disease progression after transitioning to amastigotes. Similarly, LPG is a known virulence factor for L. major promastigotes, but the structural change in lso− parasites may be relatively minor compared to those of LPG synthetic-null mutants (51, 67). Overall, the fitness loss displayed by lso− parasites is similar in severity to that of smt− and less dramatic than that of c14dm− mutants (18, 19).
Based on our characterization of the smt− and lso− mutants, it appears that Leishmania could develop resistance to Amp B and only suffer mild to moderate fitness loss. However, Amp B has been used to treat leishmaniasis, especially visceral leishmaniasis (caused by L. infantum and L. donovani), since the 1960s, and reports of resistance have been scarce (4, 68). In addition, studies on several visceral leishmaniasis clinical isolates suggest that Amp B treatment failure is not due to resistance but other host/parasite factors (69, 70). Since our smt− and lso− mutants were generated in L. major, it would be of interest to determine the degree of fitness loss in equivalent mutants in L. infantum or L. donovani. While C14DM could be deleted from L. major, it is essential for L. donovani (19, 71), so perhaps L. major can better withstand changes in sterol synthesis than those responsible for visceral leishmaniasis infection.
In summary, our study shed new light on the biological roles of LSO in Leishmania sterol synthesis, growth, stress response, and virulence. Along with previous reports on smt− and c14dm− mutants (18, 19), these findings reveal the potential fitness costs associated with mutations conferring Amp B resistance and may offer strategies to counter the development of drug resistance.
MATERIALS AND METHODS
Materials.
M199 medium, cholesta-3,5-diene, phenyl-Sepharose CL-4B, and alkaline phosphatase (from Escherichia coli) were purchased from Sigma-Aldrich (St. Louis, MO). Itraconazole (ITZ) and amphotericin B (Amp B) were purchased from LKT Laboratories, Inc. (St. Paul, MN), and EMD Chemicals, Inc. (San Diego, CA), respectively. MitoXpress oxygen probe was purchased from Luxcel Biosciences (Cork, Ireland). AG50W-X12 cation-exchange and AG1-X8 anion-exchange resins were purchased from Bio-Rad (Hercules, CA). All other chemicals were purchased from Thermo Fisher Scientific unless specified otherwise.
Molecular constructs.
The predicted open reading frame (ORF) of L. major LSO (LmjF.23.1300, 302 amino acids) was amplified by PCR from L. major LV39 WT genomic DNA with primers 649 and 650 (see Table S1 in the supplemental material). The PCR product was digested with BamHI and ligated into the pXG vector to generate pXG-LSO. A modified ORF of LSO was amplified by using primers 649 and 651 to remove the stop codon and then used to generate pXG-LSO-GFP for localization study.
List of oligonucleotides used in this study; sequences in lowercase represent restriction enzyme recognition sites. Download Table S1, PDF file, 0.1 MB (85.6KB, pdf) .
Copyright © 2020 Ning et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To generate knockout constructs, the upstream and downstream flanking sequences (∼550 bp each) of LSO were amplified with primer pairs 645/646 and 647/659, respectively. These flanking sequences were digested and ligated into the cloning vector pUC18. Genes conferring resistance to nourseothricin (SAT) and blasticidin (BSD) were inserted between the upstream and downstream flanking sequences to generate pUC18-KO-LSO::SAT and pUC18-KO-LSO::BSD. All the molecular constructs were confirmed by restriction enzyme digestion and/or sequencing. Oligonucleotides used in this study are summarized in Table S1.
Leishmania culture and genetic manipulation.
Unless otherwise specified, L. major strain LV39 clone 5 (Rho/SU/59/P), L. major strain Friedlin V1 (MHOM/IL/80/Friedlin), L. donovani strain 1S2D (MHOM/SD/00/1S-2D), and L. donovani strain LV82 (MHOM/ET/67:LV82) promastigotes were cultivated at 27°C in M199 medium (pH 7.4, with 10% fetal bovine serum and other supplements) (72). The infective metacyclic promastigotes (metacyclics) were isolated from day 3 stationary-phase promastigotes using the density centrifugation method (73). To generate the LSO-null mutants, the two LSO alleles in L. major LV39 WT parasites were replaced with BSD and SAT by homologous recombination (17). The resulting heterozygous (LSO+/−) and homozygous (lso−) mutants were confirmed by Southern blotting. Briefly, genomic DNA samples were digested and resolved on a 0.7% agarose gel, transferred to a nitrocellulose membrane, and hybridized with [32P]-labeled DNA probes targeting the ORF or a 500-bp upstream region of endogenous LSO. To restore LSO expression, pXG-LSO or pXG-LSO-GFP was introduced into lso− parasites by stable transfection, resulting in lso−/+LSO or lso−/+LSO-GFP parasites, respectively.
Sterol analysis by GC-MS.
