Acinetobacter johnsonii has been severely understudied and its population structure and the presence of antibiotic resistance genes (ARGs) are very much uncertain. Our phylogeographical analysis shows that intercontinental transmission has occurred frequently and that different lineages are circulating within single countries; notably, clinical and nonclinical strains are not well differentiated from one another. Importantly, in this species recombination is a significant source of single nucleotide polymorphisms.
KEYWORDS: Acinetobacter johnsonii, population structure, antibiotic resistance, emerging pathogens, horizontal gene transfer, population genomics
ABSTRACT
Acinetobacter johnsonii has been severely understudied and its population structure and the presence of antibiotic resistance genes (ARGs) are very much uncertain. Our phylogeographical analysis shows that intercontinental transmission has occurred frequently and that different lineages are circulating within single countries; notably, clinical and nonclinical strains are not well differentiated from one another. Importantly, in this species recombination is a significant source of single nucleotide polymorphisms. Furthermore, our results show this species could be an important reservoir of ARGs since it has a significant amount of ARGs, and many of them show signals of horizontal gene transfer. Thus, this study clearly points out the clinical importance of A. johnsonii and the urgent need to better appreciate its genomic diversity.
OBSERVATION
Acinetobacter johnsonii has not been studied as much as A. baumannii, and few studies have been carried out to examine this species. A. johnsonii has been found in aquatic sources, human skin, and animals (1, 2). However, it also causes severe human infections (3–6), highlighting its clinical importance. For instance, Turton et al. showed that 1.7% of 690 nonduplicate Acinetobacter isolates associated with bacteremia were A. johnsonii (6). Moreover, Cleland et al. identified A. johnsonii as a relevant pathogen involved in chronic rhinosinusitis (7), and some studies have described A. johnsonii isolates carrying antibiotic resistance genes (ARGs) (3, 8, 9). For example, different carbapenemase genes, such as blaNDM-1 and blaOXA-58, have been identified in A. johnsonii (3, 9). The first description of blaNDM-1-positive A. johnsonii occurred in two isolates recovered from sewage in China in 2010 (9), and another isolate (also collected from sewage) was found to coproduce the plasmid-encoded carbapenemases NDM-1, OXA-58, and PER-1 (3). Interestingly, NDM-1 has also been found in phages not only in A. johnsonii (10) but also in A. baumannii (11). Hence, this species could be a potential reservoir of ARGs against last-line antibiotics, which is particularly worrying since these genes can be transferred to other clinically relevant microorganisms.
Population genomics studies are needed to achieve a better understanding of the phylogeny and the ARGs within A. johnsonii. Although two previous studies conducted some comparative genomics analyses of A. johnsonii (3, 4), these only considered a very small number of genomes. Thus, our aim was to characterize the phylogeography and ARGs in A. johnsonii using all the genomes available to date. The lack of information on A. johnsonii is clear; as of 16 March 2020, there were only 31 genomes on the National Center for Biotechnology Information database. We downloaded these genomes (see Table S1 in the supplemental material) and corroborated that they were A. johnsonii by conducting an average nucleotide identity (ANI) analysis via OrthoANI (12). All but one genome (UBA3112) belonged to A. johnsonii since they shared ANI values higher than the 95% (the cutoff value for species demarcation) when they were compared. Of note, UBA3112 and UBA8888 were not included in downstream analyses because they did not have high-quality genomes according to CheckM (13) (see also the footnote for Table S1).
List of the genomes and their metadata used for this study. BioSample number, source, and country of origin are provided for the isolates. In addition, it is indicated whether the isolate is related to hospital settings or not (HA, fifth column). The last column indicates cluster to which the isolate belonged according to the population structure analysis. Download Table S1, DOCX file, 0.02 MB (18.8KB, docx) .
Copyright © 2020 Castillo-Ramírez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
A pangenome analysis through Roary (14) yielded a total of 13,531 groups of homologous genes (GHGs), most of them (89%) within the accessory genome (see Table S2). The strict core genome consisted of 1,538 GHGs and the majority (∼67%) was found in 15% or fewer of the genomes (see Table S2). Moreover, this is an open pangenome (see Fig. S1), since the number of GHGs kept growing as more genomes were considered without tailing off; we therefore did not fully sample the gene repertoire of this species. Then, to evaluate the level of synteny, we conducted a genome alignment considering five genomes (one from each of the clusters identified in the population structure analysis [see below]) using progressiveMauve (15). Figure S2 shows that a significant number of inversions and large-scale changes occurred within these genomes, indicating that this species has undergone a considerable amount of genome rearrangement.
Pangenome analysis of A. johnsonii. The numbers of core homologous groups (conserved genes) and the total numbers of homologous groups are plotted versus the number of genomes. This is an open pangenome because the total number of homologous groups (dashed line) keeps growing as more genomes are added, without any signal of reaching a plateau. Download FIG S1, PDF file, 0.01 MB (13.4KB, pdf) .
Copyright © 2020 Castillo-Ramírez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genome alignment of five A. johnsonii genomes, one from each of the clusters defined by the population structure analysis. The alignment was generated using progressiveMauve. Download FIG S2, JPG file, 0.8 MB (866.8KB, jpg) .
Copyright © 2020 Castillo-Ramírez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Details about the pangenome analysis. The numbers of genes for the core and accessory genomes are provided. Download Table S2, DOCX file, 0.01 MB (13.1KB, docx) .
Copyright © 2020 Castillo-Ramírez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
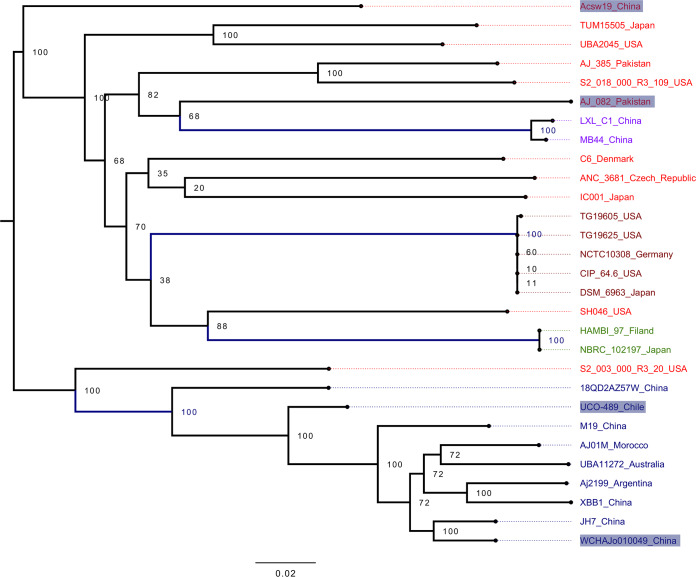
To establish the population structure and the evolutionary relationships of these isolates, maximum-likelihood phylogeny using PhyML (16) (model GTR+R+I) and population structure analyses via hierBAPS (17) (in Rstudio, with K = 20) were conducted on the core genome alignment, which had 161,087 segregating sites and a nucleotide diversity of 0.0295. We found five genetically differentiated clusters (colored labels in Fig. 1), and four seemed to be real populations, since they were monophyletic groups according to the phylogeny (blue, maroon, green, and purple labels in the figure); in contrast, cluster 2 appeared to be an exclusion group (red labels, Fig. 1). Some of the real clusters had isolates from different continents. For instance, cluster 1 (blue labels) had isolates from South America (Chile and Argentina), Africa (Morocco), Asia (China), and Australia, whereas cluster 3 (maroon labels) contained isolates from Asia (Japan), Europe (Germany), and North America (USA). In addition, different lineages can be circulating in the same country. For instance, isolates from China were found in three of the clusters; this pattern also applies for the Japanese and U.S. strains. Remarkably, we noted that in cluster 2 the clinical isolates (XBB1, Aj2199, and UCO-489) grouped together with environmental isolates such as JH7 (recovered from mine tailings), WCHAJo010049 (collected from sewage), or 18QD2AZ57W (sampled from pig feces).