Total lipids were extracted from mid-log-phase promastigotes (3 × 106 to 7 × 106 cells/ml) by following the method of Folch et al. (74). An internal standard, cholesta-3,5-diene (formula weight, 368.34), was provided at 2.0 × 107 molecules/cell during extraction. Lipid samples were dissolved in methanol at 1.0 × 109 cell equivalents/ml. Equal amounts of each lipid extract in methanol were transferred to separate vial inserts, evaporated to dryness under nitrogen, and derivatized with 50 μl of BSTFA plus 1% TMCS-acetonitrile (1:3), followed by heating at 70°C for 30 min. GC-MS analysis was conducted on an Agilent 7890A GC coupled with Agilent 5975C MSD in electron ionization mode. Derivatized samples (2 μl each) were injected with a 10:1 split into the GC column with the injector and transfer line temperatures set at 250°C. The GC temperature started at 180°C and was held for 2 min, followed by 10°C/min increase until 300°C and then held for 15 min. To confirm that the unknown GC peak retention time matched that of the episterol standard, we also used a second temperature program started at 80°C for 2 min, ramped to 260°C at 50°C/min, held for 15 min, and increased to 300°C at 10°C/min and held for 10 min. A 25-m Agilent J & W capillary column (DB-1; inner diameter, 0.25 mm; film thickness, 0.1 μm) was used for the separation.
Immunofluorescence microscopy.
For LSO-GFP localization, lso−/+LSO-GFP parasites were labeled with rabbit anti-T. brucei BiP antiserum (1:2,000) for 20 min, followed by goat anti-rabbit IgG-Texas Red antibody (1:1,000) for 20 min. Localizations of LPG were determined as previously described (19). To label acidocalcisomes, stationary day 3 promastigotes were permeabilized with ice-cold ethanol and stained with rabbit anti-T. brucei VP1 (TbVP1) antiserum (1:800) (44) for 30 min, followed by goat anti-rabbit IgG-Texas Red antibody (1:1,000). DNA staining was performed with 1.5 μg/ml Hoechst 33342 for 10 min. Images were acquired using an Olympus BX51 upright fluorescence microscope equipped with a digital camera. To quantify the overlap between LSO-GFP and anti-BiP staining, 30 randomly selected cells were analyzed using Image J JACoP (Just Another Colocalization Plugin) (75). The fluorescence intensity of TbVP1 staining in WT, lso−, and lso−/+LSO parasites was measured from metacyclic-like cells (100 each) using Image J.
Cell growth, stress response, and drug sensitivity.
To measure parasite growth under regular conditions, log-phase promastigotes were inoculated in M199 medium (pH 7.4) at 1.0 × 105 cells/ml and incubated at 27°C. Culture densities were determined daily using a hemocytometer. Percentages of round cells and metacyclics in stationary phase were determined as previously described (19). Parasite growth under the acidic condition was determined using an acidic M199 medium (same as complete M199 medium, except that the pH was adjusted to 5.0 with hydrochloric acid).
To assess cell viability under stress, mid-log-phase promastigotes were incubated in complete M199 medium (pH 7.4) at 37°C (heat stress) in an acidic M199 medium (pH 5.0) at 27°C (acidic stress) or in phosphate-buffered saline (PBS) at 27°C (starvation stress). Cell viability over time was determined by flow cytometry after staining with 5 μg/ml of propidium iodide (dead cells were highly positive for propidium iodide).
To determine sensitivity to drugs, log-phase promastigotes were inoculated in complete M199 medium at 2.0 × 105 cells/ml with different concentrations of Amp B (0.01 to 0.6 μM) or ITZ (0.01 to 13 μM). Percentages of growth were calculated after 48 h by comparing culture densities from drug-treated cells to cells grown in the absence of drugs (18).
To determine sensitivity to detergent, log-phase promastigotes were inoculated in complete M199 medium with 0.0125% Triton X-100 at 2.0 × 105 cells/ml. Cell viability was measured at different time points by flow cytometry after staining with propidium iodide.
Intracellular pH measurement.
Intracellular pH was measured using a pH-sensitive fluorescent indicator, BCECF-AM (76). Briefly, promastigotes were inoculated in the regular (pH 7.4) medium or acidic (pH 5.0) medium at 1.0 × 105 cells/ml as described above. After 3 days, 1.0 × 107 cells were washed once with PBS and resuspended in 500 μl of buffer A (136 mM NaCl, 2.68 mM KCl, 0.8 mM MgSO4, 11.1 mM glucose, 1.47 mM KH2PO4, 8.46 mM Na2HPO4, 1 mM CaCl2, and 20 mM HEPES, pH 7.4) with 10 μM BCECF-AM. After 30 min of incubation, cells were washed twice and resuspended in buffer A. The emission intensity at 535 nm was measured using a microplate reader when samples were excited at 490 nm and 440 nm at the same time. The fluorescence intensity ratio (emission intensity at 535 nm when excited at 490 nm/emission intensity at 535 nm when excited at 440 nm) was converted into an intracellular pH value using a calibration curve, which was generated by measuring fluorescence intensity ratios of cells prepared in pH 5.0-pH 8.0 buffer A containing 10 μM BCECF-AM and 5 μg/ml the ionophore nigericin (77, 78).
Acidocalcisome isolation and analysis of short-chain and long-chain polyphosphate.
Acidocalcisome fractions were isolated from log-phase and stationary-phase promastigotes as described for T. brucei and T. cruzi (79). The amounts of short-chain and long-chain polyphosphate in acidocalcisome fractions were determined as previously described (80).