FIG 1.
Phylogeny and population structure of A. johnsonii. The phylogeny was made on the core genome alignment. Strains are colored according to the clusters found in the population structure analysis and are coded as follows: blue, cluster 1; red, cluster 2; maroon, cluster 3; green, cluster 4; and purple, cluster 5. Gray rectangles denote the isolates having the carbapenemase NDM-1 gene. The numbers by the nodes give the bootstrap values for the nodes, and the scale bar shows the number of substitutions per site.
Thus, these analyses reveal a clear population structure in this species, where some clusters are composed of isolates from distant geographic regions, showing that intercontinental transmission has occurred frequently. Furthermore, different lineages circulate within single countries, implying that several introduction events have happened in the same country. Importantly, there seems to be no clear delimitation between clinical and nonclinical isolates. We used Gubbins (18) to assess the impact of homologous recombination. Clearly, recombination is of paramount importance since the average per-branch recombination/mutation ratio was 4.64, implying that recombination is introducing almost five times more single nucleotide polymorphisms than does mutation.
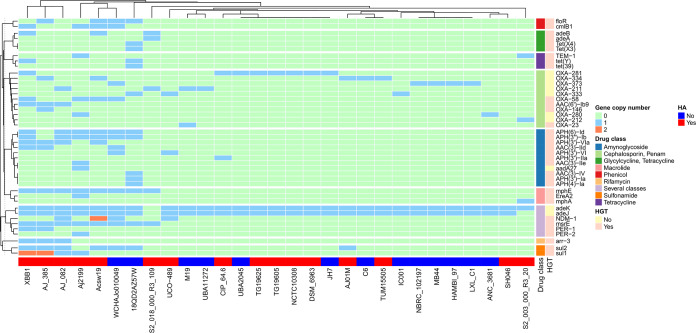
Finally, we conducted an in silico prediction of ARGs by conducting BLAST searches (similarity criteria, ≥80% identity and ≥70% coverage) of the A. johnsonii proteomes against the Comprehensive Antibiotic Resistance Database (19). Notably, all the strains, even the environmental ones, had at least two ARGs (see Fig. 2); for instance, isolates C6 and LXL_C1 both had oxacillinases and multidrug efflux resistance-nodulation-division (RND) transporter genes. We found resistant determinants for several drug classes in many isolates (see Fig. 2, drug class). We also looked for mutations conferring resistance to fluoroquinolones via ResFinder (20), but we did not find any. In agreement with previous studies (3, 4), we found some β-lactamase genes (blaNDM-1, blaPER-1, blaPER-2, and blaOXA-58). In addition to some clinical isolates, two sewage strains (Acsw19 and WCHAJo010049) and a strain collected from pig feces (18QD2AZ57W) had the largest amount of ARGs. In this regard, Tang et al. determined that strain Acsw19 has 12 ARGs in plasmids and in the chromosome (10). Considering the OXA β-lactamases, we found several families: OXA-211-like, OXA-58-like, and OXA-23-like. However, the most abundant—OXA-281, OXA-334 and OXA-373—belong to the OXA-211-like family, which was described rather recently in non-baumannii Acinetobacter spp. Remarkably, many ARGs have undergone horizontal gene transfer (HGT) since 81% of them had identical sequences in other bacteria from clinically relevant genera such as Salmonella, Klebsiella, Vibrio, etc. (see Fig. 2 and Table S3). As a case in point, the carbapenemase NDM-1 was present in four isolates (see gray rectangles in Fig. 1) on noncontiguous branches of the tree, implying independent acquisitions of this gene, and identical sequences of this gene were found in many genera other than Acinetobacter (see Table S3). Taken together, these results show that many strains, both clinical and nonclinical, had ARGs with signals of HGT and thus could function as a reservoir of ARGs for other bacteria.
FIG 2.
Antibiotic resistance genes in A. johnsonii. ARGs in A. johnsonii were predicted in silico. A heat map shows the frequency of ARGs in the A. johnsonii isolates. Antibiotic classes are color-coded. A dendrogram at the top of the figure shows a hierarchical clustering analysis of the strains according to ARG presence. Next to the drug class column, there is a column (HGT key) specifying whether the ARG had identical sequences in other species (salmon) or not (yellow). The row below the heat map indicates whether (red) or not (blue) the isolates are associated with hospitals (HA key).
ARGs with identical sequences in other bacteria. All the species that had sequences identical to the ARG in A. johnsonii are listed in the organism column. When there were no identical sequences in other species, only A. johnsonii is listed in organism column. Download Table S3, XLSX file, 0.01 MB (13.9KB, xlsx) .
Copyright © 2020 Castillo-Ramírez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In conclusion, we highlight the clinical relevance of this species, since environmental and clinical strains are intermingled with one another, and all the strains show ARGs. Further (genomic and functional) studies of clinical and nonclinical strains are needed to fully understand the clinical nature of this species.
ACKNOWLEDGMENTS
S.C.-R. and V.M.-E. are very grateful to Alfredo José Hernández Álvarez and Victor Manuel del Moral Chávez for installing some of the programs used for this study on our servers.
This study was supported by National Fund for the Scientific and Technological Development (FONDECYT) of Chile (FONDECYT-Iniciación, grant number 11190602) to A.O.-C. and by the ANID Millennium Science Initiative/ Millennium Initiative for Collaborative Research on Bacterial Resistance, MICROB-R, NCN17_081, to A.O.-C. and G.G.-R. This study was also partially supported by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (grant IN206019) and the Consejo Nacional de Ciencia y Tecnología Ciencia Básica 2016 (grant 284276) to S.C.-R.
All authors report no potential conflicts of interest.
REFERENCES
- 1.Guardabassi L, Dalsgaard A, Olsen JE. 1999. Phenotypic characterization and antibiotic resistance of Acinetobacter spp. isolated from aquatic sources. J Appl Microbiol 87:659–667. doi: 10.1046/j.1365-2672.1999.00905.x. [DOI] [PubMed] [Google Scholar]
- 2.Seifert H, Dijkshoorn L, Gerner-Smidt P, Pelzer N, Tjernberg I, Vaneechoutte M. 1997. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. J Clin Microbiol 35:2819–2825. doi: 10.1128/JCM.35.11.2819-2825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y, Yang P, Wang X, Zong Z. 2016. Characterization of Acinetobacter johnsonii isolate XBB1 carrying nine plasmids and encoding NDM-1, OXA-58 and PER-1 by genome sequencing. J Antimicrob Chemother 71:71–75. doi: 10.1093/jac/dkv324. [DOI] [PubMed] [Google Scholar]
- 4.Montaña S, Schramm STJ, Traglia GM, Chiem K, Parmeciano Di Noto G, Almuzara M, Barberis C, Vay C, Quiroga C, Tolmasky ME, Iriarte A, Ramírez MS. 2016. The genetic analysis of an Acinetobacter johnsonii clinical strain evidenced the presence of horizontal genetic transfer. PLoS One 11:e0161528. doi: 10.1371/journal.pone.0161528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seifert H, Strate A, Schulze A, Pulverer G. 1993. Vascular catheter-related bloodstream infection due to Acinetobacter johnsonii (formerly Acinetobacter calcoaceticus var. lwoffi): report of 13 cases. Clin Infect Dis 17:632–636. doi: 10.1093/clinids/17.4.632. [DOI] [PubMed] [Google Scholar]