Western blots.
To determine LPG and GP63 expression, promastigotes were washed once in PBS and resuspended at 5.0 × 107 cells/ml in 1× SDS sample buffer. Samples were boiled for 5 min and resolved by SDS-PAGE, followed by immunoblotting with mouse-anti-L. major LPG monoclonal antibody WIC79.3 (1:1,000) (47), mouse-anti-GP63 monoclonal antibody 235 (1:1,000) (81), mouse-anti-L. donovani LPG monoclonal antibody CA7AE (1:500), or mouse-anti-α-tubulin antibody (1:1,000), followed by a goat anti-mouse IgG-horseradish peroxidase (1:2,000). To examine the expression of LSO-GFP, immunoblotting was performed using a rabbit anti-GFP antiserum (1:1,000) followed by a goat anti-rabbit IgG-horseradish peroxidase (1:2,000). To confirm LPG purification, 5 μg of purified LPG isolated from each strain was subjected to immunoblotting as described above using monoclonal antibody WIC79.3 (1:1,000).
Glycoconjugate extraction, purification, preparation, and FACE.
LPG and GIPLs from Leishmania promastigotes (2 to 4 × 1010 cells each) were extracted in solvent E (H2O-ethanol-diethyl ether-pyridine-NH4OH; 15:15:5:1:0.017) and chloroform-methanol-water (10:10:3), respectively. The extracts were dried by N2 evaporation, resuspended in 0.1 M acetic acid–0.1 M NaCl, and applied to a column of phenyl-Sepharose (2 ml), equilibrated in the same buffer. LPG and GIPLs were eluted using solvent E (82).
To prepare LPG repeat units, the LPG samples were depolymerized by mild acid hydrolysis (0.02 M HCl, 5 min, 100°C). This would generate a mixture of phosphorylated repeat units and core-PI anchor, which were separated after n-butanol–water (2:1) partitioning. Repeat units were collected in the aqueous phase and dephosphorylated with alkaline phosphatase in 15 mM Tris-HCl, pH 9.0 (1 U/ml, 16 h, 37°C). After enzymatic treatment, the repeat units were desalted by passage through a two-layered column of AG50W-X12 (H+) over AG1-X8 (acetate) (55). GIPLs were depolymerized after strong acid hydrolysis (2 M trifluoroacetic acid, 3 h, 100°C) to obtain neutral monosaccharides, samples were dried in a speed-vac, and acid was removed by toluene wash (twice) under N2. GIPL samples were resuspended in 500 ml of water and desalted as described above (59).
LPG repeat units and GIPL monosaccharides then were subjected to FACE analysis. Purified LPG samples were fluorescently labeled with 8-aminonaphthalene-1,3,6-trisulfate and subjected to FACE analysis, and the gel was visualized by a UV imager as described previously (55). To determine the monosaccharide composition of GIPLs, depolymerized and desalted GIPL samples were fluorescently labeled with 0.1 M 2-aminoacridone in 5% acetic acid and 1 M cyanoborohydride. Labeled sugars were subjected to FACE, and the gel was visualized under UV light. Oligo-glucose ladders (G1 to G7) and monosaccharides (d-galactose, d-glucose, and d-mannose) were used as standards for oligosaccharides and monosaccharide gels, respectively (59).
Mitochondria membrane potential, mitochondrial ROS, and oxygen consumption.
Mitochondrial membrane potential was determined as described previously (18). Log- or stationary-phase promastigotes were resuspended at 1.0 × 106/ml in PBS with 100 nM TMRE. After incubation at 27°C for 15 min, cells were washed once with PBS and analyzed by an Attune acoustic flow cytometer. To evaluate superoxide accumulation in mitochondria, promastigotes were resuspended in PBS at 1.0 × 107/ml and labeled with 5 μM MitoSox for 25 min. Cells were washed once with PBS and analyzed by flow cytometry (18). To determine the oxygen consumption rate, log-phase promastigotes were resuspended in a respiration buffer (Hanks’ balanced salt solution with 5.5 mM sodium pyruvate, 5.5 mM 2-deoxy-d-glucose) at 2.0 × 107/ml, and oxygen consumption was measured with 1 μM MitoXpress as described previously (65). WT parasites treated with 10 μM antimycin A were included as a negative control (65).
Mouse footpad infection.
BALB/c mice (female, 7 to 8 weeks old) were purchased from Charles River Laboratories International (Wilmington, MA). Mice were housed and cared for in a facility operated by the Animal Care and Resources Center at Texas Tech University. Procedures involving live mice were approved by the Animal Care and Use Committee at Texas Tech University (PHS-approved animal welfare assurance no. A3629-01). To determine parasite virulence, 2.0 × 105 metacyclics or 2.0 × 104 lesion-derived amastigotes were injected into the footpad of each mouse (5 mice per group). The progression of footpad lesions was monitored weekly using a Vernier caliper. Anesthesia was applied via isoflurane inhalation during footpad injection and measurement. Euthanasia was achieved by CO2 asphyxiation. Parasite loads from infected footpads were determined by the limiting-dilution assay (83).
Statistical analysis.