- 6.Turton JF, Shah J, Ozongwu C, Pike R. 2010. Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: evidence for emerging species. J Clin Microbiol 48:1445–1449. doi: 10.1128/JCM.02467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleland EJ, Bassiouni A, Vreugde S, Wormald P-J. 2016. The bacterial microbiome in chronic rhinosinusitis: richness, diversity, postoperative changes, and patient outcomes. Am J Rhinol Allergy 30:37–43. doi: 10.2500/ajra.2016.30.4261. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez CH, Nastro M, Dabos L, Barberis C, Vay C, Famiglietti A. 2014. First isolation of Acinetobacter johnsonii co-producing PER-2 and OXA-58 beta-lactamases. Diagn Microbiol Infect Dis 80:341–342. doi: 10.1016/j.diagmicrobio.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Zong Z, Zhang X. 2013. blaNDM-1-carrying Acinetobacter johnsonii detected in hospital sewage. J Antimicrob Chemother 68:1007–1010. doi: 10.1093/jac/dks505. [DOI] [PubMed] [Google Scholar]
- 10.Tang L, Shen W, Zhang Z, Zhang J, Wang G, Xiang L, She J, Hu X, Zou G, Zhu B, Zhou Y. 2020. Whole-genome analysis of two copies of blaNDM-1 gene carrying Acinetobacter johnsonii strain Acsw19 isolated from Sichuan, China. Infect Drug Resist 13:855–865. doi: 10.2147/IDR.S236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Leal G, Santamaria RI, Cevallos MÁ, Gonzalez V, Castillo-Ramírez S. 2020. Prophages encode antibiotic resistance genes in Acinetobacter baumannii. Microb Drug Resist doi: 10.1089/mdr.2019.0362. [DOI] [PubMed] [Google Scholar]
- 12.Lee I, Ouk Kim Y, Park SC, Chun J. 2016. OrthoANI: an improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol 66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- 13.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, Holden MT, Fookes M, Falush D, Keane JA, Parkhill J. 2015. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darling AE, Mau B, Perna NT. 2010. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 17.Cheng L, Connor TR, Siren J, Aanensen DM, Corander J. 2013. Hierarchical and spatially explicit clustering of DNA sequences with BAPS software. Mol Biol Evol 30:1224–1228. doi: 10.1093/molbev/mst028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, Parkhill J, Harris SR. 2015. Rapid phylogenetic analysis of large samples of recombinant bacterial whole-genome sequences using Gubbins. Nucleic Acids Res 43:e15-e15. doi: 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FS, Wright GD, McArthur AG. 2017. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of the genomes and their metadata used for this study. BioSample number, source, and country of origin are provided for the isolates. In addition, it is indicated whether the isolate is related to hospital settings or not (HA, fifth column). The last column indicates cluster to which the isolate belonged according to the population structure analysis. Download Table S1, DOCX file, 0.02 MB (18.8KB, docx) .
Copyright © 2020 Castillo-Ramírez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Pangenome analysis of A. johnsonii. The numbers of core homologous groups (conserved genes) and the total numbers of homologous groups are plotted versus the number of genomes. This is an open pangenome because the total number of homologous groups (dashed line) keeps growing as more genomes are added, without any signal of reaching a plateau. Download FIG S1, PDF file, 0.01 MB (13.4KB, pdf) .
Copyright © 2020 Castillo-Ramírez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genome alignment of five A. johnsonii genomes, one from each of the clusters defined by the population structure analysis. The alignment was generated using progressiveMauve. Download FIG S2, JPG file, 0.8 MB (866.8KB, jpg) .
Copyright © 2020 Castillo-Ramírez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Details about the pangenome analysis. The numbers of genes for the core and accessory genomes are provided. Download Table S2, DOCX file, 0.01 MB (13.1KB, docx) .
Copyright © 2020 Castillo-Ramírez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ARGs with identical sequences in other bacteria. All the species that had sequences identical to the ARG in A. johnsonii are listed in the organism column. When there were no identical sequences in other species, only A. johnsonii is listed in organism column. Download Table S3, XLSX file, 0.01 MB (13.9KB, xlsx) .
Copyright © 2020 Castillo-Ramírez et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.