All experiments were repeated at least three times, except for the Southern blotting. All graphs were made using SigmaPlot 13.0 (Systat Software Inc, San Jose, CA). Differences between two groups were determined by the Student's t test. P values indicating statistical significance were grouped into values of <0.05 (*), <0.01 (**), and <0.001 (***).
ACKNOWLEDGMENTS
We thank Hannah Burks (Texas Tech University, Lubbock, Texas, USA) for technical assistance; W. Robert McMaster (University of British Columbia, Vancouver, Canada) and Jay Bangs (University at Buffalo, State University of New York) for kindly providing the anti-GP63 monoclonal antibody 235 and the rabbit anti-T. brucei BiP polyclonal antiserum, respectively; and Catherine Wakeman (Texas Tech University, Lubbock, Texas, USA) for use of the BioTek synergy 4 fluorescence microplate reader.
This work was supported by National Institutes of Health grants AI099380 (K.Z.), P30DK020579, P30DK056341, and P41GM103422 (Mass Spectrometry Resource of Washington University). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Desjeux P. 2004. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Sunter J, Gull K. 2017. Shape, form, function, and Leishmania pathogenicity: from textbook descriptions to biological understanding. Open Biol 7:170165. doi: 10.1098/rsob.170165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Croft SL, Olliaro P. 2011. Leishmaniasis chemotherapy–challenges and opportunities. Clin Microbiol Infect 17:1478–1483. doi: 10.1111/j.1469-0691.2011.03630.x. [DOI] [PubMed] [Google Scholar]
- 4.Ponte-Sucre A, Gamarro F, Dujardin J-C, Barrett MP, López-Vélez R, García-Hernández R, Pountain AW, Mwenechanya R, Papadopoulou B. 2017. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis 11:e0006052. doi: 10.1371/journal.pntd.0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Payne AH, Hales DB. 2004. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 6.Goad LJ, Holz GG, Jr, Beach DH. 1984. Sterols of Leishmania species. Implications for biosynthesis. Mol Biochem Parasitol 10:161–170. doi: 10.1016/0166-6851(84)90004-5. [DOI] [PubMed] [Google Scholar]
- 7.Parks LW. 1978. Metabolism of sterols in yeast. CRC Crit Rev Microbiol 6:301–341. doi: 10.3109/10408417809090625. [DOI] [PubMed] [Google Scholar]
- 8.Cournia Z, Ullmann GM, Smith JC. 2007. Differential effects of cholesterol, ergosterol and lanosterol on a dipalmitoyl phosphatidylcholine membrane: a molecular dynamics simulation study. J Phys Chem B 111:1786–1801. doi: 10.1021/jp065172i. [DOI] [PubMed] [Google Scholar]
- 9.Lemke A, Kiderlen AF, Kayser O. 2005. Amphotericin B. Appl Microbiol Biotechnol 68:151–162. doi: 10.1007/s00253-005-1955-9. [DOI] [PubMed] [Google Scholar]
- 10.Mesa-Arango AC, Trevijano-Contador N, Román E, Sánchez-Fresneda R, Casas C, Herrero E, Argüelles JC, Pla J, Cuenca-Estrella M, Zaragoza O. 2014. The production of reactive oxygen species is a universal action mechanism of amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob Agents Chemother 58:6627–6638. doi: 10.1128/AAC.03570-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jha TK, Giri YN, Singh TK, Jha S. 1995. Use of amphotericin B in drug-resistant cases of visceral leishmaniasis in north Bihar, India. Am J Trop Med Hyg 52:536–538. doi: 10.4269/ajtmh.1995.52.536. [DOI] [PubMed] [Google Scholar]
- 12.Diro E, Lynen L, Ritmeijer K, Boelaert M, Hailu A, van Griensven J. 2014. Visceral Leishmaniasis and HIV coinfection in East Africa. PLoS Negl Trop Dis 8:e2869. doi: 10.1371/journal.pntd.0002869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mbongo N, Loiseau PM, Billion MA, Robert-Gero M. 1998. Mechanism of amphotericin B resistance in Leishmania donovani promastigotes. Antimicrob Agents Chemother 42:352–357. doi: 10.1128/AAC.42.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purkait B, Kumar A, Nandi N, Sardar AH, Das S, Kumar S, Pandey K, Ravidas V, Kumar M, De T, Singh D, Das P. 2012. Mechanism of amphotericin B resistance in clinical isolates of Leishmania donovani. Antimicrob Agents Chemother 56:1031–1041. doi: 10.1128/AAC.00030-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pountain AW, Weidt SK, Regnault C, Bates PA, Donachie AM, Dickens NJ, Barrett MP. 2019. Genomic instability at the locus of sterol C24-methyltransferase promotes amphotericin B resistance in Leishmania parasites. PLoS Negl Trop Dis 13:e0007052. doi: 10.1371/journal.pntd.0007052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mwenechanya R, Kovářová J, Dickens NJ, Mudaliar M, Herzyk P, Vincent IM, Weidt SK, Burgess KE, Burchmore RJS, Pountain AW, Smith TK, Creek DJ, Kim D-H, Lepesheva GI, Barrett MP. 2017. Sterol 14alpha-demethylase mutation leads to amphotericin B resistance in Leishmania mexicana. PLoS Negl Trop Dis 11:e0005649. doi: 10.1371/journal.pntd.0005649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz A, Beverley SM. 1990. Gene replacement in parasitic protozoa. Nature 348:171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee S, Xu W, Hsu FF, Patel J, Huang J, Zhang K. 2019. Sterol methyltransferase is required for optimal mitochondrial function and virulence in Leishmania major. Mol Microbiol 111:65–81. doi: 10.1111/mmi.14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu W, Hsu F-F, Baykal E, Huang J, Zhang K. 2014. Sterol biosynthesis is required for heat resistance but not extracellular survival in Leishmania. PLoS Pathog 10:e1004427. doi: 10.1371/journal.ppat.1004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McConville MJ, Thomas-Oates JE, Ferguson MA, Homans SW. 1990. Structure of the lipophosphoglycan from Leishmania major. J Biol Chem 265:19611–19623. [PubMed] [Google Scholar]
- 21.Kelly SL, Lamb DC, Kelly DE, Manning NJ, Loeffler J, Hebart H, Schumacher U, Einsele H. 1997. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett 400:80–82. doi: 10.1016/s0014-5793(96)01360-9. [DOI] [PubMed] [Google Scholar]
- 22.Young LY, Hull CM, Heitman J. 2003. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob Agents Chemother 47:2717–2724. doi: 10.1128/aac.47.9.2717-2724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arthington BA, Bennett LG, Skatrud PL, Guynn CJ, Barbuch RJ, Ulbright CE, Bard M. 1991. Cloning, disruption and sequence of the gene encoding yeast C-5 sterol desaturase. Gene 102:39–44. doi: 10.1016/0378-1119(91)90535-j. [DOI] [PubMed] [Google Scholar]
- 24.Matsushima M, Inazawa J, Takahashi E, Suzumori K, Nakamura Y. 1996. Molecular cloning and mapping of a human cDNA (SC5DL) encoding a protein homologous to fungal sterol-C5-desaturase. Cytogenet Cell Genet 74:252–254. doi: 10.1159/000134427. [DOI] [PubMed] [Google Scholar]
- 25.Brumfield KM, Moroney JV, Moore TS, Simms TA, Donze D. 2010. Functional characterization of the Chlamydomonas reinhardtii ERG3 ortholog, a gene involved in the biosynthesis of ergosterol. PLoS One 5:e8659. doi: 10.1371/journal.pone.0008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gachotte D, Husselstein T, Bard M, Lacroute F, Benveniste P. 1996. Isolation and characterization of an Arabidopsis thaliana cDNA encoding a delta 7-sterol-C-5-desaturase by functional complementation of a defective yeast mutant. Plant J 9:391–398. doi: 10.1046/j.1365-313x.1996.09030391.x. [DOI] [PubMed] [Google Scholar]
- 27.Osumi T, Nishino T, Katsuki H. 1979. Studies on the delta 5-desaturation in ergosterol biosynthesis in yeast. J Biochem 85:819–826. [PubMed] [Google Scholar]
- 28.Poklepovich TJ, Rinaldi MA, Tomazic ML, Favale NO, Turkewitz AP, Nudel CB, Nusblat AD. 2012. The cytochrome b5 dependent C-5(6) sterol desaturase DES5A from the endoplasmic reticulum of Tetrahymena thermophila complements ergosterol biosynthesis mutants in Saccharomyces cerevisiae. Steroids 77:1313–1320. doi: 10.1016/j.steroids.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darnet S, Schaller H. 2019. Metabolism and biological activities of 4-methyl-sterols. Molecules 24:451. doi: 10.3390/molecules24030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waterman MR, Lepesheva GI. 2005. Sterol 14 alpha-demethylase, an abundant and essential mixed-function oxidase. Biochem Biophys Res Commun 338:418–422. doi: 10.1016/j.bbrc.2005.08.118. [DOI] [PubMed] [Google Scholar]
- 31.Smith SJ, Parks LW. 1993. The ERG3 gene in Saccharomyces cerevisiae is required for the utilization of respiratory substrates and in heme‐deficient cells. Yeast 9:1177–1187. doi: 10.1002/yea.320091104. [DOI] [PubMed] [Google Scholar]
- 32.Kamthan A, Kamthan M, Datta A. 2017. Expression of C-5 sterol desaturase from an edible mushroom in fission yeast enhances its ethanol and thermotolerance. PLoS One 12:e0173381. doi: 10.1371/journal.pone.0173381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alcazar-Fuoli L, Mellado E, Garcia-Effron G, Buitrago MJ, Lopez JF, Grimalt JO, Cuenca-Estrella JM, Rodriguez-Tudela JL. 2006. Aspergillus fumigatus C-5 sterol desaturases Erg3A and Erg3B: role in sterol biosynthesis and antifungal drug susceptibility. Antimicrob Agents Chemother 50:453–460. doi: 10.1128/AAC.50.2.453-460.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanglard D, Ischer F, Parkinson T, Falconer D, Bille J. 2003. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob Agents Chemother 47:2404–2412. doi: 10.1128/aac.47.8.2404-2412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bangs JD, Uyetake L, Brickman MJ, Balber AE, Boothroyd JC. 1993. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. J Cell Sci 105:1101–1113. [DOI] [PubMed] [Google Scholar]
- 36.Sacks DL, Perkins PV. 1984. Identification of an infective stage of Leishmania promastigotes. Science 223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 37.Sundar S, Chakravarty J. 2010. Liposomal amphotericin B and leishmaniasis: dose and response. J Glob Infect Dis 2:159–166. doi: 10.4103/0974-777X.62886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Podinovskaia M, Descoteaux A. 2015. Leishmania and the macrophage: a multifaceted interaction. Future Microbiol 10:111–129. doi: 10.2217/fmb.14.103. [DOI] [PubMed] [Google Scholar]
- 39.Moradin N, Descoteaux A. 2012. Leishmania promastigotes: building a safe niche within macrophages. Front Cell Infect Microbiol 2:121. doi: 10.3389/fcimb.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pal DS, Abbasi M, Mondal DK, Varghese BA, Paul R, Singh S, Datta R. 2017. Interplay between a cytosolic and a cell surface carbonic anhydrase in pH homeostasis and acid tolerance of Leishmania. J Cell Sci 130:754–766. doi: 10.1242/jcs.199422. [DOI] [PubMed] [Google Scholar]
- 41.Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SN. 2005. Acidocalcisomes—conserved from bacteria to man. Nat Rev Microbiol 3:251–261. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- 42.Docampo R, Jimenez V, Lander N, Li ZH, Niyogi S. 2013. New insights into roles of acidocalcisomes and contractile vacuole complex in osmoregulation in protists. Int Rev Cell Mol Biol 305:69–113. doi: 10.1016/B978-0-12-407695-2.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodrigues CO, Scott DA, Docampo R. 1999. Presence of a vacuolar H+-pyrophosphatase in promastigotes of Leishmania donovani and its localization to a different compartment from the vacuolar H+-ATPase. Biochem J 340:759–766. doi: 10.1042/0264-6021:3400759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemercier G, Dutoya S, Luo S, Ruiz FA, Rodrigues CO, Baltz T, Docampo R, Bakalara N. 2002. A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei. J Biol Chem 277:37369–37376. doi: 10.1074/jbc.M204744200. [DOI] [PubMed] [Google Scholar]
- 45.Brown DA, London E. 1998. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol 164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 46.Kelleher M, Bacic A, Handman E. 1992. Identification of a macrophage-binding determinant on lipophosphoglycan from Leishmania major promastigotes. Proc Natl Acad Sci U S A 89:6–10. doi: 10.1073/pnas.89.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Ibarra A, Howard J, Snary D. 1982. Monoclonal antibodies to Leishmania tropica major: specificities and antigen location. Parasitology 85:523–531. doi: 10.1017/S0031182000056304. [DOI] [PubMed] [Google Scholar]
- 48.Sacks DL. 1992. The structure and function of the surface lipophosphoglycan on different developmental stages of Leishmania promastigotes. Infect Agents Dis 1:200–206. [PubMed] [Google Scholar]
- 49.Dobson DE, Scholtes LD, Valdez KE, Sullivan DR, Mengeling BJ, Cilmi S, Turco SJ, Beverley SM. 2003. Functional identification of galactosyltransferases (SCGs) required for species-specific modifications of the lipophosphoglycan adhesin controlling Leishmania major-sand fly interactions. J Biol Chem 278:15523–15531. doi: 10.1074/jbc.M301568200. [DOI] [PubMed] [Google Scholar]
- 50.Dobson DE, Mengeling BJ, Cilmi S, Hickerson S, Turco SJ, Beverley SM. 2003. Identification of genes encoding arabinosyltransferases (SCA) mediating developmental modifications of lipophosphoglycan required for sand fly transmission of Leishmania major. J Biol Chem 278:28840–28848. doi: 10.1074/jbc.M302728200. [DOI] [PubMed] [Google Scholar]
- 51.Spath GF, Epstein L, Leader B, Singer SM, Avila HA, Turco SJ, Beverley SM. 2000. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A 97:9258–9263. doi: 10.1073/pnas.160257897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spath GF, Lye LF, Segawa H, Sacks DL, Turco SJ, Beverley SM. 2003. Persistence without pathology in phosphoglycan-deficient Leishmania major. Science 301:1241–1243. doi: 10.1126/science.1087499. [DOI] [PubMed] [Google Scholar]
- 53.Tolson DL, Turco SJ, Beecroft RP, Pearson TW. 1989. The immunochemical structure and surface arrangement of Leishmania donovani lipophosphoglycan determined using monoclonal antibodies. Mol Biochem Parasitol 35:109–118. doi: 10.1016/0166-6851(89)90113-8. [DOI] [PubMed] [Google Scholar]
- 54.McConville MJ, Schnur LF, Jaffe C, Schneider P. 1995. Structure of Leishmania lipophosphoglycan: inter- and intra-specific polymorphism in Old World species. Biochem J 310:807–818. doi: 10.1042/bj3100807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soares RP, Barron T, McCoy-Simandle K, Svobodova M, Warburg A, Turco SJ. 2004. Leishmania tropica: intraspecific polymorphisms in lipophosphoglycan correlate with transmission by different Phlebotomus species. Exp Parasitol 107:105–114. doi: 10.1016/j.exppara.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 56.McConville MJ, Turco SJ, Ferguson MA, Sacks DL. 1992. Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. EMBO J 11:3593–3600. doi: 10.1002/j.1460-2075.1992.tb05443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turco SJ, Descoteaux A. 1992. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol 46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 58.McConville MJ, Homans SW, Thomas-Oates JE, Dell A, Bacic A. 1990. Structures of the glycoinositolphospholipids from Leishmania major. A family of novel galactofuranose-containing glycolipids. J Biol Chem 265:7385–7394. [PubMed] [Google Scholar]
- 59.Assis RR, Ibraim IC, Noronha FS, Turco SJ, Soares RP. 2012. Glycoinositolphospholipids from Leishmania braziliensis and L. infantum: modulation of innate immune system and variations in carbohydrate structure. PLoS Negl Trop Dis 6:e1543. doi: 10.1371/journal.pntd.0001543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Medina J, Rodrigues J, De Souza W, Atella G, Barrabin H. 2012. Tomatidine promotes the inhibition of 24-alkylated sterol biosynthesis and mitochondrial dysfunction in Leishmania amazonensis promastigotes. Parasitology 139:1253–1265. doi: 10.1017/S0031182012000522. [DOI] [PubMed] [Google Scholar]
- 61.Kessler RL, Soares MJ, Probst CM, Krieger MA. 2013. Trypanosoma cruzi response to sterol biosynthesis inhibitors: morphophysiological alterations leading to cell death. PLoS One 8:e55497. doi: 10.1371/journal.pone.0055497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pérez-Moreno G, Sealey-Cardona M, Rodrigues-Poveda C, Gelb MH, Ruiz-Pérez LM, Castillo-Acosta V, Urbina JA, González-Pacanowska D. 2012. Endogenous sterol biosynthesis is important for mitochondrial function and cell morphology in procyclic forms of Trypanosoma brucei. Int J Parasitol 42:975–989. doi: 10.1016/j.ijpara.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt CL, Grey M, Schmidt M, Brendel M, Henriques JA. 1999. Allelism of Saccharomyces cerevisiae genes PSO6, involved in survival after 3‐CPs+ UVA induced damage, and ERG3, encoding the enzyme sterol C‐5 desaturase. Yeast 15:1503–1510. doi:. [DOI] [PubMed] [Google Scholar]
- 64.Crowley LC, Christensen ME, Waterhouse NJ. 2016. Measuring mitochondrial transmembrane potential by TMRE staining. Cold Spring Harb Protoc 2016:pdb.prot087361. doi: 10.1101/pdb.prot087361. [DOI] [PubMed] [Google Scholar]
- 65.Cardenas D, Carter PM, Nation CS, Pizarro JC, Guidry J, Aiyar A, Kelly BL. 2015. LACK, a RACK1 ortholog, facilitates cytochrome c oxidase subunit expression to promote Leishmania major fitness. Mol Microbiol 96:95–109. doi: 10.1111/mmi.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Capul AA, Hickerson S, Barron T, Turco SJ, Beverley SM. 2007. Comparisons of mutants lacking the Golgi UDP-galactose or GDP-mannose transporters establish that phosphoglycans are important for promastigote but not amastigote virulence in Leishmania major. Infect Immun 75:4629–4637. doi: 10.1128/IAI.00735-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forestier CL, Gao Q, Boons GJ. 2014. Leishmania lipophosphoglycan: how to establish structure-activity relationships for this highly complex and multifunctional glycoconjugate? Front Cell Infect Microbiol 4:193. doi: 10.3389/fcimb.2014.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alvar J, Croft S, Olliaro P. 2006. Chemotherapy in the treatment and control of leishmaniasis. Adv Parasitol 61:223–274. doi: 10.1016/S0065-308X(05)61006-8. [DOI] [PubMed] [Google Scholar]
- 69.Lachaud L, Bourgeois N, Plourde M, Leprohon P, Bastien P, Ouellette M. 2009. Parasite susceptibility to amphotericin B in failures of treatment for visceral leishmaniasis in patients coinfected with HIV type 1 and Leishmania infantum. Clin Infect Dis 48:e16–e22. doi: 10.1086/595710. [DOI] [PubMed] [Google Scholar]
- 70.Durand R, Paul M, Pratlong F, Rivollet D, Dubreuil-Lemaire ML, Houin R, Astier A, Deniau M. 1998. Leishmania infantum: lack of parasite resistance to amphotericin B in a clinically resistant visceral leishmaniasis. Antimicrob Agents Chemother 42:2141–2143. doi: 10.1128/AAC.42.8.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCall LI, El Aroussi A, Choi JY, Vieira DF, De Muylder G, Johnston JB, Chen S, Kellar D, Siqueira-Neto JL, Roush WR, Podust LM, McKerrow JH. 2015. Targeting ergosterol biosynthesis in Leishmania donovani: essentiality of sterol 14 alpha-demethylase. PLoS Negl Trop Dis 9:e0003588. doi: 10.1371/journal.pntd.0003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kapler GM, Coburn CM, Beverley SM. 1990. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol 10:1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spath GF, Beverley SM. 2001. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol 99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 74.Folch J, Lees M, Stanley GS. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509. [PubMed] [Google Scholar]
- 75.Bolte S, Cordelieres FP. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 76.Vieira L, Lavan A, Dagger F, Cabantchik ZI. 1994. The role of anions in pH regulation of Leishmania major promastigotes. J Biol Chem 269:16254–16259. [PubMed] [Google Scholar]
- 77.Pal DS, Mondal DK, Datta R. 2015. Identification of metal dithiocarbamates as a novel class of antileishmanial agents. Antimicrob Agents Chemother 59:2144–2152. doi: 10.1128/AAC.05146-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manzano JI, Carvalho L, Perez-Victoria JM, Castanys S, Gamarro F. 2011. Increased glycolytic ATP synthesis is associated with tafenoquine resistance in Leishmania major. Antimicrob Agents Chemother 55:1045–1052. doi: 10.1128/AAC.01545-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang G, Moreno SNJ, Docampo R. 2020. Isolation and characterization of acidocalcisomes from trypanosomatids. Methods Mol Biol 2116:673–688. doi: 10.1007/978-1-0716-0294-2_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruiz FA, Rodrigues CO, Docampo R. 2001. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi. J Biol Chem 276:26114–26121. doi: 10.1074/jbc.M102402200. [DOI] [PubMed] [Google Scholar]
- 81.Connell ND, Medina-Acosta E, McMaster WR, Bloom BR, Russell DG. 1993. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guerin expressing the Leishmania surface proteinase gp63. Proc Natl Acad Sci U S A 90:11473–11477. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paranaíba LF, de Assis RR, Nogueira PM, Torrecilhas AC, Campos JH, Silveira A. C d O, Martins-Filho OA, Pessoa NL, Campos MA, Parreiras PM, Melo MN, Gontijo N. d F, Soares RPP. 2015. Leishmania enriettii: biochemical characterisation of lipophosphoglycans (LPGs) and glycoinositolphospholipids (GIPLs) and infectivity to Cavia porcellus. Parasit Vectors 8:31. doi: 10.1186/s13071-015-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Titus RG, Marchand M, Boon T, Louis J. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol 7:545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sterol biosynthesis pathway in L. major WT and lso− parasites. I to VII represent sterol intermediates or final products (underlined). I, lanosterol; II, zymosterol; III, fecosterol; IV, episterol; V, 5-dehydroepisterol; VI, ergosterol; VII, ergosta-7,22-dienol. C14DM, sterol C14α demethylase. SMT, Sterol C24-methyl transferase. LSO, lathosterol oxidase. Download FIG S1, PDF file, 0.4 MB (402.8KB, pdf) .
Copyright © 2020 Ning et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
LSO-GFP expression complements lso− mutants. (A) Whole-cell lysates from log-phase promastigotes of lso−/+LSO-GFP (clones 1 and 2). c14dm−/+C14DM-GFP and WT were analyzed by Western blotting (upper, anti-GFP; lower, anti-α-tubulin). (B to E) Partial GC-MS spectra of lipids from WT (B), lso− (C), lso−/+LSO (D), and lso−/+LSO-GFP (E) promastigotes. The blue dashed lines indicate the shift of peaks in lso− mutants compared to WT and add-backs. Download FIG S2, PDF file, 0.7 MB (736.7KB, pdf) .
Copyright © 2020 Ning et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Short-chain and long-chain phosphate contents are not changed in lso− mutants. Acidocalcisomes from log phase or day 3 stationary-phase promastigotes of WT, lso−, and lso−/+LSO strains were isolated and adjusted to an equivalence of 1.3 × 109 cells/ml. The concentrations of short-chain polyphosphate (A) and long-chain polyphosphate (B) were determined as previously described. Error bars represent standard deviations from 3 repeats. Download FIG S3, PDF file, 0.3 MB (270.4KB, pdf) .
Copyright © 2020 Ning et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
lso− mutants exhibit mild mitochondrial defects. (A and B) Log-phase and stationary-phase (day 1 to 4) promastigotes were labeled with 100 nM TMRE for 15 min for mitochondrial membrane potential (A) or 5 μM MitoSox Red for 25 min for mitochondrial ROS level (B). Mean fluorescence intensities were determined by flow cytometry. (C) Log-phase promastigotes were resuspended in a respiration buffer (HBSS plus 5 mM 2-deoxyglucose plus 5 mM sodium pyruvate), and oxygen consumption over time was measured after labeling with 1 μM MitoXpress. Error bars represent standard deviations from 3 experiments (*, P < 0.05; **, P < 0.01). Download FIG S4, PDF file, 0.04 MB (40.4KB, pdf) .
Copyright © 2020 Ning et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
List of oligonucleotides used in this study; sequences in lowercase represent restriction enzyme recognition sites. Download Table S1, PDF file, 0.1 MB (85.6KB, pdf) .
Copyright © 2020 Ning et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.